Abstract
Weight gain and obesity are driving the global epidemic of type-2 diabetes through metabolic and inflammatory pathways that cause insulin resistance and impair pancreatic β-cell function, the two important factors that are directly responsible for the development of this disease in susceptible populations. Lifestyle methods and modest weight loss are powerful at preventing and managing type-2 diabetes, but sustaining substantial weight loss is problematic. Bariatric surgery provides exceptional sustained weight loss and remission of type-2 diabetes in 50–85% of subjects, especially if treated early before irreparable β-cell damage has occurred. In addition, there is substantial evidence that bariatric surgery provides additional comorbidity and quality-of-life improvements and reduces mortality in patients with type-2 diabetes. There is an association between the extent of weight loss and remission of type-2 diabetes. Diversionary bariatric procedures such as gastric bypass and biliopancreatic diversion induce a rapid non-weight-loss-associated improvement in glycemic control. Several mechanisms have been proposed for this exciting and novel effect that may provide key insights into the pathogenesis of type-2 diabetes. A range of novel surgical, endoluminal procedures/devices, and pharmacologic therapies are likely to evolve when we better understand how bariatric surgery enables long-term changes in energy balance and non-weight-related metabolic improvements. Bariatric surgery should be considered for adults with BMI ≥ 35 kg/m2 and type-2 diabetes, especially if the diabetes is difficult to control with lifestyle and pharmacologic therapy. Although all bariatric procedures produce exceptional results in the management of type-2 diabetes, choice of procedure requires a careful risk–benefit analysis for the individual patient.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
The diabesity epidemic
Obesity and type-2 diabetes are likely to be the two greatest public health problems of the coming decades [1]. There is a strong relationship between obesity and type-2 diabetes, and the term “diabesity,” introduced by Shafrir, has been coined to suggest a single problem [2, 3]. The adjusted relative risk of developing type-2 diabetes in those with a body mass index (BMI) of greater than 35 kg/m2 is 93 (95% confidence interval (CI) = 81–107) for women [4] and 42 (95% CI = 22–81) for men [5] compared with those of one with a BMI <22 and <23 kg/m2, respectively. Approximately half of those diagnosed with type-2 diabetes are obese [6]. In addition, the increase in the prevalence of diabetes over recent decades has been disproportionately included people extremely obese [7].
A substantial portion of the health costs attributed to obesity is related to type-2 diabetes. The socioeconomic impact of type-2 diabetes and its complications are substantial to individuals, their families, and to society [8]. In particular, it is an inexorably progressive disease that leads to deterioration of multiple organs and systems and is the most common cause of adult blindness, limb amputations, and renal failure in Western countries as well as the leading independent risk factor for coronary artery disease [9].
Pathophysiology: obesity and type-2 diabetes
Type-2 diabetes is a heterogeneous group of conditions broadly characterized by insulin resistance, a state of reduced responsiveness of insulin-mediated glucose uptake to circulating insulin, and an inadequacy of the pancreatic β cells to provide sufficient insulin for current requirements [10].
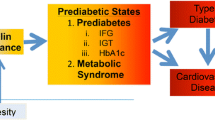
Adipose tissue is an important endocrine organ that communicates with the brain and peripheral tissues regulating appetite and metabolism [11]. Excess adipose tissue, especially visceral fat, delivers a range of metabolic and inflammatory factors, including free fatty acids [12] and proinflammatory cytokines such as tumor necrosis factor α and interleukin-6 [13], that via a number if intracellular pathways, induce insulin resistance in key metabolic organs, including liver, skeletal muscle, and adipose tissue [14]. One adipose tissue factor, adiponectin, improves insulin sensitivity, but circulating levels are unfortunately lower in obesity, metabolic syndrome, and type-2 diabetes [15]. Indeed, obesity, especially central obesity, sets up a complex metabolic and inflammatory cascade that drives much of the more serious weight-related disease (Fig. 1).
