Abstract
Background
Detection of normal and pathologic parathyroid glands often is difficult due to their variability in number and location. We have implemented photosensitizer-induced fluorescence for the routine intraoperative identification of parathyroids for the surgical treatment of hyperparathyroidism.
Methods
From 2004 to 2007, 25 patients suffering from primary and secondary hyperparathyroidism underwent minimally invasive videoscopic-assisted parathyroidectomy after oral photosensitization with aminolevulinic acid (ALA).
Results
Fluorescence was sufficiently strong in 48% of patients to aid faster detection of the glands in situ. In an additional 44%, the fluorescence behavior supported the identification of the glands in situ and after excision, yielding a total of 92% of glands whose identity could be confirmed by the fluorescence technique.
Conclusions
Fluorescence-guided minimally invasive parathyroidectomy is technically feasible and may support the surgeon in detecting and confirming the parathyroid glands. As the fluorescence method requires only moderate additional technical efforts and clinical expenditure, it is a valuable add-on component in parathyroid surgery to facilitate the operation.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Intraoperative identification of the parathyroid glands can be a challenge even for experienced surgeons. The glands are small, soft, well-camouflaged, and variable in position and number, necessitating a meticulous dissection during surgery for hyperparathyroidism (HPT) [1].
Fluorescence diagnosis using aminolevulinic acid (ALA) has been described to identify normal parathyroid glands [2, 3] and enlarged glands in hyperparathyroidism [4] in an experimental setting. As an intermediate of the heme biosynthesis, ALA is a naturally occurring substance and precursor of the fluorescent protoporphyrin IX (PpIX), which can act as a photosensitizer. After systemic application of ALA, the photosensitizer accumulated within the parathyroid glands of rats. By specific blue light illumination, using a xenon light source, the D-Light, the parathyroids were detectable by the emitted red fluorescence [5].
After successful experimental evaluation, we were the first to transfer this technique to human surgery for intraoperative localization of parathyroid glands in patients with primary and secondary hyperparathyroidism [6, 7].
This article reports our initial experience gained with fluorescence-guided minimally invasive parathyroidectomy in the first series of patients. The purpose of the study was to assess the feasibility of this new technique and to evaluate its potential role in the management of hyperparathyroidism.
Patients and methods
This was a prospective, nonrandomized and nonblinded clinical study undertaken in the surgical department of the medical center Mannheim of the University of Heidelberg. Beginning in 2004, patients who required surgery with the diagnosis of primary and secondary hyperparathyroidism were evaluated for fluorescence-guided minimally invasive parathyroidectomy. All patients eligible for participating in the study were fully informed about the study, which had been approved by the ethics committee of the Faculty of Medicine, University of Heidelberg.
Twenty-five patients (15 women and 10 men of median age 52 years, range 21–76 years) had no exclusion criteria (e.g., younger than aged 18 years, porphyria, participation in another study) and were entered after written consent into the study. Twenty-one patients suffered from primary hyperparathyroidism (pHPT) and 4 patients from secondary hyperparathyroidism (sHPT).
Preoperative technetium-99m-2-methoxyisobutylisonitrile (Tc-MIBI or sestamibi) scan was available in 19 patients. Preoperative ultrasonography was performed in 19 patients and CT or NMR imaging was performed in 6 patients.
Photosensitization
All patients were photosensitized with 30 mg/kg BW of ALA (Photonamic, Wedel, Germany) dissolved in water 4 h before surgery. The patients were shielded from direct light exposure for 48 h to avoid photobleaching and phototoxic reactions.
Blood samples to determine the serum transaminases were taken before photosensitization and 4, 8, and 12 h after ALA application. All patients were monitored regularly for heart rate and blood pressure. In addition, potential side effects, such as nausea and vomiting, were recorded.
