Abstract
Background
The aim of this study was to compare the impact of preexisting diabetes mellitus (pre-DM), posttransplant DM (PTDM), and non-DM on the long-term outcomes of renal transplant recipients (RTRs).
Methods
This is a retrospective observational cohort study of 427 RTRs who underwent transplantation from 1999 to 2008. Patients were divided into non-DM, pre-DM, and PTDM groups. The primary outcome was the composite of doubling of the serum creatinine (SCr) level, graft failure, or death. Secondary outcomes were biopsy-proven acute rejection (BPAR), biopsy-proven interstitial fibrosis and/or tubular atrophy (IF/TA), and individual components of the primary outcome.
Results
A total of 70 patients (16.4%) had pre-DM, 104 (24.2%) had PTDM, and 253 (59.3%) had non-DM. Kaplan–Meier analysis indicated significant differences in the development of the primary outcome: p = 0.003 (log rank test). Relative to the non-DM group, the pre-DM group had a 6.36-fold increased risk [95% confidence interval (CI) 2.43–16.33; p < 0.001), and the PTDM group had a 2.00-fold increased risk (95% CI 1.08–3.73; p = 0.029) for development of the primary outcome. Patients in the pre-DM group had 6.73-fold (95% CI 2.46–18.42; p < 0.001), 4.56-fold (95% CI 1.77–11.78; p = 0.002), and 13.95-fold (95% CI 2.96–65.75; p < 0.001) increased risks for the development of SCr doubling, biopsy-proven IF/TA, and death-censored graft failure, respectively. Patients in the PTDM group had a 2.09-fold (95% CI 1.10–3.99; p = 0.025), increased risk for the development of SCr doubling.
Conclusions
The presence of pre-DM or PTDM significantly impaired kidney allograft outcome.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
End-stage renal disease (ESRD) is a significant public health problem in the United States, United Kingdom, and especially Taiwan. Diabetic kidney disease is the major cause of the high prevalence and incidence of ESRD [1–3]. Most patients with ESRD and diabetes mellitus (DM) also have multiple chronic co-morbid macrovascular complications, such as congestive heart failure, ischemic heart disease, and peripheral vascular diseases, all of which contribute to the high mortality and morbidity associated with ESRD. In Taiwan, the number of patients with ESRD who require dialysis has increased over the last decade. The survival rates of dialysis patients with DM versus those without DM are significantly lower at 1 year (81.5% vs. 89.4%), 5 years (37.1% vs. 63.5%), and 10 years (17.5% vs. 44.0%) (p < 0.001) [4].
Kidney transplantation is one type of renal replacement therapy for pretransplant DM (pre-DM) patients with ESRD. However, previous studies showed that the presence of pre-DM was the major predictor of poor graft and patient survival in renal transplant recipients (RTRs) [5–7]. In addition, previous research has shown that DM recipients appear to have a higher risk for delayed graft function [odds ratio (OR) 1.67, 95% confidence interval (CI) 1.46–1.93; p < 0.01) [8].
Post-transplant DM (PTDM) is a significant morbidity that can occur after kidney transplantation. PTDM has a significant impact on renal allograft outcomes, cardiovascular diseases, and infection [9, 10]. The United States Renal Data System (USRDS), which was the largest epidemiologic study to examine PTDM incidence in kidney recipients, indicated an elevated incidence of PTDM (14–16%) during the first posttransplant year, followed by an annual incidence of 4–6% [11]. PTDM is influenced by genetic background and immunosuppressive protocols [12]. Joss et al. reported that the patients with PTDM had 5- and 10-year survival rates of 86.1% and 67.1%, respectively, whereas those without DM had 5- and 10-year survival rates of 90.0% and 81.9%, respectively [12]. Additionally, Siraj et al. demonstrated that relative to patients without DM, patients with PTDM had a higher risk of cardiovascular disease (22% vs. 6%, p = 0.033), infection (52% vs. 30%, p = 0.036), and graft rejection (47% vs. 23%, p = 0.018) [13].
The metabolic influences in pre-DM and PTDM patients are similar, and the two groups have similar adverse outcomes from allografts. We utilized the longitudinal results of a 10-year follow-up study at a single Taiwanese tertiary care university-affiliated hospital to compare the impact of pre-DM and PTDM on the long-term outcomes of RTRs.
