Abstract
Background
Some patients with autonomously functioning thyroid nodules (AFTN) are not suitable for surgery or radioiodine therapy. Therefore, minimally invasive alternative treatments, such as ethanol ablation or radiofrequency ablation (RFA), are necessary.
Methods
This study included nine patients (4 toxic and 5 pretoxic patients; male to female ratio = 1:8; mean age, 47 ± 17 (range, 25–71) years) who were not eligible for surgery or radioiodine therapy. All of the patients showed hot nodule with suppression of normal thyroid gland in 99mTc pertechnetate scintigraphy. RFA was performed using a 17- and 18-gauge internally cooled electrode. Nodule volume, thyroid function, scintigraphy, symptom score (visual analogue scale, 0–10 cm), cosmetic grading score (4-point scale), and complications were evaluated before treatment and at 1, 3, and 6 months follow-up.
Results
Mean volume of the index nodule was 14.98 ± 25.53 (range, 0.29–82.29) mL. After RFA it decreased at 1 month (12.01 ± 25.97 mL, p = 0.015), 3 months (7.27 ± 15.13 mL, p = 0.011), 6 months (8.27 ± 21.29 mL, p = 0.008), and the last month (7.57 ± 19.99 mL, p = 0.008). Initial mean T3, fT4, and TSH were 156.2 ± 42.1 ng/dL, 1.73 ± 0.40 ng/dL, and 0.052 ± 0.087 mU/mL, respectively. A significant improvement of mean T3, fT4, and TSH were observed at last follow-up (T3: 116.8 ± 20.7 ng/dL, p = 0.015; fT4: 1.37 ± 0.26 ng/dL, p = 0.036; TSH: 1.454 ± 1.756 mU/mL, p = 0.012). After ablation, four patients became a cold or normal scan and five remained as a hot nodule. The mean symptom and cosmetic grading score was reduced from 2.4 ± 1.7 to 0.6 ± 0.7 (p = 0.011) and from 3.1 ± 1.2 to 1.4 ± 1.0 (p = 0.017), respectively. No major complications were encountered.
Conclusions
RFA seems to be effective and safe for the treatment of AFTN.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Approximately 10% of solitary thyroid nodules are hot nodules, half of which are autonomously functioning. Subclinical hyperthyroidism is present in 75% of patients with an autonomously functioning thyroid nodules (AFTN) [1, 2]. Treatment may be indicated by compression of adjacent structures, cosmetic complains, hyperthyroid symptoms, and to avoid progression of hyperthyroidism (annual risk approximately 4%) [1, 3]. Because subclinical hyperthyroidism has a detrimental effect, particularly on the skeletal and cardiovascular systems, treatment often is recommended, especially in cases of elderly patients [3–7].
Surgery and radioiodine (131I) therapy are the most common options for definitive treatment of the thyroid nodules. However, some patients refuse these treatment options and others are unsuitable for surgery because of high surgical risk; therefore, alternative therapeutic strategies have been proposed. Also, the reluctance to use radioactive iodine, particularly in southern Europe, has lead to the introduction of ethanol ablation (EA) of AFTNs. The results have been promising, but there are limitations of EA, such as the difficulty in predicting the diffusion of the ethanol within the nodule, leakage-induced pain, and the possibility of extraglandular fibrosis impeding subsequent surgery. Ultrasonography (US)-guided percutaneous laser ablation (LA) also has been introduced for the treatment of AFTN with a good results [8–10].
Radiofrequency ablation (RFA) is a nonsurgical, minimally invasive technique that has been widely used to treat hepatoma, as well as other benign and malignant tumors [11–13]. In the thyroid gland, RFA has been applied to recurrent cancer and benign nodules [13–15]. Recently, treatment of AFTN using RFA has been introduced [15, 16].
We previously described radiofrequency ablation for an autonomously function thyroid nodule in a 37-year-old woman [16]. We report our experience with nine patients with AFTNs. This study was designed to define the efficacy and safety of RFA using an internally cooled electrode for the treatment of AFTN.
