Abstract
Background
Radiofrequency ablation (RFA) is a relatively novel procedure in the management of benign nodular goiter. This study was conducted to evaluate the safety and efficacy of ultrasound (US)-guided percutaneous RFA for benign symptomatic thyroid nodules as an alternative to surgery.
Methods
The study involved patients for whom a fine needle aspiration biopsy had proved a diagnosis of benign nodular goiter and had nodule-related symptoms such as dysphagia, cosmetic problems, sensation of foreign body in the neck, hyperthyroidism due to autonomous nodules or fear of malignancy. Percutaneous RFA was performed as an outpatient procedure under local anesthesia. The primary outcome was an evaluation of the changes in symptom scores (0–10) for pain, dysphagia and foreign body sensation at the 1st, 3rd, and 6th months after the RFA procedure. Secondary outcomes were assessing volume changes in nodules, complication rates, and changes in thyroid function status.
Results
A total of 33 patients (24 % female, 76 % male) and a total of 65 nodules were included into the study. More than one nodule was treated in 63.6 % of the patients. We found a statistically significant improvement from baseline to values at the 1st, 3rd, and 6th months, respectively, as follows: pain scores (2.9 ± 2.7, 2.3 ± 2.01, 1.8 ± 1.7, and 1.5 ± 1.2, p 0.005), dysphagia scores (3.9 ± 2.7, 2.6 ± 1.9; 1.7 ± 1.6, and 1.1 ± 0.3, p 0.032), and foreign body sensation scores 3.6 ± 3, 2.5 ± 2.2; 1.6 ± 1.5, and 1.1 ± 0.4, p 0.002).The mean pre-treatment nodule volume was 7.3 ± 8.3 mL. There was a statistically significant size reduction in the nodules at the 1st, 3rd, and 6th months after RFA (3.5 ± 3.8, 2.7 ± 3.4, and 1.2 ± 1.7 mL, p 0.002). The volume reduction was found to be 74 % at 6th months following the RFA (p 0.005). 8 patients had autonomously functioning nodules in the pre-treatment period, 50 % (n: 4) became euthyroid at the 6th month after RFA. There were no complaints other than pain (12 %).
Conclusion
RFA can be an alternative treatment modality in the management of benign symptomatic thyroid nodules. The results showed that it is a safe and effective procedure.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Thyroid nodules are a common clinical finding, prevalence by palpation alone ranges from 4 to 7 %, and there is an estimated 10 % lifetime probability for developing a thyroid nodule [1, 2]. Autopsy findings reveal that the prevalence of clinically unapparent nodules is up to 65 % [3] The risk of thyroid nodular disease increases with age, is more prevalent in women than in men, occurs more frequently in iodine-deficient regions, and in people who experience secondary exposure to ionizing radiation [4, 5]. The reported prevalence of malignancy in thyroid nodules evaluated by biopsy ranges from 4.0 to 6.5 % and is largely independent of the nodule size [4].
Nodular thyroid comprises wide spectrum of clinical entities such as multinodular goiter, solitary thyroid nodules, nodular goiter with autoimmune thyroid disease and non-palpable nodules (incidentilomas). Rates of advanced radiological imaging for medical evaluation (computed tomography, magnetic resonance imaging, 18 FDG-PET scanning, high resolution ultrasound) increased recently and these techniques reveal thyroid nodules that remained undiscovered before [6].
Ultrasound (US) detects clinically unapparent nodules in 20–76 % of the adult population [7]. It is a useful tool in patients with the diagnosis of nodular thyroid, to measure nodule number and size, to rule out malignancy in nodules, and to select lesions that require US-guided fine needle aspiration (FNA) biopsy. Although no single characteristic can distinguish benign and malignant nodules, several US features (irregular or indistinct nodule margins, microcalcifications with scanty or no posterior acoustic shadowing, chaotic appearance of intranodular vascular images, more tall than wide morphology, extension beyond the thyroid capsule) help to predict malignancy [8, 9]. When combined with FNA biopsy, precision rate to guide the biopsy needle to desired location increases. Also, US-guided FNA decreases non-diagnostic rates from 15 to 3.5 % [10].