Materials and methods
For this retrospective observational cohort study, we recruited 427 patients who had undergone renal transplantation from December 1999 to January 2008. We based the enrollment of patients on a two-sided type I error rate of 5%, 80% power, and a study that showed an 8-year patient survival of 80% in the non-DM group, 63% in the PTDM group, and 29% in the pre-DM group. Hjelmesaeth et al. conducted that study, in which they observed 201 patients from 1995 to 2004 in the Norwegian Renal Registry [14].
We enrolled 70 patients in the pre-DM group and 104 patients in the PTDM group and compared them to 253 patients in the non-DM group. Clinical data, including age, sex, human leukocyte antigen, parental DM, follow-up duration after transplantation, hypertension (HTN), cardiovascular events—cerebrovascular accident (CVA), coronary artery disease (CAD), peripheral artery occlusive disease (PAOD)—and use of tacrolimus-based or cyclosporine-based immunosuppressive regimens, were recorded. HTN was defined as a systolic blood pressure ≥140 mmHg, a diastolic blood pressure ≥90 mmHg, a prior diagnosis of HTN, or treatment with anti-HTN medications [15]. CVA was defined as having a history of a transient ischemic attack or a CVA [16]. CAD was diagnosed by cardiac catheterization. PAOD was defined as a confirmed ankle-brachial index of <0.9 [17]. HTN, CVA, CAD, and PAOD were diagnosed before the patients underwent renal transplantation. The follow-up period began on the date of the kidney transplantation and continued until a primary outcome or the end of the study (December 2009). The institutional review board of our hospital approved this study.
Each patient was placed in a non-DM, pre-DM, or PTDM group. Patients were defined as having pre-DM if they met the criteria of type 2 DM or used an oral anti-DM medication or insulin prior to renal transplantation. PTDM was diagnosed according to the American Diabetes Association guidelines [18] using the criteria: fasting blood glucose of at least 126 mg/dl; or symptoms of diabetes plus plasma glucose concentration of at least 200 mg/dl at any time of day; or 2-h postload glucose of at least 200 mg/dl during an oral glucose tolerance test. Patients who did not meet the definitions of pre-DM or PTDM were assigned to the non-DM group.
Primary composite outcome
The primary outcome was a composite endpoint at the time of first event, which comprised doubling of serum creatinine (SCr), graft failure, or patient death. Graft failure was defined as the return to dialysis or retransplantation and was censored for death. Doubling of SCr, graft failure, and patient survival were chosen as the primary composite outcome because they encompassed well-defined, clinically relevant outcomes for both patients and physicians [19, 20].
Secondary outcomes
The secondary outcomes included individual components of the primary outcome: biopsy-proven acute rejection (BPAR) and biopsy-proven interstitial fibrosis and/or tubular atrophy (IF/TA). BPAR and IF/TA were confirmed histologically. For patients who underwent several biopsies with repeated reports of acute rejection or chronic IF/TA, the time of the first biopsy was recorded. Diagnoses of BPAR and biopsy-proven IF/AT were based on the Banff classification [21].
Statistical analysis
The Kruskal–Wallis H test and the χ2 test were used for analysis of continuous and categorical data, respectively. Results are presented as means ± standard deviations or as percentages. Graft survival was calculated from the date of transplantation until the date of graft loss (i.e., first retransplantation, graft nephrectomy, maintenance dialysis). Patients who were alive or who died with a functioning graft at the date of the last-known follow-up were right censored. The cumulative incidence and difference between time-to-event probabilities of primary and secondary outcomes were analyzed by the Kaplan–Meier method and the log-rank test. The adjusted Cox regression model considered confounders of HTN and those with p < 0.05 in demographic comparison—age, sex, hepatitis C virus (HCV) infection, parental DM, CVA, CAD, PAOD, and duration of renal replacement therapy—for calculating the adjusted hazard ratio (aHR) of pre-DM and PTDM relative to non-DM. A value of p < 0.05 was considered statistically significant. All data were analyzed using Medcalc version 11.2 statistical software.
