Abstract
Background
This study was designed to analyze the prognostic significance of the staging system based on the ratio of metastatic lymph nodes (TRM) compared with the TNM staging system in patients with gastric cancer.
Methods
We reviewed the data of 219 patients who underwent gastrectomy for node-positive M0 cancer. Node Ratio (NR) categories (NR1: 1–15%; NR2: 16–40%; NR3: >40%) were determined by the best cutoff approach at log-rank test. After confirming the prognostic validity of NR in a collinearity-free Cox regression analysis, among different combinations of pT and NR categories we selected five prognostically homogeneous TRM classes. To compare the prognostic power of the TRM vs. TNM systems, we plotted TRM and TNM classes against the mortality estimates.
Results
Both of the TRM and TNM classifications significantly stratified patients outcomes (p < 0.0001), but the TRM system identified prognostic subgroups more homogeneous than the TNM system.
Conclusions
The TRM staging system is a simple and reliable tool to stratify patients with gastric cancer and has a higher prognostic power than the current system.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The Tumour, Nodes, and Metastasis (TNM) staging system is a worldwide benchmark for reporting the extent of malignant disease and is a major prognostic factor for predicting the outcome of patients with cancer [1].
For gastric cancer, the optimal staging system still represents a matter of intense debate because in all TNM editions, the definition of lymph node status (N) has always been affected by the extension of lymph node dissection or by the degree to which specimens are examined. Many authors have suggested that more appropriate pathologic staging and a potential survival advantage may be provided by D2 or even more extended lymph node dissections [2–4]. Conversely several other authors have argued that such extensive procedures resulted only in improved staging with increase of morbidity rather than therapeutic benefit [5, 6].
With these premises, a pragmatic approach could be represented by a lymph node staging system based on a classification not dependent by the extent of lymph node dissection. Along with many authors we have recently discussed the significance of the metastatic Nodes Ratio (NR), which is the number of metastatic lymph nodes related to the total number of dissected lymph nodes [7–18]. NR has been indicated as the best classification from the viewpoint of predicting patient survival and avoiding stage migration errors, obviating risks of overtreating or understaging incurred with current staging systems [7–18].
In the present study, 219 patients with gastric cancer with lymph node metastases were reviewed and the prognostic significance of the NR was evaluated by comparing the prognostic power of the traditional TNM system with that of a hypothetical staging system based on lymph node ratio (TRM system).
Materials and methods
A total of 219 consecutive patients with M0 gastric adenocarcinoma (with the exclusion of tumors located in the esophagogastric junction), who underwent surgery between January 1992 and December 2007, and with documented metastatic lymph nodes were reviewed. Pretreatment tumor stage was determined in all cases by conventional imaging techniques, such as chest x-ray, upper gastrointestinal (GI) series, computed tomography (CT), ultrasonography (US), and bone scan (BS). During the last 5 years, endoscopic ultrasonography (EUS) was introduced in the routine preoperative staging protocol.
The extent of the lymph-node dissection depended on surgeon’s attitude: D2 lymphadenectomy according to JGCA recommendations [19] was performed in 147 patients (67%); D1 lymphadenectomy was performed in 64 patients (29%). In eight patients (4%) anatomical extent of lymphadenectomy was not specified. In all cases, a member of the surgical team detected and dissected single nodal stations postoperatively from the resected specimens, classifying and numbering all nodes before conventional pathological examination. The median number of examined lymph nodes was 27 (mean, 30 ± 18; range, 3–93) for all 219 patients. The median number of involved lymph nodes was five (mean, 9 ± 10; range, 1–62).
For comparative purposes, we classified lymph node status by two staging systems:
-
UICC/AJCC TNM (6th edition): pN1, pN2, pN3 [20]
-
NR: NR1 (ratio <15%), NR2 (ratio 15–40%), NR3 (ratio >40%).
We categorized the NR parameter by the best cutoff approach according to the log-rank test and we evaluated the prognostic relevance of NR and pN by multivariate analysis.
Finally, after combining NR with pT we selected five classes to compare the prognostic power of the TRM vs. TNM system.
Statistical analysis
The final date for follow-up was January 30, 2008. The median follow-up from the date of surgery was 24 (range, 1–172) months for all patients and 41 (range, 1–171) months for survivors. Five-year survival rates were calculated according to the Kaplan-Meier method; the log-rank test was used to assess the statistical difference between groups.