Besides high detection rate, mostly nodules are small, benign, remain asymptomatic, and do not warrant treatment. A proportion of them, however, grow over time and cause local symptoms. Studies have reported different degrees of effectiveness with levothyroxine suppression therapy and radioactive iodine treatment in reducing the nodule size of nontoxic multinodular goiters [11, 12]. l-tyroxine treatment has side effects like reducing bone density and atrial fibrillation; also lifelong suppression was found to be unsuitable for most patients [13]. Radioactive iodine treatment can cause complications to other organs due to radiation exposure [14–16].
Surgical indications for nodular goiter include malignancy, compression of the trachea or esophagus causing dyspnea and/or dysphagia, mediastinal extension, and cosmetic purposes [17]. However, surgery carries the risk of laryngeal nerve injury, hypoparathyroidism, bleeding, and hematoma. Therefore, non-surgical techniques like ethanol ablation and percutaneous laser ablation have been proposed for the treatment of symptomatic benign thyroid nodules [18, 19].
Surgeons encounter most benign nodular goiters in outpatient settings. Radiofrequency ablation (RFA) is a minimally invasive technique reported to be safe and effective in the treatment of benign thyroid nodules or recurrent thyroid cancer [20–22]. A recent guideline was developed including recommendations for optimal use of RFA in treatment of benign thyroid nodules [23]. It is also a relatively easy technique for surgeons that substantial portion of them make thyroid US examination in their practice.
The purpose of our study was to assess the efficacy and safety of US-guided percutaneous RFA in the treatment of benign symptomatic thyroid nodules.
Patients and methods
Participants
The study was a single-institution, prospective, safety and efficacy study. From January 2013 to January 2014, the patients with a diagnosis of benign nodular goiters having one or more nodule-related symptoms such as dysphagia, a sensation of foreign bodies in the neck, neck discomfort, cosmetic problems, or hyperthyroidism due to autonomous thyroid nodules or patients having a fear of malignancy were evaluated for their eligibility for the ablation procedure.
Ethics
With approval from the institutional ethical committee, we prospectively started RFA procedures and data collection. Written informed consent was obtained from all eligible patients. The patients were informed about adverse effects of the procedure (hematoma, infection, hypothyroidism, vocal cord palsy, tracheal injury, skin burns).
Outcome measures
The primary outcome was the evaluation of changes in symptom scores for pain, dysphagia, and foreign body sensation at the 1st, 3rd, and 6th months after the RFA procedure. Secondary outcomes were assessment of volume changes in nodules, complication rates, and changes in thyroid function status at the follow-up period.
Pre-procedural assessments
Images of nodules and of the neck region were obtained with a 10-MHz linear probe on a real-time US system in order to characterize the size, shape, echogenicity, calcification status, solid/cystic proportions, internal vascularity, and margins of each nodule. Presence of any lymph nodes was recorded. Two different samples were obtained with a fine needle under US guidance in order to check whether the nodules were benign. The cytopathology of the thyroid tissue was reported using the Bethesda system [24]. Patients were excluded from the study and reevaluated for surgery if there were suspicious ultrasonography findings concerning nodule structures or the accompanying lymph nodes or when the Bethesda scores were 3–5. On this basis, cytology-proven benign nodules with >50 % solid components were found to be suitable for RFA (Fig. 1).
US examination included volumetric measurements of each nodule using the equation: V = πabc/6 (a = the largest diameter, b and c = the other perpendicular diameters in millimeters).
Laboratory tests included a complete blood count, and tests for thyrotrophin, thyroid hormones, prothrombin time, and the activated partial thromboplastin time. The patients under anticoagulant treatment were withdrawn from their treatments for 7–10 days to assure normal coagulation tests before the ablation procedure. Also, all the participants were assessed for vocal cord function by an experienced laryngologist before the ablation procedure.
Scales
-
(A)
Patients’ subjective symptoms such as pain, dysphagia, and foreign body sensation were measured with a 10-cm visual analog scale (0–10 cm).