Results
There were 427 eligible patients who received renal transplants from December 1999 to January 2008 (Table 1). A total of 240 patients (56%) were male and 187 patients (44%) were female, and the mean age at surgery was 54.3 ± 10.7 years. Prior to renal transplantation, 69.1% of the patients underwent hemodialysis, 15.2% had peritoneal dialysis, and the others (15.7%) had medical therapy without dialysis. The mean duration of renal replacement therapy was 25.9 months (range 0–204 months). Based on American Diabetes Association (ADA) criteria, 70 patients (16.4%) and 104 patients (24.2%) were diagnosed with pre-DM and PTDM, respectively. The prevalences of HTN, CVA, CAD, PAOD, hepatitis B virus (HBV) and HCV infections were 82.7% (353/427), 3% (13/427), 6.1% (26/427), 4.2% (18/427), 11.5% (49/427), and 9.8% (42/427), respectively. The average posttransplant follow-up duration was 72.7 ± 23.3 months (range 11.2–124.6 months). There were significant differences in age (p < 0.001); sex (p < 0.001); the presence of CVA (p = 0.013), CAD (p < 0.001), PAOD (p = 0.004), HCV infection (p = 0.027), parental DM (p < 0.001); and duration of dialysis (p = 0.01) among the groups.
Primary composite outcome
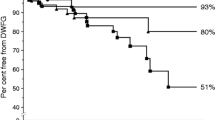
A total of 9.9% of patients (25/253) in the non-DM group, 25.7% of patients (18/70) in the pre-DM group, and 16.3% of patients (17/104) in the PTDM group reached the primary outcome (Table 2). Kaplan–Meier analysis indicated a significant difference in the percentage of patients reaching the primary outcome (p = 0.003, log rank test) (Fig. 1). Subgroup analysis indicated that patients in the pre-DM group were more likely to develop the primary outcome than those in the non-DM group (p < 0.001, log-rank test), but the difference between the pre-DM and PTDM groups was not significant. Cox proportional regression analysis indicated that relative to the non-DM group there was a 6.36-fold increased risk for developing the primary outcome in the pre-DM group (95% CI 2.43–16.63; p < 0.001) and a 2.00-fold increased risk in the PTDM group (95% CI 1.08–3.73; p = 0.029) (Table 3).
Secondary outcomes
A total of 65 patients (15.2%), 53 patients (12.4%), 54 patients (12.6%), 12 patients (2.8%), and 17 patients (4.0%) were diagnosed with BPAR, biopsy-proven IF/AT, doubling of SCr, graft failure, and patient death, respectively. Table 2 compares the percentage of patients of each group who reached each component of the secondary outcomes. A χ2 test indicated significant differences in SCr doubling (p = 0.014), IF/TA (p = 0.015), and graft failure (p = 0.043) for the three groups. The BPAR (p = 0.284) and patient survival (p = 0.077) were similar for the three groups.
For the secondary outcomes, Kaplan–Meier analysis indicated that the three groups had significantly different time-to-event probabilities of the SCr doubling time, biopsy-proven IF/TA, and death-censored graft failure (p = 0.018, 0.014, and 0.037, respectively; log rank tests). Patients in the pre-DM group had a 6.73-fold (95% CI 2.46–18.42; p < 0.001), 4.56-fold (95% CI 1.77–11.78; p = 0.002), and 13.95-fold (95% CI 2.96–65.75; p < 0.001) increased risk for the development of SCr doubling, biopsy-proven IF/TA, and death-censored graft failure, respectively. Patients in the PTDM group had a 2.09-fold (95% CI 1.1–3.99; p = 0.025) increased risk for the developing SCr doubling (Table 3), but was comparable to the non-DM group in regard to developing biopsy-proven IF/TA (aHR 1.29; 95% CI 0.63–2.65; p = 0.49) and graft failure (aHR 0.44; 95% CI 0.05–3.73; p = 0.456). The BPAR and patient survival of the three groups were comparable (p = 0.238 and 0.401, respectively, log-rank tests).
Subgroup analysis demonstrated that the pre-DM group had a higher probability for developing SCr doubling, IF/TA, and death-censored graft survival than the non-DM group (p = 0.004, 0.004, and 0.039, respectively; log-rank tests). The pre-DM group had higher probabilities for developing biopsy-proven IF/TA and death-censored graft failure than the PTDM group (p = 0.046 and 0.029, respectively; log-rank tests). All components of the secondary outcomes in the PTDM and the non-DM groups were comparable (Fig. 2). The risk of all-cause mortality of the three groups were comparable (p = 0.401, log-rank test) (Fig. 3).