Operation and fluorescence technique
Fluorescence-guided, minimally invasive parathyroidectomy in terms of a video-assisted gasless open technique was performed under general anesthesia with the patient in a supine position and the neck slightly extended. Two different neck incisions were evaluated: in 23 patients with pHPT and all patients with sHPT, a small equilateral suprasternal incision was made. The cervical fascia was incised in the midline and the left strap muscles were retracted laterally. The thyroid lobe was rotated anteriorly and medially to expose the posterior surface of the portion of the thyroid with the parathyroid glands. In two patients with pHPT and an unequivocal localization of the adenoma, a unilateral approach with collar incision directly over the site of the gland and overlying the medial border of the sternocleidomastoid muscle was performed.
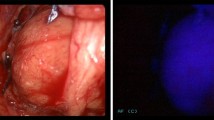
Tissue dissection was performed under normal white light conditions using the D-Light™ system (Karl Storz, Tuttlingen, Germany) and a specific 4-mm scope (Hopkins™ II, 30°, Karl Storz) connected to a modified CCD camera (Tricam SL PDD™, Karl Storz). The D-Light™ system is based on a 300-W xenon short arc lamp with special optical properties to focus high intensities of light. It allows two different illumination modes: (1) a conventional, white light mode; and (2) a specific blue light (380–440 nm) mode for ALA fluorescence excitation [5].
After preparation of the anatomical region where the enlarged parathyroid was suspected, the white light was switched to blue light. All fluorescent areas were further isolated from surrounding tissue and examined. Structures that macroscopically appeared to be parathyroid gland were evaluated by their fluorescence pattern and intensity during the process of surgical preparation. The removed parathyroid glands were medially incised to examine the parenchymatous fluorescence.
The value of fluorescence-guidance was determined by its capability to speed up identification of the parathyroid glands resulting in reduced operation time. In addition, the success of the procedure was evaluated by the reliability of the fluorescence to allow an “optical biopsy.”
Histology and biochemical analysis
The diagnosis of parathyroid adenoma in pHPT or hyperplasia in sHPT was achieved by histological examinations, aided by frozen sections, and gross morphology of all removed glands. In addition, confirmation of surgical success was attained by a significant decrease (>50%) of intraoperative parathyroid hormone (PTH) from baseline serum concentration 15 minutes after removal of the glands.
Results
By definition, fluorescence-guided minimally invasive parathyroidectomies for pHPT resulted in removal of the enlarged gland. Parathyroidectomies for sHPT resulted in subtotal removal of the hyperplastic glands in one patient, in total removal with autotransplantation of a morcellated portion of one gland into the sternocleidomastoid muscle in three cases, and without autotransplantation in one patient.
In 28% (7 patients), preoperative imaging was wrong and misleading: in four patients MIBI scintigraphy was unable to demonstrate any enlarged gland; in one case scintigraphy suggested a nonexisting supernumeric adenoma; and in two patients preoperative diagnosis localized the gland in the inferior position when, in fact, the patient had a superior adenoma.
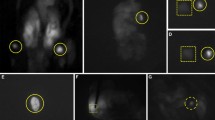
In 92% (23 patients), the chosen ALA dose was effective to achieve a sufficient photosensitization of the parathyroid glands. In 12 of these patients (48%), high fluorescence intensities could be observed within the parenchyma of the glands (Figs. 1, 2). The homogenous fluorescence allowed precise differentiation of macroscopically resembling parathyroid glands and soft tissue. In these cases, fluorescence guidance was considered beneficial for faster detection of the glands, as well as, immediate confirmation of the glands by their fluorescence behaviour, in terms of an “optical biopsy.” Although a direct comparison was not possible due to the noncomparative study design, it was felt that the total operation time was reduced by shortening the intraoperative search of the glands.
In the remaining 11 patients (44%), photosensitization led to an inhomogeneous fluorescence pattern with much localized small fluorescence spots mainly at the edges of the glands (Fig. 3). In these cases, the extent of fluorescence was not enough to help parathyroid tissue stand out in contrast to background soft tissue and thyroid gland, limiting the macroscopic detectability of these glands in the blue light mode. Therefore, the technique did not allow for faster identification and dissection of the parathyroid glands in these patients. In these cases, the advantage of fluorescence diagnosis was solely seen in the immediate optical confirmation of the gland, replacing the results of frozen sections, due to the high specificity of the fluorescence technique: In all 25 cases, we observed only three false-positive findings: in one patient with previous metastatic mammary carcinoma a lymph node showed a strong red fluorescence, although it was stated tumor-free by the pathologist; in two other cases, fluorescent thyroid nodules were found, which were histologically classified as adenomas.