Patients and methods
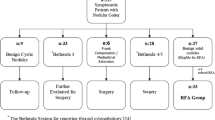
From October 2006 to July 2007, nine patients (8 females, 1 male; aged 47 ± 17 (mean ± SD; range 25–71) years) were treated using RFA on an outpatient basis. Five patients showed pretoxic nodules and four showed toxic nodules. All patients had only one hyperfunctioning thyroid nodule, and all had previously refused surgery or radioiodine therapy. They were referred to the Radiological Interventional Department at our thyroid center for RFA. All nodules were benign on cytology at least two times. None had previous radiation to the neck or US findings suggestive of a malignancy. This study is a retrospective study design and was approved by the institutional review board of Daerim St. Mary’s Hospital. A written, informed consent document was obtained from all patients before the procedure.
Preablation assessment
The US examination, fine-needle aspiration biopsy (FNAB), laboratory data, 99mTc pertechnetate scintigraphy, and clinical symptoms were examined in all patients. Two radiologists (JHB and YSK) performed the US examination and FNAB by using a 10-Mhz linear probe on a real-time US system (Aplio SSA-770A, Toshiba, Otawara-shi, Japan). Three orthogonal diameters of the nodules (the largest diameter and two other perpendicular ones) were measured before initiating RFA. The volume of the nodule was calculated using the following equation: V = πabc/6 (V: volume, a: the largest diameter, b and c: the other two perpendicular diameters). The composition of the nodules was assessed subjectively by the two above-mentioned examiners, and was classified as mainly solid (having a solid portion > 80%), mainly cystic (having a cystic portion > 80%), or mixed type. Tumor vascularity was classified with a five-point scale: 0 (no signal in the tumor), 1 (a few spotty signals in the tumor), 2 (signals in < 25% of the tumor), 3 (signals in 25–50% of the tumor), and 4 (signals in > 50% of the tumor).
Laboratory studies included serum thyrotrophin (TSH; normal range 0.4–4.0 mU/mL) determined by CEIA (IMMULITE2000, Siemens, Los Angeles, CA), serum total triiodothyroxine (T3; normal range 61–173 ng/dL) determined by CEIA (IMMULITE2000, Siemens, Gwynedd, United Kingdom), serum-free thyroxine (Free T4; normal range 0.8–1.9 ng/dL) determined by CEIA (IMMULITE2000, Siemens, Los Angeles, CA), serum anti-thyroid peroxidase antibodies (anti-TPOAb; normal range 0–35 IU/mL) determined by CEIA (IMMULITE2000, Siemens, Gwynedd, United Kingdom), serum TSH receptor antibody (TSH-R-Ab; normal range 0–10 U/L) determined by RIA (assay for thyrotrophin autoantibodies, RSR, Cardiff, England), serum thyroglobulin antibody (anti-TGAb; normal range 0-40 IU/mL) determined by CEIA (IMMULITE2000, Siemens, Gwynedd, United Kingdom), and serum calcitonin (normal range 0–10 pg/mL) determined by IRMA (IRMA-HCT, CIS, Cedex, France). 99mTc pertechnetate scintigraphy was performed in all patients before ablation. Clinical symptoms were evaluated using the symptom grading score (visual analogue scale, 0–10 cm) and the cosmetic grading score (grade 1: no palpable mass, grade 2: invisible but palpable mass, grade 3: visible mass only by experienced clinician’s eyes, grade 4: easily visible mass). Two older patients with hyperthyroid symptoms were treated with methimazole for 1 month before and 1 week after ablation.