Discussion
This 10-year observational cohort study used a composite endpoint for comparing long-term allograft outcomes among the pre-DM, non-DM, and PTDM populations. Our results indicate that RTRs with pre-DM had the highest risk (aHR 6.36, 95% CI 2.43–16.63) for developing the primary composite outcome (SCr doubling, graft failure, patient survival), and that RTRs with PTDM had a higher secondary risk (aHR 2.0, 95% CI 1.08–3.73) than the non-DM RTRs. Moreover, analysis of the secondary outcomes indicated that the three groups also had significantly different risks for development of SCr doubling, biopsy-proven IF/TA, and graft survival, but not BPAR.
Increasing evidence indicates that type 2 DM leads to an elevated risk for developing chronic kidney disease and long-term mortality and morbidity following a diagnosis of ESRD [2–4, 22, 23]. Also, long-term hyperglycemia is associated with the development of microvascular and cardiovascular diseases. In particular, the atherogenic properties of hyperglycemia are particularly significant in RTRs, who have marked predispositions for CVD [10, 24]. Previous studies have suggested implementation of better glucose control for these patients because RTRs with DM have poorer survival, poorer graft survival [7], and delayed graft function [8].
Joss et al. conducted a retrospective observational study of 787 RTRs from 1994 to 2004 in western Scotland [12]. Among patients who died with functional grafts, 14.0% had pre-DM, 10.9% had PTDM, and 7.7% had no DM (p = 0.01). These results indicate that DM patients (pre-DM and PTDM) had poorer survival than non-DM patients [12]. Wiesbauer et al. suggested that an elevated glucose level is independently associated with higher patient mortality in pre-DM RTRs and that tight glycemic control is necessary to prevent the typical pathohistologic changes associated with diabetic nephropathy in these patients [5].
The prevalence of PTDM in the present study was about 24.2%, somewhat higher than that reported by several previous observational studies (9.0–103.8 months), which reported ranges of 7.4% to 23.0% [13, 25]. The median onset time of PTDM in our RTRs was 5.3 months (25–75% IQR: 1.4–35.6 months). Based on the DIRECT study conducted by Vincenti et al. [26], however, the prevalence of PTDM in the non-DM population (no DM before transplantation) at 6 months after transplantation was 26% (73/281) in cyclosporine-treated patients and 33.6% (95/286) in tacrolimus-treated patients (p = 0.046). The overall prevalence of PTDM in their study was 29.6% (168/567). In our study, the overall prevalence of PTDM was 24.2% (104/427). However, after excluding those who were diagnosed to have DM before undergoing renal transplantation, the prevalence was 29.1% (104/357), which was similar to the DIRECT study. The previously identified risks that predispose patients to PTDM are advanced age, obesity, male sex, non-Caucasian race, family history of diabetes, use of immunosuppressive medications, and HCV infection [10, 13, 27]. These risk factors are all related to inherited or acquired defects in insulin sensitivity and beta cell function, which contribute to glucose dysregulation [27, 28]. The atherogenic property of hyperglycemia contributes to a marked predisposition to CVD in RTRs [10]. Recently, we reported that homozygosity of the 5G allele of the PAI-1 gene provides protection against the development of PTDM [29], similar to the results of another study of gestational DM [30]. Moreover, Hjelmesaeth et al. reported that PTDM patients were at higher risk for major cardiovascular events than non-DM patients [14]. PTDM patients are also at higher risk of adverse graft effects and poorer patient survival [9, 10, 12, 13]. In the present study, RTRs with PTDM had greater primary composite outcome and SCr doubling than RTRs with no DM (aHR 2.0, 95% CI 1.08–3.73 and aHR 2.09, 95% CI 1.1–3.99, respectively) after adjusting with the confounders of age, sex, HCV infection, parental DM, HTN, CVA, CAD, PAOD, and duration of renal replacement therapy.