Factors considered were: age (≤64 or >64 years, median value), sex (male or female), tumor site (upper or medium/distal third), tumor depth (pT1, pT2, pT3, pT4), tumor diameter (≤40 or >40 mm, median value), residual tumor (R0, R1, and R2), Lauren type (intestinal or diffuse), grading (G1–2 or G3), and nodal status, considered both as NR (NR1, ratio ≤15%; NR2, ratio 15–40%; NR3, ratio >40%) and pN (pN1, pN2, pN3).
Multivariate analysis was performed using the Cox proportional hazard model selected in backward stepwise regression: hazard ratios for pN and NR were calculated by two different models. To evaluate the reliability of the two models, we used the maximum log likelihood (ML) as a measure of goodness of model fit.
For comparative purposes, we selected TRM classes by K-means cluster analysis of mean survival times of the subgroups, determined according to the different combinations of tumor depth (pT) and NR categories. K value was set to 5 to identify the same number of TNM stages.
To compare the prognostic power of the TRM vs. TNM system, we plotted the 95% confidence intervals (CIs) mortality rate against new TRM classes as well as against TNM classes.
Values of p < 0.05 were considered significant. The statistical analysis was performed with SPSS (16th edition) software for Windows™.
Results
The 5-year survival rate was 41% for all 219 patients (SE 3.8). When the patients were classified by the NR classification, the 5-year survival rates were 64% for NR1 (SE 6.3), 39% for NR2 (SE 7.0), and 16% for NR3 (SE 5.0) (p < 0.0001; Fig. 1a).
The 5-year survival rates of the patients staged by pN classification were 56% for N1 (SE 5.1), 23% for N2 (SE 7.4), and 14% for N3 (SE 5.8) (p < 0.0001; Fig. 1b). Clinical and histopathological records of the 219 patients and observed 5-year survival rates with univariate analysis are shown in Table 1.
NR Cox model and pN model included the same covariates (all with p < 0.1 at log-rank test): regression analyses showed that lymph nodal status indicators (pN and NR) were both independent prognostic factors, together with pT. According to our results, the maximum log likelihood (ML) of the multivariate analysis, which considered the NR-based classification, showed higher value compared with that including the TNM classification (Table 2). Moreover, the hazard ratios for NR and pN were different (Table 2).
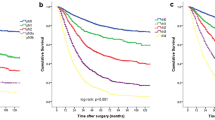
K-means cluster analysis selected five prognostically homogeneous subgroups, including the different combinations of tumor depth (T) and NR categories (Table 3). The 5-year survival of TRM groups was 100% for TR1, 71% for TR2 (SE 6.7), 34% for TR3 (SE 6.3), 12% for TR4 (SE 4.6), and 0% for TR5 (p < 0.0001; Fig. 2a). The 5-year survival of TNM classes was 83% for IB (SE 15.2), 72% for II (SE 6.8), 43% for IIIA (SE 7.9), 25% for IIIB (SE 10.4), and 12% for IV (SE 4.2) (p < 0.0001; Fig. 2b).
When the five TRM stages were plotted against 95% CIs mortality rate, no overlapping was observed, whereas an evident overlapping was observed when the five TNM subsets were plotted against 95% CIs mortality rate (Fig. 3a, b).
Discussion
To date, there is still much debate about the possible therapeutic efficacy related with the extension of lymph node dissection in gastric cancer [21]. At the same time the current TNM staging system based on the number of metastatic lymph nodes is certainly affected by the extension of lymphadenectomy: in fact, when less than 15 nodes are harvested or examined, pN stage cannot be applied [20].
Because of its simplicity, reproducibility, and potential advantage of reducing the “stage migration” phenomenon, the node ratio classification has been closely investigated in recent years [7–17]. Consistent with several authors, in a previous experience we identified the NR classification as an independent prognostic factor stronger than AJCC/UICC and JGCA classifications, and we suggested that the N ratio might be usefully implemented in any clinical practice, not being influenced by differences in the routine extension of lymphadenectomy [8, 10, 11, 15–18].