Procedure
Before ablation, an intravenous route was made via the antecubital vein of the arm; however, premedication was not administered. The patients were placed in the supine position with the neck extended. Two grounding pads were attached to both thighs. An RF generator (Cool-tip RF system, Radionics, Valleylab, CO) and an internally cooled electrode was used. The internally cooled electrodes used were 17-gauge (Radionics, Burlington, MA) and 18-gauge 1-cm active tips (Taewoong Medical Co., Koyang, Korea). We developed a modified, internally cooled electrode for the thyroid gland with technical support from Taewoong Medical Co. This electrode is shorter and thinner than a conventional electrode designed for easy control and minimizing normal tissue injury (Fig. 1). The patients were treated with 2% lidocaine (Huons, Hwasung, Korea) for the local anesthesia of the puncture site and around the thyroid gland. The skin was not incised to prevent unnecessary scar formation. An electrode was inserted into the thyroid nodule under US guidance along the short axis of the nodule using the trans-isthmic approach method [14, 16]. Initially, the electrode tip was positioned in the deepest and most remote portion of the nodule. Based on our previous experience, we treated the nodule using a “moving-shot technique” beginning with 30-W of power. If the formation of a transient hyperechoic zone at the electrode tip did not appear within 5–10 seconds, power was increased in 10-W increments, reaching full capacity at 80 W. In cases of mixed and mainly cystic nodules, we performed RFA after aspiration of internal fluid [17, 18]. If the patient could not tolerate the pain during ablation, the power was reduced or turned off. During ablation, both thighs were checked frequently to prevent skin burn. Ablation was terminated when all imaginary units of the nodule had changed to transient hyperechoic zones [14].
Posttreatment care
RFA was performed on an outpatient basis. At the end of the procedure, we evaluated the complications and the patients remained under observation for 1–2 hours with compression of the neck lasting 10–20 minutes. Oral analgesics (acetaminophen) were prescribed for 1 day.
Follow-up
US examination, laboratory data, 99mTc pertechnetate scintigraphy, and clinical symptoms were examined. A follow-up US examination was performed immediately after ablation and at the 1, 3, 6, and 12-month follow-ups. On the US examination, changes in size, intranodular vascularity, and echogenicity were evaluated. The volume reduction was assessed by US imaging and was calculated by the following equation: volume reduction (%) = {[initial volume (ml) – final volume (ml)] × 100}/initial volume. The laboratory test, 99mTc pertechnetate scintigraphy, and symptom/cosmetic grading score were evaluated at 1, 3, 6, and 12 months after ablation. T3, fT4, and TSH were checked at every follow-up; however, anti-TPOAb and anti-TGAb were checked at the 6-month follow-up. Hot nodules have problems related to hormone and mass effect. Therefore, we terminated the procedure when TSH level and cosmetic problems were normalized. Repeat RFA was performed 1–2 months after first RFA because we previously reported that volume reduction of the nodule was rapid during the first month after RFA [14]. The complications during and after the procedure were evaluated by the clinical signs and symptoms.
Statistical analysis
Statistical analyses were performed using SPSS for Windows (version 13.0; SPSS, Inc., Chicago, IL). At each treatment interval, the nodule volume change and % volume reduction were compared by using the Wilcoxon signed-rank test. The level of significance was defined as at p < 0.05.
The relationship of nodular characteristics (initial volume, solid composition, and vascularity) and technical parameters (number of treatment session, fractional energy per volume) with treatment response variables (TSH, % volume reduction and clinical scale) was calculated using the Spearman rank correlation test.
Results
Each patient’s data are summarized in Table 1.
Treatment characteristics
The number of treatment sessions ranged from 1 to 4 sessions (mean ± SD, 2.2 ± 1.0). The ablation time and power ranged from 5 to 22 minutes and from 30 to 80 W, respectively. Total energy deposition ranged from 7440 to 177540 J (mean ± SD, 56280 ± 49224 J). The mean energy deposited at the first treatment (n = 9) was 22153 ± 12480 J, during the second treatment (n = 7) was 29503 ± 22448 J and during the third treatment (n = 3) was 18140 ± 16881 J. The fourth ablation was performed in only one patient and the energy deposited was 46200 J. The mean total energy delivered per milliliter of the pretreatment nodule volume was 10818 ± 15609 J.
Ultrasonographic evaluation
Follow-up periods ranged from 6 to 17 (mean, 11.0 ± 4.2) months. The largest diameter of the index nodules ranged from 1.1 to 6.5 (mean, 3.21 ± 1.43) cm. The volume of the index nodules ranged from 0.29 to 82.29 (mean, 14.98 ± 25.53) mL. A significant decrease in nodule volume was observed at 1 month (12.01 ± 25.97 mL, p = 0.015), 3 months (7.27 ± 15.13 mL, p = 0.011), 6 months (8.27 ± 21.29 mL, p = 0.008), and the last month (7.57 ± 19.99 mL, p = 0.008). The volume reduction was 36.4 ± 26.1% at the 1-month follow-up and 70.7 ± 22.9% at the 6 month follow-up (Fig. 2a).