-
(B)
Goiters were graded according to the World Health Organization’s grading system in the following fashion: grade 0: impalpable/invisible; grade 1a and 1b: palpable but invisible even in full extension or palpable in neutral position/visible in extension; grade 2: visible but no palpation required to make diagnosis; grade 3: visible at a distance. The patients’ vocal cord motility was examined by direct laryngoscopy for the presence of any nerve injury prior to the procedure.
-
(C)
The US examination was done at the 1st, 3rd, and 6th months following RFA for evaluation of the changes in volume, echogenicity, intranodular vascularity and for the presence of a viable portion within the nodule. In each US examination the volume reduction ratio (VRR) was calculated in percentages by an equation: VRR (%) = (index volume (mL)−final volume (mL) × 100)/index volume (mL).
RF procedure
All RF ablation procedures were done on an outpatient basis. The patient was placed in a supine position with a mild neck extension. We used an internally cooled electrode (Star electrode, 18 gage, with 7 mm active tip) and an RF generator (Viva RF system, Starmed, Korea.). According to the position of the nodule, local anesthesia with 2 % lidocaine was applied to the skin puncture site. We made a 2-mm incision with the tip of a scalpel. After positioning the electrode in the deepest portion of the nodule, the ablation was started using the ‘moving shot technique’ [20]. Briefly, the primary ablation energy was set at 25 W, and when the hyperechoic zone was seen within the nodule after 20–45 s, the electrode was moved to another area of the nodule. If the hyperechoic zone was not seen after 45 s, the power was increased in 5 W steps up to 60 W. The ablation was discontinued when all zones were hyperechoic allowing 5–10 min for each nodule (Fig. 2). The procedure was done on an outpatient basis, and all patients were given oral analgesics and discharged 2 h after the procedure. On the next day, we made phone calls and asked them about any complaints.
Follow-up
The patients were called back to the hospital at the end of the1st, 3rd, and 6th months following the RFA. Blood samples were drawn for laboratory tests (complete blood count, TSH, thyroid hormones). The cosmetic grading and scoring for complaints were repeated at each admission. Also the VRRs were calculated at each visit. Patients were examined for development of any new clinical signs.
Statistical analysis
Statistical analyses were performed using SPSS for Windows (version 16.0; SPSS, Chicago, Ill., USA). We compared the pre-treatment and post-treatment values of nodule volume, VRR, symptoms, and cosmetic scores. All values were given as mean ± SD. One-way analysis of variance (ANOVA) test was used to compare symptom scores, changes in nodule volume, VRR; the McNemar's test was used to make comparison among the grade 2 and grade 3 cosmetic scores; and multiple linear regression analysis was done to evaluate the correlation between patients’ ages and the VRR. The level of significance was defined as p less than 0.05.
Results
The study included 33 patients (8 female, 24 % and 25 male, 76 %) and a total of 65 nodules. More than one nodule (range 2–4) was treated in 21 patients (63.6 %). The mean nodule diameter was 26.4 ± 9.9 mm. The study included 33 patients (8 female, 24 % and 25 male, 76 %) and a total of 65 nodules. More than one nodule (range 2–4) was treated in 21 patients (63.6 %). The mean nodule diameter was 26.4 ± 9.9 mm.
Symptom scores
Symptoms like pain, foreign body sensation, and dysphagia were scored for the pre- and post-treatment periods. The mean pain score was 2.9 ± 2.7 for the pre-treatment period, and at the end of the 1st, 3rd, and 6th months following the RFA, the scores were 2.3 ± 2.01, 1.8 ± 1.7, and 1.5 ± 1.2, respectively. The decreases in pain scores were statistically significant (p 0.005).
In pre-treatment period, the mean dysphagia score was 3.9 ± 2.7. At the 1st, 3rd, and 6th months, the mean dysphagia scores were 2.6 ± 1.9, 1.7 ± 1.6, and 1.1 ± 0.3, respectively. The improvement in mean dysphagia scores was statistically significant (p 0.032).
The mean score for foreign body sensation was 3.6 ± 3 at pre-treatment and following the RFA at 1st, 3rd, and 6th months, the mean scores were 2.5 ± 2.2; 1.6 ± 1.5, and 1.1 ± 0.4, respectively. The differences in the scores at all follow-up periods were found to be statistically significant (p 0.002) (Table 1).