Misuse of stepwise regression analysis has been advocated as a major methodological bias in studies regarding NR [22]; in fact, the inclusion in the same model of two variables, which convey essentially the same information about nodal status (pN and NR) and imply a same value for pN0 and NR0, leads to a multicollinearity problem. Having dealt with multicollinearity in our previous report [18], to verify the true prognostic power of NR, in the present study we shifted to a different statistical method: (1) patients without lymph node metastases were excluded; (2) two different regression models were designed (Table 2): for a separate nodal status description model A included pN (without NR) and model B included NR (without pN).
In this retrospective study, multivariate analysis confirmed that the lymph node status “per se,” NR as well as pN, is a powerful independent prognostic factor, similar to our previous results and other data from literature [8, 10, 11, 15–18]. Nevertheless, the Cox model, including NR, has evidenced a better reliability (ML: −905.548 vs. −906.875) discriminating different nodal status categories more strongly than the model, including pN (NR3/NR2/NR1 = 2.982/1.716/1 vs. pN3/pN2/pN1 = 2.550/2.091/1).
Of note, both Cox models retained pT as the most significant prognostic factor, confirming that in a correct staging system for M0 gastric cancer, tumor depth, and node involvement should be combined for a refined prognostic estimation. Therefore, by k-means clusters analysis, five new combinations of pT and NR were identified, allowing us to perform a comparison between TNM classification and a novel staging system based on pT plus NR (TRM).
Comparing different combinations of pT and NR with survival, we observed that TRM was more effective than TNM in distinguishing groups with different prognosis: remarkably, no overlapping of 95% CIs of survival estimates for TRM stages was displayed (Table 3; Figs. 2, 3). Furthermore, this classification identified a group of N+ patients with T1 cancer (TR1) which showed to have a 100% survival rate (Fig. 2a), similarly to that of patients with early gastric cancer without metastatic nodes. This figure indirectly supports the idea that NR in some way could reflect the interaction between the host immune defense mechanism (total number of dissected nodes as the denominator) and tumor aggressiveness (number of the metastatic nodes as the numerator), apart from TNM stage.
On the other hand, TRM system homogeneously selected in the last stage (TR5) all patients with the worst prognosis: it must be stressed that TR5 category includes only T4-NR2 patients, which seems to show a lower survival rate than T4-NR3 patients (Table 3). This paradoxical result probably reflected the fact that four of five patients (80%) of TR5 stage presented a macroscopic residual tumor (R2) (5/17; 29.4% among T4-NR3 patients). Because previous survival analysis showed that nodal status affected prognosis more than R factor, we included this variable in our regression models [23, 24]. Actually, the literature data demonstrate that R2 resection negatively affects overall survival [25, 26]: therefore, the inclusion of R2 patients in our analysis could be considered a limitation of this study. Moreover, the small size of population study did not allow us to consider our survival analysis about R2 group sufficiently reliable.
Despite the limitations of this retrospective analysis, our results are relevant, because we have combined pT and NR in a new staging system (TRM) after confirming the prognostic validity of NR in a multivariate analysis. These results cannot be generalized and should be validated in larger series. If confirmed by validation studies, TRM will be able to improve the prognostic accuracy compared with current TNM classification; it can be considered a reliable staging system with the peculiar feature of being less affected by the extension of lymph node dissection in predicting patient outcome.
Historically, the TNM classification system has been modified by a decision-making process based on expert opinion from several national TNM committees and from the individual membership of the UICC. We believe that there is sufficient amount of scientific evidence about the staging accuracy of node ratio; it should be taken into account in the forthcoming revisions to the TNM classification, mainly to improve its prognostic ability and to keep pace with the demands of clinical practice.