Analysis of the USRDS registry demonstrated that pre-DM and PTDM were independent risk factors for myocardial infarction within the first 36 months after transplantation [31]. Cosio et al. showed that RTRs with pre-DM had a significantly higher incidence of posttransplant cardiovascular events and all-cause mortality than non-DM patients [32]. Hjelmesaeth et al. reported that the 8-year survival was 80% for non-DM patients, 63% for PTDM patients, and 29% for pre-DM patients [14]. Additionally, in the First National Health and Nutrition Examination Survey (U.S. population, 1971–1993), Gu et al. reported that in a general population the mortality rates were highest for those with at least 15 years of DM [33]. In the present study, we found that RTRs with pre-DM had the poorest long-term outcome and that those with PTDM had intermediate risk of poor outcome. These differences appear to be associated with the duration of exposure to the hyperglycemia milieu.
Previous studies have reported that the mortality rates of DM patients in Taiwan and the United States were 39.0 and 141.8 per 1000 person-years, respectively, higher than in the general populations without DM; they also found that DM patients who are elderly and male had poorer outcomes [32–34]. We found significant differences among our three groups based on sex (p < 0.001), age (p < 0.001), and parental DM (p < 0.001); in contrast, in patients with CVA (p = 0.013), CAD (p < 0.001), PAOD (p = 0.004), or HCV infection (p = 0.027), the all-cause death rate was comparable between groups after adjusting for these risk factors. It is unknown whether these comparable patient survival rates are due to the improved metabolic control by newly developed multiagents or to the lower mortality rate of DM patients in Taiwan than in the United States.
The limitations of this study include its retrospective observational characteristics and the limited number of enrolled patients in only one center. For example, it is possible that in patients with DM various changes might have occurred in their immunosuppression in attempts to achieve better diabetic control during the course of the follow-up. Such events might have an impact on the results of a retrospective study.
Conclusions
Our results indicate that patients with pre-DM or PTDM have a more adverse long-term primary composite outcome. Also, we infer that the duration of DM appears to play a major role in the susceptibility to a deleterious allograft outcome.
References
Foley RN, Collins AJ (2007) End-stage renal disease in the United States: an update from the United States Renal Data System. J Am Soc Nephrol 18:2644–2648
Boddana P, Caskey F, Casula A et al (2009) UK Renal Registry 11th Annual Report (December 2008): Chapter 14 UK Renal Registry and international comparisons. Nephron Clin Pract 111(Suppl 1):c269–c276
Hwang SJ, Yang WC, Lin MY et al (2010) Impact of the clinical conditions at dialysis initiation on mortality in incident haemodialysis patients: a national cohort study in Taiwan. Nephrol Dial Transpl 25:2616–2624
Yang WC, Hwang SJ (2008) Incidence, prevalence and mortality trends of dialysis end-stage renal disease in Taiwan from 1990 to 2001: the impact of national health insurance. Nephrol Dial Transpl 23:3977–3982
Wiesbauer F, Heinze G, Regele H et al (2010) Glucose control is associated with patient survival in diabetic patients after renal transplantation. Transplantation 89:612–619
Kuo HT, Sampaio MS, Vincenti F, et al (2010) Associations of pretransplant diabetes mellitus, new-onset diabetes after transplant, and acute rejection with transplant outcomes: an analysis of the Organ Procurement and Transplant Network/United Network for Organ Sharing (OPTN/UNOS) Database. Am J Kidney Dis 1127–1139
Einollahi B, Jalalzadeh M, Taheri S et al (2008) Outcome of kidney transplantation in type 1 and type 2 diabetic patients and recipients with posttransplant diabetes mellitus. Urol J 5:248–254
Parekh J, Bostrom A, Feng S (2010) Diabetes mellitus: a risk factor for delayed graft function after deceased donor kidney transplantation. Am J Transpl 10:298–303
Montori VM, Basu A, Erwin PJ et al (2002) Posttransplantation diabetes: a systematic review of the literature. Diabetes Care 25:583–592
Bloom RD, Crutchlow MF (2008) Transplant-associated hyperglycemia. Transpl Rev (Orlando) 22:39–51