In 8% (2 patients), the intraoperative application of fluorescence diagnosis was without any benefit. In these cases, the parathyroid glands showed no areas of fluorescence and remained dark. All patients had prompt resolution of the elevated PTH serum concentrations the day after operation.
Photosensitization with ALA did not cause any clinical phototoxic side effects. The protection of the patients under subdued room light for 48 h after surgery did not burden the patients. A slight-to-moderate elevation of liver enzymes was observed in 12 patients (48%) within the first postoperative week (alanine aminotransferase: mean 149 U/l, range 34–380 U/l; aspartate aminotransferase: mean 97 U/l, range 39–240 U/l) with no development of hepatic symptoms.
Conclusions
After 1925, Felix Mandl performed the first successful parathyroidectomy [8]. The technique of parathyroidectomy has traditionally involved a bilateral exploration of the neck with the intent of visualizing all parathyroids and resecting pathologically enlarged glands. However, 80–90% of patients suffering from pHPT have a solitary parathyroid adenoma, which requires the excision only of this particularly gland for cure. Hence, a unilateral approach was first introduced by Tibblin et al. [9]. In keeping with current trends toward less invasive surgery, various minimally invasive parathyroidectomy procedures have been developed, ranging from fully endoscopic approaches with gas insufflation to video-assisted gasless techniques and minimally invasive open parathyroidectomies.
The success of a minimally invasive approach is based mainly on preoperative imaging studies. MIBI scintigraphy, which is currently considered to be the routine “gold standard” with the highest sensitivity, can accurately localize a high proportion of solitary adenomas but its usefulness is diminished by its inability to consistently identify smaller glands and those in the upper position [10]. In secondary HPT, the effectiveness of scintigraphy appears to be even lower than in primary HPT [11].
Hence, intraoperative diagnostic modalities have been developed to serve as useful adjuncts to the safe and successful conduct of parathyroid surgery. However, all tested techniques, such as radioguided parathyroidectomy by intraoperative use of MIBI scintigraphy [12–14] or methylene blue staining [1] of parathyroid glands, were not accepted for routine use due to their complexity and inaccuracy.
In 2001 we were able to prove the capability of rodent parathyroid glands to emit fluorescence after systemic photosensitization with aminolevulinic acid [2]. After intense evaluation of this phenomenon in an experimental setting with normal and pathologically enlarged glands [3, 4], our institution was the first to apply fluorescence-guided minimally invasive parathyroidectomy with a video-assisted gasless open technique in 2004 for the treatment of pHPT [6] and sHPT [7].
Photosensitizer-induced fluorescence, formerly known as photodynamic diagnosis (PDD), is increasingly used to distinguish healthy from diseased tissue in various medical disciplines. The technique is based on the accumulation of administered or metabolized agents, so-called photosensitizers, usually in tumors and inflamed tissue. After systemic or topical application, the agent concentrates in diseased cells and remains inactive until exposed to light of a specific wavelength. When light is delivered to the site, it causes fluorescence of the photosensitizers. Whereas earlier generations of photosensitizers, such as Photofrin, were fluorescent at the time of application (exogenous photosensitizers), the photosensitizer aminolevulinic acid (ALA), a natural precursor of the heme pathway, requires endogenous metabolism to the fluorescent protoporphyrin IX (PpIX) (endogenous photosensitizer). When PpIX is stimulated by a defined wavelength within its absorption spectrum, it emits a typical red fluorescence at approximately 635 nm. In contrast to earlier photosensitizers, ALA is characterized by a low incidence of side effects, such as skin sensitivity (phototoxicity), nausea, and vomiting. Transiently elevated liver transaminase levels can be observed a few days after application of ALA without a risk to the patient, as confirmed by our data [15].