Pancreatic β cells are usually able to adapt to a changing metabolic load. Diabetes ensues as a result of inadequate adaptation of β-cell function to cater to sufficient insulin secretion. While there are inherited tendencies to β-cell failure, the current diabesity epidemic is driven by an acquired reduction in β-cell mass, mainly due to β-cell apoptosis or programmed cell death [16]. As the diabetes state progresses there is continued β-cell deterioration and a point of no return is reached when a permanent diabetes state arises, one that must be treated with insulin [17]. The metabolic stress of obesity and type-2 diabetes is associated with lipotoxicity, glucotoxicity, inflammatory cytokines, leptin, and islet cell amyloid—all factors that act in a vicious cycle of reduced β-cell mass and failure [18, 19]. Preserving and enhancing β-cell function should be a fundamental aim in the prevention and management of type-2 diabetes. Early and intensive treatment of type-2 diabetes is known to improve health outcomes and quality of life [20–23].
Nonsurgical weight loss and type-2 diabetes
Weight control is perhaps the most important way of preventing and treating type-2 diabetes, with weight loss reducing morbidity and mortality [24]. The durable effect of modest weight loss associated with practical achievable lifestyle interventions in preventing the development of type-2 diabetes in those at high risk of the disease has been shown to be a 58% risk reduction in both the US and Finnish diabetes prevention studies [25, 26]. Recently, the early results from the intensive lifestyles intervention arm of the US “Look Ahead” study demonstrated improved glycemic control and reduced cardiovascular risk associated with weight loss [27]. Intentional weight loss through diet and exercise is associated with better control, fewer complications, and reduced mortality [24, 28, 29]. A systematic review indicates that improvement in blood glucose control is closely related to degree of weight loss [30].
Unfortunately, despite the impressive effect of weight loss, currently available lifestyle, dietary, and pharmacologic strategies provide only small to modest levels of weight loss, with diabetics experiencing greater difficulty in losing weight than nondiabetics [30–33], and achieving and sustaining substantial weight loss in those with type-2 diabetes has been an elusive goal [32].
Bariatric surgery and type-2 diabetes
An early study to show this clinical improvement consisted of 608 patients followed for up to 14 years after a Greenville-type open gastric bypass [34]. Weight loss was 55% of excess weight at 10 years and 49% at 14 years. There were 146 type-2 diabetic patients, and 121 (83%) achieved and maintained a nondiabetic clinical state with normal fasting plasma glucose, HbA1c, and serum insulin levels. In addition, 150 of 152 patients with impaired glucose tolerance became normoglycemic. The weight loss was accompanied by major improvements or remission of other obesity-related comorbidity, including hypertension, sleep apnea, and infertility. This study also showed a significant reduction in mortality in the patients treated surgically when compared to a similar morbidly obese group who did not proceed with operation, principally due to reduced cardiovascular deaths [29]. However, remission of type-2 diabetes was less likely in older subjects and in those who had a longer history of type-2 diabetes [35]. In addition, the authors of this study were the first to observe rapid remission of type-2 diabetes and hypothesize that the diversionary component of some bariatric procedures may provide benefit beyond that of weight loss [36].
Numerous subsequent studies have demonstrated high rates of type-2 diabetes remission with bariatric surgery, but most have serious methodologic weakness [37]. Many did not enrol consecutive patients, report biochemical measures, or accurately define diabetes remission, and follow-up was often very poor. Few studies meet the minimum GRADE criteria standards and report follow-up rates of at least 80% [38, 39]. Remission rates reported in the ten studies that reached the minimal GRADE quality were 92–97% (n = 2) for the malabsorptive procedures biliopancreatic diversion of the duodenal switch variant (BPD and BPD-DS), 78–83% (n = 4) for roux-en Y gastric bypass (RYGB), and 45–73% (n = 4) for procedures described as restrictive, i.e., laparoscopic adjustable gastric banding (LAGB) and vertical banded gastroplasties (VBG). The only data considered to be of moderate or high quality were from three studies of largely restrictive procedures where the extent of weight loss appears critical in the delivery of benefit to blood pressure, dyslipidemia, and glycemic control [40–42]. A summary of these three studies follows.
The extent of weight loss is important
The Swedish Obese Subjects study group reported major reductions in the prevalence and incidence of type-2 diabetes after a range of bariatric surgical procedures, with the majority being gastroplasty. This study involved over 2000 subjects electing to undergo bariatric surgical procedure who were then compared to well-matched controls. A major reduction in the prevalence of type-2 diabetes at 2, 8, and now 10 years following surgical intervention was reported, and more recent mortality data indicate a major reduction in the mortality of patients with diabetes in the surgically treated group [43–45]. This study provides the most detailed and compelling evidence of the effect of bariatric surgery on the natural history of type-2 diabetes.