Changes in nodule volume
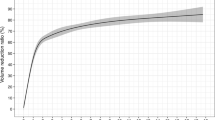
The mean pre-treatment volume of the nodules was 7.3 ± 8.3 mL (range 0.16–51). The mean volumes at 1st, 3rd, and 6th months after the procedure were 3.5 ± 3.8 mL (range 0.1–18), 2.7 ± 3.4 mL (range 0.04–18), and 1.2 ± 1.7 mL (range 0–8), respectively. At the post-procedural 1st, 3rd, and 6th months, nodule volumes were compared with the index volume and were found to be significantly different at p 0.002. Figure 3 shows changes in nodule diameter under US examination at 1st, 3rd, and 6th months of the RFA procedure.
The VRRs were calculated and the mean percentage reduction of volumes after the procedure at 1st month was 47 %, at 3rd month was 59 %, and at 6th month was 74 %. The reduction overtime was statistically significant (p 0.005). Multiple regression analysis showed no correlation between patient’s age and VRR having the correlation coefficient as 0.273 and p values as 0.679 (Table 2).
Cosmesis
Cosmetic scores were graded from 0 to 3: Prior to RFA, 36 % had grade 0 score, 27 % had grade 1 score, 24 % had grade 2 score, and 13 % had grade 3 score. At 1st month, 51 % had a grade 0 score, 22 % had a grade 1 score, 20 % had a grade 2 score, and 7 % had a grade 3 score. At 3rd month, 57 % had a grade 0 score, 20 % had a grade 1 score, 16 % had a grade 2 score, and 7 % had a grade 3 score. At the last evaluation at 6th month of the procedure, 69 % had a grade 0 score, 14 % had a grade 1 score, 12 % had a grade 2 score, and 5 % had a grade 3 score. When the index grade 2 and 3 scores and the last grade 3 score were compared, the improvements in cosmetic scores were found to be statistically significant, respectively (p 0.024 and p 0.003) (Table 3).
Thyroid function status
Thyroid functions of the patients were evaluated; prior to ablation, 85 % (n: 25) of the patients were euthyroid, and 12 % (n: 3) of this group was under levothyroxine treatment. 15 % (n:8) had autonomously functioning thyroid nodules. In the pre-treatment period, the patients were placed on methimazole treatment. At the 3rd month of the RFA, 50 % (n: 4) of the hyperthyroid patients became euthyroid. The other half continued to require lower doses of anti-thyroid medications to remain euthyroid in follow-up.
Complications
There were no deaths. The patients were observed for 1–2 h and discharged. Patients complained of some degree of pain during the RFA. The pain ceased by decreasing the power output. 12 % (n: 4) of the patients reported continuation of the painful feeling at the neck radiating to the teeth and head for 3 days after the ablation. They had pain relief with oral paracetamol. Also, we did not observe any skin burns, hematomas, and infection. All the participants were assessed for vocal cord functions by direct laryngoscopy. There was neither vocal cord dysfunction nor voice changes detected after the RFA procedure.
Discussion
There are minimal invasive alternatives to surgery in the treatment of benign thyroid nodules. Ethanol ablation (EA) and percutaneous laser ablation (PLA) were used and yielded exciting results [19, 25, 26]. EA is generally saved for cystic nodules rather than solid nodules [27]. PLA is also considered to be effective in the treatment of solid nodules [18, 28].
Levothyroxine suppression treatment is another approach in the management of thyroid nodules of euthyroid patients although its efficacy is controversial [29]. Levothyroxine therapy has also some adverse effects like atrial fibrillation and reduction of bone density [13]. In patients having fear of malignancy, hemithyroidectomy is an option in the management of thyroid nodules. Hypothyroidism ranging from 5 to 49 % was reported following hemithyroidectomy [30–32] In a previous study with 1,051 patients, who underwent hemithyroidectomy, postoperative hormone requirement was reported to be up to 28 % [33].
The use of RF energy was proposed because of the ease of handling, perfect consistency, and controllability of the ablation site [23, 34]. Surgeons are familiar to RF ablation, which is generally used in treatment of liver malignancies [35, 36]. In this study, we presented our experience with RFA in management of benign symptomatic thyroid nodules.