Woodward RS, Schnitzler MA, Baty J et al (2003) Incidence and cost of new onset diabetes mellitus among U.S. wait-listed and transplanted renal allograft recipients. Am J Transpl 3:590–598
Joss N, Staatz CE, Thomson AH et al (2007) Predictors of new onset diabetes after renal transplantation. Clin Transpl 21:136–143
Siraj ES, Abacan C, Chinnappa P et al (2010) Risk factors and outcomes associated with posttransplant diabetes mellitus in kidney transplant recipients. Transpl Proc 42:1685–1689
Hjelmesaeth J, Hartmann A, Leivestad T et al (2006) The impact of early-diagnosed new-onset post-transplantation diabetes mellitus on survival and major cardiac events. Kidney Int 69:588–595
Chobanian AV, Bakris GL, Black HR et al (2003) The seventh report of the Joint National Committee on prevention, detection, evaluation, and treatment of high blood pressure: the JNC 7 report. JAMA 289:2560–2572
Abelha FJ, Botelho M, Fernandes V et al (2009) Determinants of postoperative acute kidney injury. Crit Care 13:R79
Merino J, Planas A, Elosua R et al (2010) Incidence and risk factors of peripheral arterial occlusive disease in a prospective cohort of 700 adult elderly men followed for 5 years. World J Surg 34:1975–1979. doi:10.1007/s00268-010-0572-7
2005 Clinical Practice Recommendations 2005. Diabetes Care 28(Suppl 1):S1–S79
Fellstrom B, Holdaas H, Jardine AG et al (2005) Risk factors for reaching renal endpoints in the Assessment of Lescol in Renal Transplantation (ALERT) trial. Transplantation 79:205–212
Knoll GA, Cantarovitch M, Cole E et al (2008) The Canadian ACE-inhibitor trial to improve renal outcomes and patient survival in kidney transplantation: study design. Nephrol Dial Transpl 23:354–358
Solez K, Colvin RB, Racusen LC et al (2007) Banff ‘05 meeting report: differential diagnosis of chronic allograft injury and elimination of chronic allograft nephropathy (‘CAN’). Am J Transpl 7:518–526
Longenecker JC, Coresh J, Powe NR et al (2002) Traditional cardiovascular disease risk factors in dialysis patients compared with the general population: the CHOICE Study. J Am Soc Nephrol 13:1918–1927
Kuo HW, Tsai SS, Tiao MM et al (2007) Epidemiological features of CKD in Taiwan. Am J Kidney Dis 49:46–55
Villar E, Rabilloud M, Berthoux F et al (2004) A multicentre study of registration on renal transplantation waiting list of the elderly and patients with type 2 diabetes. Nephrol Dial Transpl 19:207–214
Araki M, Flechner SM, Ismail HR et al (2006) Posttransplant diabetes mellitus in kidney transplant recipients receiving calcineurin or mTOR inhibitor drugs. Transplantation 81:335–341
Vincenti F, Friman S, Scheuermann E et al (2007) Results of an international, randomized trial comparing glucose metabolism disorders and outcome with cyclosporine versus tacrolimus. Am J Transpl 7:1506–1514
Sharif A, Baboolal K (2010) Risk factors for new-onset diabetes after kidney transplantation. Nat Rev Nephrol 6:415–423
Van Hooff JP, Christiaans MH, van Duijnhoven EM (2005) Tacrolimus and posttransplant diabetes mellitus in renal transplantation. Transplantation 79:1465–1469
Chang HR, Yang SF, Tsai JP et al (2010) Plasminogen activator inhibitor-1 5G/5G genotype is a protecting factor preventing posttransplant diabetes mellitus. Clin Chim Acta 412:322–326
Leipold H, Knoefler M, Gruber C et al (2006) Plasminogen activator inhibitor 1 gene polymorphism and gestational diabetes mellitus. Obstet Gynecol 107:651–656
Lentine KL, Brennan DC, Schnitzler MA (2005) Incidence and predictors of myocardial infarction after kidney transplantation. J Am Soc Nephrol 16:496–506
Cosio FG, Hickson LJ, Griffin MD et al (2008) Patient survival and cardiovascular risk after kidney transplantation: the challenge of diabetes. Am J Transpl 8:593–599
Gu K, Cowie CC, Harris MI (1998) Mortality in adults with and without diabetes in a national cohort of the U.S. population, 1971–1993. Diabetes Care 21:1138–1145
Tseng CH (2004) Mortality and causes of death in a national sample of diabetic patients in Taiwan. Diabetes Care 27:1605–1609
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Tsai, JP., Lian, JD., Wu, SW. et al. Long-Term Impact of Pretransplant and Posttransplant Diabetes Mellitus on Kidney Transplant Outcomes. World J Surg 35, 2818–2825 (2011). https://doi.org/10.1007/s00268-011-1287-0
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00268-011-1287-0