The composition of the nodules was mainly solid (n = 6), mixed (n = 2), and mainly cystic (n = 1). During the RFA, the electrode was always clearly visualized with US monitoring as a hyperechoic line. After ablation, color and power doppler US showed significant reduction of the peripheral and/or intranodular vascular signals as a consequence of necrosis induced by RF (vascular scale, initial vs. 6 month, 1.78 ± 1.09 vs. 0.20 ± 0.44, p = 0.016; Fig. 3a and b). Echogenicity of the nodule was increased markedly at the end of the procedure due to microbubbles. However, on the follow-up US examination, the echogenicity was lower than that observed before ablation.
Laboratory data, scintigraphy, and clinical findings
Initial mean T3, fT4, and TSH were 156.2 ± 42.1 ng/dL, 1.73 ± 0.4 ng/dL, and 0.052 ± 0.087 mU/mL, respectively. A significant improvement of mean T3, fT4, and TSH were observed at last follow-up (T3: 116.8 ± 20.7 ng/dL, p = 0.015; fT4: 1.37 ± 0.26 ng/dL, p = 0.036; TSH: 1.454 ± 1.756 mU/mL, p = 0.012; Fig. 2b–d). Serum level of TSH was normalized in five of nine patients, improved but still decreased in three patients, and elevated in one patient. Before RFA, elevation of anti-TPOAb and/or anti-TGAb was observed in three patients. After ablation one patient with elevated anti-TGAb developed subclinical hypothyroidism (TSH 5.6 mU/mL) by the 12-month follow-up and aggravated at the 15-month follow-up (TSH 36.7 mU/mL). At enrolment, all had normal levels of serum TSH-R-Ab and calcitonin. During the follow-up period, no one newly developed elevation of anti-TPOAb and anti-TGAb.
After ablation, four patients showed a cold or normal scan and five remained as a hot nodule (Fig. 3c and d). Three of four patients with cold or normal scans recovered normal serum hormone levels. However, in one patient the serum TSH level was slightly improved but still decreased. Five patients remained as a hot nodule showing variable serum levels of TSH (2 normal, 1 low, 2 high serum levels of TSH). One patient, incompletely recovered thyroid function, has been treated with medication and others wanted only observation.
The mean symptom and cosmetic grading score was reduced from 2.4 ± 1.7 to 0.6 ± 0.7 (p = 0.011) and from 3.1 ± 1.2 to 1.4 ± 1.0 (p = 0.017), respectively. No one complained of aggravation of hyperthyroid symptoms after treatment.
Factors affecting the treatment response
The last TSH level and clinical scale were not correlated with any tumor characteristics or treatment parameters. In contrast, the % volume reduction was significantly correlated with the tumor vascularity at the initial US (Spearman rho = 0.788, p = 0.012).
Complications
During RFA, most patients complained of mild pain and/or a sensation of heat in the neck, sometimes radiating to the head, shoulders, teeth, and chest. However, none complained of symptoms that made it necessary to stop the procedure. No major complications were encountered, such as voice change, skin burning, hematoma formation, infection, or damage to the vital structures of the neck.
Discussion
US-guided EA and LA have been proposed as an effective and safe alternative to radioiodine therapy and surgery for the treatment of AFTNs [8–10]. The advantage of thermal ablation, such as LA, compared with EA, is the precision in inducing a well-defined area of necrosis [19–21]. RFA is another method of thermal ablation to induce thyroid tissue necrosis, as demonstrated by Kanauchi et al. [22] in an experimental animal study. So far, RFA has been applied for both cold and hot thyroid nodules, both with good results [14–16, 23].
After ablation significant volume reduction was achieved in this study. The volume reduction by the 1-month follow-up of our study (38.6 ± 25.5%) was similar to the data of Deandrea et al. (33.1 ± 15.6%) [15]. However the volume reduction of our study (70.7 ± 22.9%) at the 6-month follow-up was similar or somewhat superior to those of LA (74%, 44 ± 5%), and RFA (52.6 ± 16.3%) study for AFTN [8, 10, 15].