ALA-induced PpIX is cleared within 48 h, which reduces the risk for phototoxic skin reactions. No serious side-effects have been reported after systemic or topical administration of ALA in humans so far.
The data of our study prove the technical feasibility of fluorescence-guided minimally invasive parathyroidectomy as a routine operation. The fluorescence method requires only moderate additional technical and clinical expenditure for a hospital already performing minimally invasive surgery. Mandatory prerequisites are a special light source, capable of emitting the blue excitation light, and a camera system equipped with spectral filters to allow the visualization of the fluorescence. An optical system, such as the D-Light™ by Karl Storz, is already commercially available for diagnostic applications (e.g., in urology).
In our study, surgery for most patients with pHPT was performed through a small skin incision, resulting in a satisfactory cosmetic outcome and an overall less invasive procedure; the mini-incision open technique is thought to be the least invasive of all parathyroidectomy procedures. Patients with sHPT required a larger incision due to the bilateral neck exploration and the need for more working space.
During surgery, as well as 48 h after the operation, all patients were shielded from direct light exposure. No photobleaching or phototoxic reactions were observed. The precaution to operate under subdued light conditions in the operating room with the light directed only toward the operative site was initially unaccustomed. However, the measures in this extent were probably exaggerated as the utilized light intensities and ALA serum levels are too low to cause relevant side effects.
The fluorescence technique was clearly beneficial in 48% of patients in helping to detect the glands in the operation area. The emitted red fluorescence allowed definite identification of the glands and increased dissection speed. During preparation and resection of the glands, the fluorescence demarcated the edges of the parathyroid tissue and guaranteed complete removal of the enlarged glands (Fig. 4). In patients with pHPT parathyroid tissue that was unintentionally remaining after resection was discovered by its fluorescence and consecutively removed to avoid recurrences (Fig. 5).
In contrast, after subtotal parathyroidectomy for sHPT, the red fluorescence allowed the verification of parathyroid tissue left to maintain parathyroid hormone activity (Fig. 6). After total parathyroidectomy, the fluorescence technique helped to identify endocrinologic active tissue during autotransplantation and control the result by seeing fluorescent tissue in the sternocleidomastoid muscle after reinsertion (Fig. 7).
In 44%, the fluorescence technique was helpful in terms of an “optical biopsy”: the advantage of the fluorescence method was seen in the immediate optical confirmation of an adequate removal of the right tissue. Due to a low false-positive rate, fluorescent tissue could be determined with a relatively high likelihood as parathyroid gland. Therefore, cumbersome resection of parathyroid resembling tissue and its time-consuming histopathologic examination in frozen sections was avoided in some cases. Unfortunately, the fluorescence pattern and intensity within the glands was not homogenous or strong enough to speed up their intraoperative identification.
A relevant drawback of the fluorescence technique is given by the biophysical property of light-penetrating tissue; the blue light which is necessary to stimulate PpIX within its absorption spectrum reaches a penetration depth of only a few millimeters. Therefore, surgery with broad exposure of the area close to the glands is still mandatory, characterizing the fluorescence technique as “near-field” detection method. This also means that the fluorescence technique is not a substitute for careful and meticulous dissection by a reasonably experienced parathyroid surgeon, as well as knowledge of normal parathyroid anatomy and its potential variations. It is merely a useful, additional tool to help guide dissection down to a preoperatively localized adenoma or hyperplastic gland.
It remains unelucidated why parathyroid glands are almost selectively fluorescent after ALA application. In addition it is unclear why the fluorescence pattern shows such variety, ranging from a homogenous distribution, over sporadic red spots to a complete lack of fluorescence. It is known that the localization of ALA and PpIX in tissue is a function of metabolic activity and an accumulation occurring specifically in the mitochondria. Because the cells of parathyroid glands and adenoma contain a proportionally higher number of mitochondria compared with other tissue, it can be hypothesized that the overall uptake of ALA and the subsequent fluorescence in parathyroid glands is linked to the blood flow, gland size, and mitochondrial activity—a phenomenon also observed with MIBI scintigraphy, which is released much faster from thyroid than from parathyroid tissue. However, histologic examinations of the removed glands did not prove any significant differences in microscopic structure of the glands, which could explain the various fluorescence characteristics.