An Italian study by Pontiroli et al. [40] prospectively studied 143 patients who had LAGB surgery over a 3-year period and compared the metabolic results at 1 year with those of 120 obese patients who had conventional dietary weight loss therapy. They report greater weight loss in the surgically treated group along with improvements in blood pressure, dyslipidemia, and glycemic control that were proportional to the degree of weight loss. Greater metabolic improvements were seen in those with type-2 diabetes and HbA1c levels fell significantly from a mean of 8.2% at baseline to 5.9% at 3 years. While only 45% of those with type-2 diabetes went into remission, the glycemic control for the group with diabetes was exceptional.
Currently there is only one published randomized controlled trial of surgery compared with conventional therapy for the treatment of type-2 diabetes and this was from our group in Australia [42]. Randomized patients had been diagnosed with type-2 diabetes for less than 2 years at recruitment and their BMI was in the 30–40-kg/m2 range. Remission, defined as Hba1c <6.2, fasting plasma glucose <7 mmol/l, and not on any hypoglycemic therapy, was achieved by 22 (73%) in the surgical group and 4 (13%) in the conventionally treated group. Surgical and conventional therapy groups had lost a mean of 20.7 ± 8.6% and 1.7 ± 5.2% of weight, respectively, at 2 years. Remission of type-2 diabetes was strongly related to weight loss. The pattern of weight loss, changes in HbA1c, and remission of type-2 diabetes over the 2-year period is shown in Fig. 2a–c. There was also significant improvement in dyslipidemia, with a greater mean fall in triglyceride and rise in HDL cholesterol levels in those treated surgically, and only 4 (13%) surgically treated compared with 21 (70%) fulfilled the ATPIII criteria for the metabolic syndrome at 2 years. We have recently shown that the LAGB therapy provided in this randomized study was cost effective, indeed was dominant, indicating that there would be cost savings over a patient’s lifetime if treated surgically [46, 47].
There is little doubt that achieving and sustaining weight loss in obese patients has a profound clinical effect in successfully managing type-2 diabetes and that weight loss appears to be the main mode of action in nondiversionary bariatric surgery.
Early intervention to preserve β-cell function
In an earlier study, our group in Australia reported a 64% remission in type-2 diabetes 1 year after LAGB surgery, with a further 26% having improved glycemic control, and 10% had little change [48]. Remission was strongly predicted by greater weight loss and a shorter history of diabetes. Improvement in insulin sensitivity was best predicted by the extent of weight loss, but improvement in β-cell function or secretion was predicted by a shorter history of diabetes [49]. Schauer et al. [50] showed clinical remission in 83% of patients with type-2 diabetes and the remaining 17% improved significantly following RYGB surgery. This study found that a shorter history of diabetes and milder disease was associated with an increased likelihood of remission.
Timing appears to be a key factor in achieving remission of type-2 diabetes following bariatric surgery through improvement in β-cell function. This is to be expected given the progressive deterioration of β-cell function [20, 51] which characterizes this disease. The metabolic and inflammatory milieu (Fig. 1) produces a vicious cycle of β-cell deterioration and loss of β-cell mass, largely through increased apoptosis, leading to increasing levels of hypoglycemic therapy [18]. There is a reversible component of β-cell deterioration, with weight loss improving β-cell responsiveness to glucose [52, 53]. If the bariatric surgical procedure is performed before irreversible β-cell failure has occurred, then durable weight loss will be accompanied by a high likelihood of long-term remission [34, 54].
Given the close relationship between positive energy balance, excess weight, and the development of type-2 diabetes, it is hardly surprising that weight loss provides such an impressive therapeutic effect. Indeed, the vicious cycle that drives the development of type-2 diabetes through both insulin resistance and progressive β-cell deterioration is significantly reversed through negative energy balance and significant sustained weight loss [55]. The inflammatory and metabolic milieu depicted in Fig. 1 as driving obesity-related disease is substantially reversed with weight loss. Taylor [55] has recently, perhaps provocatively, described the time course of diabetes development and its remission following bariatric surgery in a review entitled “Pathogenesis of type-2 diabetes: tracing the reverse route from cure to cause.”