After treatment of AFTNs using LA, Dossing et al. [10] reported serum TSH was normalized in 7 of 14 patients and Spiezia et al. [8] reported no recurrence up to the 12-month follow-up. Deandrea et al. [15] reported an improvement in thyroid function after RFA of AFTNs, but a complete normalization was obtained only in a few patients (approximately 24%). This could be explained by the difficulty to ablate the entire nodule, because a peripheral tissue layer is generally spared to avoid heat diffusion outside of the treated lesion. Previous studies suggested that the primary purpose of RFA for benign thyroid nodules is to perform debulking to reduce pressure symptoms rather than obtain complete ablation. However, we experienced a regrowing of the untreated peripheral portion. We designed a moving shot technique, which is a safer method to ablate the peripheral portion of the nodule [14]. In this study, serum levels of T3 and fT4 were completely recovered in all patients; however, serum levels of TSH were incompletely recovered in three patients. A more complete ablation of the hot nodule could promise a more complete improvement of serum hormone levels. In our opinion, the internally cooled electrode is more suitable for the moving shot technique than the multitined expandable electrode because the multitined expandable electrode is difficult to move easily and simultaneous US observation of multiple expandable prongs is difficult [15]. If the thyroid function or symptoms are incompletely resolved, another treatment, such as medication, would be required. RFA is a useful technique for benign thyroid nodules; however, this procedure should be performed by trained doctors or specialists of thyroid FNAC regardless of their department.
There was no report of factors affecting the treatment response in RFA or LA groups. However, in EA groups, they reported that the treatment outcome was insufficient in large hot nodules [24–26]. In this study, the only factor affecting the treatment response was the tumor vascularity.
Monzani et al. [26] reported one case of hypothyroidism among nine patients after EA, all of whom showed elevated anti-TPOAb and/or anti-TGAb before ablation. They also reported three patients with significant elevation of anti-TGAb titer during the follow-up periods; however, none of them became hypothyroid. In our study, one patient with elevated anti-TGAb developed subclinical hypothyroidism after ablation, however, no one developed antibody during the follow-up period. Therefore, if thyroid antibodies are elevated before ablation, a warning to patients about the possibility of hypothyroidism after treatment is necessary.
The limitations of this study are the small number of cases and the shorter duration of the follow-up period. Further study on a larger scale and a longer duration of follow-up will be necessary.
Conclusions
Although some patients recovered incompletely, the results of RFA showed significant improvement of thyroid function and clinical problems in patients with AFTNs who cannot undergo a surgical procedure or refuse one without major complications. However, if thyroid antibodies are elevated before ablation, a warning to patients is necessary about the possibility of hypothyroidism after the procedure.