In conclusion, the real place of the fluorescence technique in parathyroid surgery needs to be further evaluated by the inclusion of more patients. This study has demonstrated that fluorescence-guided minimally invasive parathyroidectomy is technically feasible and easy enough to be learned and applied by many surgeons. When used together with preoperative ultrasonography and sestamibi scan, the fluorescence technique may support the surgeon in detecting and confirming the parathyroid glands. At this early stage of clinical implementation, the fluorescence technique is not intended to replace any of the diagnostic tools that are part of modern minimally invasive parathyroidectomy. However, because the fluorescence method requires only moderate additional technical efforts, it can be a valuable add-on component in parathyroid surgery to facilitate the operation.
References
Orloff LA (2001) Methylene blue and sestamibi: complementary tools for localizing parathyroids. Laryngoscope 111:1901–1904
Gahlen J, Winkler S, Flechtenmacher C et al (2001) Intraoperative fluorescence visualization of the parathyroid gland in rats. Endocrinology 142:5031–5034
Prosst RL, Schroeter L, Gahlen J (2004) Kinetics of intraoperative fluorescence diagnosis of parathyroid glands. Eur J Endocrinol 150:743–747
Prosst RL, Schroeter L, Gahlen J (2005) Enhanced ALA-induced fluorescence in hyperparathyroidism. J Photochem Photobiol B 79:79–82
Gahlen J, Prosst RL, Herfarth Ch (2000) Blue light illumination for minimally-invasive fluorescence detection of tumors: technology, clinical experience and future perspectives. Minim Invasive Ther Allied Technol 9:119–124
Prosst RL, Willeke F, Schroeter L et al (2006) Fluorescence-guided minimally invasive parathyroidectomy: a novel detection technique for parathyroid glands. Surg Endosc 20:1488–1492
Prosst RL, Gahlen J, Schnuelle P et al (2006) Fluorescence-guided minimally invasive parathyroidectomy: a novel surgical therapy for secondary hyperparathyroidism. Am J Kidney Dis 48:327–331
Mandl F (1925) Therapeutischer versuch bei osteitis fibrosa generalisata mittels exstirpation eines epithelkörperchentumors. Wien Klin Wochenschr 50:1343–1344
Tibblin S, Bondeson AG, Ljungberg O (1982) Unilateral parathyroidectomy in hyperparathyroidism due to single adenoma. Ann Surg 195:245–252
Stephen AE, Roth SI, Fardo DW et al (2007) Predictors of an accurate preoperative sestamibi scan for single-gland parathyroid surgery. Arch Surg 142:381–386
Oseka T, Makarewicz W, Kaska L et al (2004) Imaging in parathyroid gland diseases with relation to surgery. Zentralbl Chir 129:87–91
Chen H, Mack E, Starling JR (2003) Radioguided parathyroidectomy is equally effective for both adenomatous and hyperplastic glands. Ann Surg 238:332–338
Goldstein RE, Billheimer D, Martin WH et al (2003) Sestamibi scanning and minimally invasive radioguided parathyroidectomy without intraoperative parathyroid hormone measurement. Ann Surg 237:722–730
Nichol PF, Mack E, Bianco J et al (2003) Radioguided parathyroidectomy in patients with secondary and tertiary hyperparathyroidism. Surgery 134:713–717
Hage M, Siersema PD, van Dekken H et al (2004) 5-Aminolevulinic acid photodynamic therapy versus argon plasma coagulation for ablation of Barrett’s oesophagus: a randomised trial. Gut 53:785–790
Grant support and conflict of interest
None
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Prosst, R.L., Weiss, J., Hupp, L. et al. Fluorescence-Guided Minimally Invasive Parathyroidectomy: Clinical Experience with a Novel Intraoperative Detection Technique for Parathyroid Glands. World J Surg 34, 2217–2222 (2010). https://doi.org/10.1007/s00268-010-0621-2
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00268-010-0621-2