References
Sobin LH (2001) TNM: principles, history, and relation to other prognostic factors. Cancer 91:1589–1592
Siewert JR, Bottcher K, Roder JD et al (1993) Prognostic relevance of systematic node dissection in gastric carcinoma. Br J Surg 80:1015–1018
Degiuli M, Sasako M, Ponti A et al (2004) Survival results of a multicentre phase II study to evaluate D2 gastrectomy for gastric cancer. Br J Cancer 90:1727–1732
Smith DD, Schwarz RR, Schwarz RE (2005) Impact of total lymph node count on staging and survival after gastrectomy for gastric cancer: data from a large US-population database. J Clin Oncol 23:7114–7124
Cuschieri A, Weeden S, Fielding J et al (1999) Patient survival after D1 and D2 resections for gastric cancer: long-term results of the MRC randomized surgical trial. Surgical Co-operative Group. Br J Cancer 79:1522–1530
Bonenkamp JJ, Hermans J, Sasako M et al (1999) Extended lymph-node dissection for gastric cancer. N Engl J Med 340:908–914
Siewert JR, Bottcher K, Stein HJ et al (1998) Relevant prognostic factors in gastric cancer: ten-year results of the German Gastric Cancer Study. Ann Surg 228:449–461
Takagane A, Terashima M, Abe K et al (1999) Evaluation of the ratio of lymph node metastasis as a prognostic factor in patients with gastric cancer. Gastric Cancer 2:122–128
Hyung WJ, Noh SH, Yoo CH et al (2002) Prognostic significance of metastatic lymph node ratio in T3 gastric cancer. World J Surg 26:323–329
Bando E, Yonemura Y, Taniguchi K et al (2002) Outcome of ratio of lymph node metastasis in gastric carcinoma. Ann Surg Oncol 9:775–784
Inoue K, Nakane Y, Iiyama H et al (2002) The superiority of ratio-based lymph node staging in gastric carcinoma. Ann Surg Oncol 9:27–34
Cheong JH, Hyung WJ, Shen JG et al (2006) The N ratio predicts recurrence and poor prognosis in patients with node-positive early gastric cancer. Ann Surg Oncol 13:377–385
Rodriguez Santiago JM, Munoz E, Marti M et al (2005) Metastatic lymph node ratio as a prognostic factor in gastric cancer. Eur J Surg Oncol 31:59–66
Kunisaki C, Shimada H, Nomura M et al (2005) Clinical impact of metastatic lymph node ratio in advanced gastric cancer. Anticancer Res 25:1369–1375
Marchet A, Mocellin S, Ambrosi A et al (2007) The ratio between metastatic and examined lymph nodes (N ratio) is an independent prognostic factor in gastric cancer regardless of the type of lymphadenectomy: results from an Italian multicentric study in 1853 patients. Ann Surg 245:543–552
Liu C, Lu P, Lu Y et al (2007) Clinical implications of metastatic lymph node ratio in gastric cancer. BMC Cancer 7:200
Celen O, Yildirim E, Berberoglu U (2007) Prognostic impact of positive lymph node ratio in gastric carcinoma. J Surg Oncol 96:95–101
Persiani R, Rausei S, Biondi A et al (2008) Ratio of metastatic lymph nodes: impact on staging and survival of gastric cancer. Eur J Surg Oncol 34:519–524
Japanese Gastric Cancer Association (1998) Japanese classification of gastric carcinoma. 2nd English edn. Gastric Cancer 1:25–30
Sobin LH, Witteking C (2002) TNM classification of malignant tumors, 6th edn. Wiley-Liss, New York
McCulloch P, Niita ME, Kazi H et al (2005) Gastrectomy with extended lymphadenectomy for primary treatment of gastric cancer. Br J Surg 92:5–13
Wang X, Wan F, Wang JJ (2008) A common misuse of stepwise regression in studies of ratio of metastatic lymph nodes for gastric cancer. Ann Surg Oncol 15:1805–1806
Kim SH, Karpeh MS, Klimstra DS et al (1999) Effect of microscopic resection line disease on gastric cancer survival. J Gastrointest Surg 3:24–33
Cho BC, Jeung HC, Choi HJ et al (2007) Prognostic impact of resection margin involvement after extended (D2/D3) gastrectomy for advanced gastric cancer: a 15-year experience at a single institute. J Surg Oncol 95:461–468
Oñate-Ocaña LF, Méndez-Cruz G, Hernández-Ramos R et al (2007) Experience of surgical morbidity after palliative surgery in patients with gastric carcinoma. Gastric Cancer 10:215–220
Miner TJ, Jaques DP, Karpeh MS et al (2004) Defining palliative surgery in patients receiving noncurative resections for gastric cancer. J Am Coll Surg 198:1013–1021
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Persiani, R., Rausei, S., Antonacci, V. et al. Metastatic Lymph Node Ratio: A New Staging System for Gastric Cancer. World J Surg 33, 2106–2111 (2009). https://doi.org/10.1007/s00268-009-0157-5
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00268-009-0157-5