Ablation of benign thyroid nodules is quite a new concept. It was shown to be efficient and safe in management [23]. Its efficacy is evaluated by calculating the VRRs in follow-ups. More than a 50 % reduction in volume ratio was designated to be successful in previous series [23, 37]. VRRs were reported as 75–97 % [38, 39]. We have seen 74 % volume reductions in 6 months. RF energy does not ablate the nodules immediately; it induces necrosis in months and also a decrease in internal vascularity of the nodules favors involution. The higher reduction ratios in other series seem to be related to longer follow-up periods [40]. In previous studies, up to six cycles of ablation were found to be tolerable with a higher rate producing transient voice changes [20].
In addition to VRR, symptom scoring, and cosmetic grading, there are complimentary methods to show the efficacy of the procedure. We have shown statistically significant decrements in symptom scores (pain, dysphagia, foreign body sensation, and improvement in cosmetic grades) in the follow-up. Also, this procedure saves the patient from an anterior neck scar following thyroidectomy; this brings additional cosmetic advantage.
In our study, we had eight hyperthyroid patients with autonomously functioning nodules confirmed by thyroid scintigraphy. At the 3rd month after RFA, 50 % (n: 4) of the hyperthyroid patients became euthyroid, and they quit anti-thyroid medication and the other half required lower doses of thyramazole at the follow-up. In a previous study, Dandrea et al. reported a normalization of thyroid function tests in 24 % of their patients [41]. Baek et al. applied RFA to 9 patients with automonously functioning nodules. They found complete recovery of T3 and free T4 levels in all patients with but incomplete recovery of TSH levels in 3 patients, and the phenomenon was explained by difficulty to ablate the entire nodule because a peripheral tissue layer remains avoiding heat diffusion outside the treated nodule [38]. In our series, the mean VRR in patients who became euthyroid after the RFA procedure was 71 %. On the other hand, the remaining patients who were still hyperthyroid, the mean VRR were 43 %. The involution could be incomplete and this can be the reason for persistence of hyperthyroid state.
The incidence of recurrent laryngeal nerve injury in thyroidectomy is 0.2–1.1 % and also there is 1 % risk of postoperative permanent hypocalcaemia [42].The safety of RFA as a minimally invasive method was questioned in previous studies and some complications like voice change (1 %), hematoma (1 %), vomiting (0.67 %), skin burn (0.27 %), hypothyroidism (0.07 %), nodule rupture with abscess (0.07 %), brachial plexus injury (0.07 %), or thyrotoxicosis have been reported [43]. The changes were reversible and patients recovered at follow-up [21, 40, 41, 44]. There are reported side effects like pain (2.6 %), vasovagal reaction (0.34 %), or coughing (0.21 %). A recent meta-analysis showed that the incidence of adverse effect (fever, hematoma, 1st degree skin burn, and edema) in thyroid RFA is 3.9 % and serious adverse effects (glandular hemorrhage or vocal cord palsy) is 0.55 %. We did not observe any serious complications after RFA except that 4 patients had 3 days of lasting pain radiating to jaw and teeth, relieved by paracetamol treatment.
Conclusion
RF ablation can be an alternative treatment modality in the management of symptomatic benign solid thyroid nodules in properly selected patients. Our study showed that RFA improves symptoms and is effective on volume reduction in patients with biopsy-proven benign solid thyroid nodules. The procedure caused minor side effects like pain in a few patients, and this was easily managed and no other major complications occurred. Further follow-up is needed to confirm its long-term effects.