References
Hegedus L (2004) Clinical practice. The thyroid nodule. N Engl J Med 351:1764–1771
Burch HB, Shakir F (1998) Fitzsimmons TR et al (1998) Diagnosis and management of the autonomously functioning thyroid nodule: the Walter Reed Army Medical Center experience, 1975–1996. Thyroid 8:871–880
Sandrock D, Olbricht T, Emrich D et al (1993) Long-term follow-up in patients with autonomous thyroid adenoma. Acta Endocrinol (Copenh) 128:51–55
Parle JV, Maisonneuve P, Sheppard MC et al (2001) Prediction of all-cause and cardiovascular mortality in elderly people from one low serum thyrotropin result: a 10-year cohort study. Lancet 358:861–865
Toft AD (2001) Clinical practice. Subclinical hyperthyroidism. N Engl J Med 345:512–516
Biondi B, Palmieri EA, Klain M et al (2005) Subclinical hyperthyroidism: clinical features and treatment options. Eur J Endocrinol 152:1–9
Gharib H, Tuttle RM, Baskin HJ et al (2005) Subclinical thyroid dysfunction: a joint statement on management from the American Association of Clinical Endocrinologists, the American Thyroid Association, and the Endocrine Society. J Clin Endocrinol Metab 90:581–587
Spiezia S, Vitale G, Di Somma C et al (2003) Ultrasound-guided laser thermal ablation in the treatment of autonomous hyperfunctioning thyroid nodules and compressive nontoxic nodular goiter. Thyroid 13:941–947
Dossing H, Bennedbaek FN, Hegedus L (2003) Ultrasound-guided interstitial laser photocoagulation of an autonomous thyroid nodule: the introduction of a novel alternative. Thyroid 13:885–888
Dossing H, Bennedbaek FN, Bonnema SJ et al (2007) Randomized prospective study comparing a single radioiodine dose and a single laser therapy session in autonomously functioning thyroid nodules. Eur J Endocrinol 157:95–100
Gazelle GS, Goldberg SN, Solbiati L et al (2000) Tumor ablation with radio-frequency energy. Radiology 217:633–646
Dupuy DE, Goldberg SN (2001) Image-guided radiofrequency tumor ablation: challenges and opportunities—part II. J Vasc Interv Radiol 12:1135–1148
Dupuy D, Monchik J, Decrea C et al (2001) Radiofrequency ablation of regional recurrence from well-differentiated thyroid malignancy. Surgery 130:971–977
Jeong WK, Baek JH, Rhim H et al (2008) Radiofrequency ablation of benign thyroid nodules: safety and imaging follow-up in 236 patients. Eur Radiol 18:1244–1250
Deandrea M, Limone P, Basso E et al (2008) US-guided percutaneous radiofrequency thermal ablation for the treatment of solid benign hyperfunctioning or compressive thyroid nodules. Ultrasound Med Biol 34:784–791
Baek JH, Jeong HJ, Kim YS et al (2008) Radiofrequency ablation for an autonomously functioning thyroid nodule. Thyroid 18:675–676
Dossing H, Bennedbaek FN, Hegedus L (2006) Beneficial effect of combined aspiration and interstitial laser therapy in patients with benign cystic thyroid nodules: a pilot study. Br J Radiol 79:943–947
Sung JY, Baek JH, Kim YS et al (2008) One-step ethanol ablation of viscous cystic thyroid nodules. AJR Am J Roentgenol 191:1730–1733
Pacella CM, Bizzarri G, Spiezia S et al (2004) Thyroid tissue: US-guided percutaneous laser thermal ablation. Radiology 232:272–280
Papini E, Pacella CM, Verde G (1995) Percutaneous ethanol injection (PEI): what is its role in the treatment of benign thyroid nodules? Thyroid 5:147–150
Pacella CM, Bizzarri G, Guglielmi R et al (2000) Thyroid tissue: US-guided percutaneous interstitial laser ablation-a feasibility study. Radiology 217:673–677
Kanauchi H, Mimura Y, Kaminishi M (2001) Percutaneous radio-frequency ablation of the thyroid guided by ultrasonography. Eur J Surg 167:305–307
Kim YS, Rhim H, Tae K et al (2006) Radiofrequency ablation of benign cold thyroid nodules: initial clinical experience. Thyroid 16:361–367
Guglielmi R, Pacella CM, Bianchini A et al (2004) Percutaneous ethanol injection treatment in benign thyroid lesions: role and efficacy. Thyroid 14:125–131
Lippi F, Ferrari C, Manetti L et al (1996) Treatment of solitary autonomous thyroid nodules by percutaneous ethanol injection: results of an Italian multicenter study. The Multicenter Study Group. J Clin Endocrinol Metab 81:3261–3264
Monzani F, Caraccio N, Goletti O et al (1997) Five-year follow-up of percutaneous ethanol injection for the treatment of hyperfunctioning thyroid nodules: a study of 117 patients. Clin Endocrinol (Oxf) 46:9–15
Acknowledgments
The authors thank Hangil Im for assistance.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Baek, J.H., Moon, WJ., Kim, Y.S. et al. Radiofrequency Ablation for the Treatment of Autonomously Functioning Thyroid Nodules. World J Surg 33, 1971–1977 (2009). https://doi.org/10.1007/s00268-009-0130-3
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00268-009-0130-3