References
Singer PA, Cooper DS, Daniels GH et al (1996) Treatment guidelines for patients with thyroid nodules and well-differentiated thyroid cancer. American Thyroid Association. Arch Intern Med 156:2165–2172
Tan GH, Gharib H (1997) Thyroid incidentalomas: management approaches to nonpalpable nodules discovered incidentally on thyroid imaging. Ann Intern Med 126:226–231
Popoveniuc G, Jonklaas J (2012) Thyroid nodules. Med Clin North Am 96:329–349
Hegedus L (2004) Clinical practice. The thyroid nodule. N Engl J Med 351:1764–1771
Gharib H, Papini E (2007) Thyroid nodules: clinical importance, assessment, and treatment. Endocrinol Metab Clin North Am 36:707–735 vi
Smith-Bindman R, Miglioretti DL, Johnson E et al (2012) Use of diagnostic imaging studies and associated radiation exposure for patients enrolled in large integrated health care systems, 1996–2010. JAMA 307:2400–2409
Guth S, Theune U, Aberle J et al (2009) Very high prevalence of thyroid nodules detected by high frequency (13 MHz) ultrasound examination. Eur J Clin Invest 39:699–706
Mandel SJ (2004) Diagnostic use of ultrasonography in patients with nodular thyroid disease. Endocr Pract 10:246–252
Marqusee E, Benson CB, Frates MC et al (2000) Usefulness of ultrasonography in the management of nodular thyroid disease. Ann Intern Med 133:696–700
Danese D, Sciacchitano S, Farsetti A et al (1998) Diagnostic accuracy of conventional versus sonography-guided fine-needle aspiration biopsy of thyroid nodules. Thyroid 8:15–21
Lima N, Knobel M, Cavaliere H et al (1997) Levothyroxine suppressive therapy is partially effective in treating patients with benign, solid thyroid nodules and multinodular goiters. Thyroid 7:691–697
Wesche MF, Tiel VBMM, Lips P et al (2001) A randomized trial comparing levothyroxine with radioactive iodine in the treatment of sporadic nontoxic goiter. J Clin Endocrinol Metab 86:998–1005
Schneider R, Schneider M, Reiners C, Schneider P (2012) Effects of levothyroxine on bone mineral density, muscle force, and bone turnover markers: a cohort study. J Clin Endocrinol Metab 97:3926–3934
Nygaard B, Faber J, Veje A et al (1999) Transition of nodular toxic goiter to autoimmune hyperthyroidism triggered by 131I therapy. Thyroid 9:477–481
Chen AY, Levy L, Goepfert H et al (2001) The development of breast carcinoma in women with thyroid carcinoma. Cancer 92:225–231
Bonnema SJ, Hegedus L (2012) Radioiodine therapy in benign thyroid diseases: effects, side effects, and factors affecting therapeutic outcome. Endocr Rev 33:920–980
Chen AY, Bernet VJ, Carty SE et al (2014) American thyroid association statement on optimal surgical management of goiter. Thyroid 24:181–189
Spiezia S, Vitale G, Di Somma C et al (2003) Ultrasound-guided laser thermal ablation in the treatment of autonomous hyperfunctioning thyroid nodules and compressive nontoxic nodular goiter. Thyroid 13:941–947
Sung JY, Baek JH, Kim YS et al (2008) One-step ethanol ablation of viscous cystic thyroid nodules. AJR Am J Roentgenol 191:1730–1733
Jeong WK, Baek JH, Rhim H et al (2008) Radiofrequency ablation of benign thyroid nodules: safety and imaging follow-up in 236 patients. Eur Radiol 18:1244–1250
Baek JH, Kim YS, Lee D et al (2010) Benign predominantly solid thyroid nodules: prospective study of efficacy of sonographically guided radiofrequency ablation versus control condition. AJR Am J Roentgenol 194:1137–1142
Monchik JM, Donatini G, Iannuccilli J, Dupuy DE (2006) Radiofrequency ablation and percutaneous ethanol injection treatment for recurrent local and distant well-differentiated thyroid carcinoma. Ann Surg 244:296–304
Na DG, Lee JH, Jung SL et al (2012) Radiofrequency ablation of benign thyroid nodules and recurrent thyroid cancers: consensus statement and recommendations. Korean J Radiol 13:117–125
Cibas ES, Ali SZ (2009) The Bethesda system For reporting thyroid cytopathology. Am J Clin Pathol 132:658–665
Papini E, Guglielmi R, Bizzarri G, Pacella CM (2004) Ultrasound-guided laser thermal ablation for treatment of benign thyroid nodules. Endocr Pract 10:276–283
Valcavi R, Frasoldati A (2004) Ultrasound-guided percutaneous ethanol injection therapy in thyroid cystic nodules. Endocr Pract 10:269–275
Kim JH, Lee HK, Lee JH et al (2003) Efficacy of sonographically guided percutaneous ethanol injection for treatment of thyroid cysts versus solid thyroid nodules. AJR Am J Roentgenol 180:1723–1726
Dossing H, Bennedbaek FN, Karstrup S, Hegedus L (2002) Benign solitary solid cold thyroid nodules: US-guided interstitial laser photocoagulation—initial experience. Radiology 225:53–57
Hegedus L, Bonnema SJ, Bennedbaek FN (2003) Management of simple nodular goiter: current status and future perspectives. Endocr Rev 24:102–132
Farkas EA, King TA, Bolton JS, Fuhrman GM (2002) A comparison of total thyroidectomy and lobectomy in the treatment of dominant thyroid nodules. Am Surg 68:678–682 discussion 682–673
Wormald R, Sheahan P, Rowley S et al (2008) Hemithyroidectomy for benign thyroid disease: who needs follow-up for hypothyroidism? Clin Otolaryngol 33:587–591
McHenry CR, Slusarczyk SJ (2000) Hypothyroidisim following hemithyroidectomy: incidence, risk factors, and management. Surgery 128:994–998
Vaiman M, Nagibin A, Hagag P et al (2008) Hypothyroidism following partial thyroidectomy. Otolaryngol Head Neck Surg 138:98–100
Gazelle GS, Goldberg SN, Solbiati L, Livraghi T (2000) Tumor ablation with radio-frequency energy. Radiology 217:633–646
Kang TW, Rhim H, Kim EY et al (2009) Percutaneous radiofrequency ablation for the hepatocellular carcinoma abutting the diaphragm: assessment of safety and therapeutic efficacy. Korean J Radiol 10:34–42
Lencioni R, Cioni D, Bartolozzi C (2001) Percutaneous radiofrequency thermal ablation of liver malignancies: techniques, indications, imaging findings, and clinical results. Abdom Imaging 26:345–360
Sung JY, Baek JH, Kim KS et al (2013) Single-session treatment of benign cystic thyroid nodules with ethanol versus radiofrequency ablation: a prospective randomized study. Radiology 269:293–300
Baek JH, Moon WJ, Kim YS et al (2009) Radiofrequency ablation for the treatment of autonomously functioning thyroid nodules. World J Surg 33:1971–1977
Lee JH, Kim YS, Lee D et al (2010) Radiofrequency ablation (RFA) of benign thyroid nodules in patients with incompletely resolved clinical problems after ethanol ablation (EA). World J Surg 34:1488–1493. doi:10.1007/s00268-010-0565-6
Spiezia S, Garberoglio R, Milone F et al (2009) Thyroid nodules and related symptoms are stably controlled two years after radiofrequency thermal ablation. Thyroid 19:219–225
Deandrea M, Limone P, Basso E et al (2008) US-guided percutaneous radiofrequency thermal ablation for the treatment of solid benign hyperfunctioning or compressive thyroid nodules. Ultrasound Med Biol 34:784–791
Bron LP, O’Brien CJ (2004) Total thyroidectomy for clinically benign disease of the thyroid gland. Br J Surg 91:569–574
Baek JH, Lee JH, Sung JY et al (2012) Complications encountered in the treatment of benign thyroid nodules with US-guided radiofrequency ablation: a multicenter study. Radiology 262:335–342
Kim YS, Rhim H, Tae K et al (2006) Radiofrequency ablation of benign cold thyroid nodules: initial clinical experience. Thyroid 16:361–367
Acknowledgment
Authors declare no conflict of interest. No competing financial interests exist. We thank to Prof. Ray Guillery for English edit.
Conflict of interest
Authors declare no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ugurlu, M.U., Uprak, K., Akpinar, I.N. et al. Radiofrequency Ablation of Benign Symptomatic Thyroid Nodules: Prospective Safety and Efficacy Study. World J Surg 39, 961–968 (2015). https://doi.org/10.1007/s00268-014-2896-1
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00268-014-2896-1