Diversionary surgery: benefits beyond that of weight loss
Despite the paucity of any medium- or high-quality clinical data regarding diversionary procedures such as RYGB and BPD, there appears to be early improvement in glucose tolerance in patients with type-2 diabetes, an effect beyond that expected for comparable weight loss [34, 38]. The evidence for this effect is now compelling and raises new questions about the gut and its role in the pathogenesis of type-2 diabetes [56] and the possibility that surgery may be manipulated to limit the weight reduction effect and to maximize its anti-diabetes effect to treat patients with type-2 diabetes who do not have a major weight problem [57]. Revealing the mechanism(s) of this non-weight-loss effect of bariatric surgery is extremely exciting as it is likely to provide a host of novel therapies for type-2 diabetes. Is the effect a rapid improvement in insulin sensitivity, stimulation of pancreatic β-cell secretion, or, perhaps, a combination of both? There are several mechanisms proposed for this early effect on glucose tolerance.
Activation of the enteroinsular axis
Incretins are gastrointestinal hormones that are released from luminal enteroendocrine cells as a response to ingested nutrients to stimulate pancreatic insulin secretion. Ingestion of glucose produces an insulin release that is greater than that of intravenous glucose—the difference is the incretin effect. The enteroinsular axis hormones glucagon-like peptide-1 (GLP-1) from L cells and glucose-dependent insulinotrophic peptide (GIP) from K cells are incretins that account for much of this effect and act on the pancreatic β cells via a common mechanism [38]. Obesity and type-2 diabetes have independent effects on incretin effects through unknown mechanisms.
The hindgut or lower intestinal hypothesis
There is now excellent evidence that nutrient ingestion following diversionary bariatric surgery has an exaggerated L-cell stimulatory effect that increases GLP-1 and peptide YY (PYY) release that may have appetite-suppressive and incretin effects [58, 59]. Direct evidence of an incretin effect was shown when comparing intravenous and oral glucose tolerance tests in nine women with type-2 diabetes 1 month post-RYGB and in ten matched dietary weight loss controls who had lost the same amount of weight. The RYGB patients had a sixfold response in GLP-1 and a fivefold greater incretin effect after oral glucose, but there was no similar effect in the diet-induced weight loss group. There was also a significantly greater reduction in postprandial glucose levels in the RYGB weight loss group [60]. There was no significant difference in GIP, fasting glucose, or peak glucose levels between groups. This provides excellent evidence of an early incretin effect and improved area-under-the-curve glucose tolerance that is not related directly to the weight loss. This exaggerated L-cell response supports the hypothesis that the hind gut may be responsible for the improved glucose tolerance. A number of studies demonstrate that ileal transposition can also stimulate L-cell activity, potentially enhancing satiety and weight loss and improving metabolic outcomes [61, 62]. These studies also support a hind gut effect of diversionary surgery. It is of interest that GLP-I receptor agonists and inhibitors of dipeptidyl peptidase (DPP)-IV, an enzyme that breaks down GLP-1, are now established therapies for type-2 diabetes. A benefit from the GLP-1 receptor agonists is that they assist with modest weight loss. There are also data from animal models that suggest GLP-I receptor agonists and DPP-IV blockers increase β-cell mass [18].
The foregut or upper gastrointestinal hypothesis
There is now a growing body of evidence that foregut intervention, in particular, exclusion of the duodenum, may improve glucose tolerance. First posed by Pories, this hypothesis was elegantly explored by Rubino in a series of experiments using animal models [36, 63]. He demonstrated, using a Goto-Kakizaki type-2 diabetic rat model, that duodenal–jejunal bypass (DJB) improved glucose tolerance with no weight difference between those with DJB and controls. In addition, he was able to switch the glucose tolerance effect on and off by reintroducing the passage of food through the duodenum. The hypothesis that there was a foregut effect through exclusion of the duodenum was thus supported, and the authors further propose that there may be a “duodenal factor,” perhaps an anti-incretin, that may explain the duodenal exclusion effect seen. His team subsequently showed that a duodenal sleeve that excludes the nutrients from the duodenal surface improved glucose tolerance. These developments have led to promising preliminary human studies of DJB and the use of endoscopically placed duodenal sleeves as a treatment for type-2 diabetes, potentially extending surgical treatment for diabetes into a weight category where substantial weight loss is not the prime target [57, 64, 65]. A range of novel procedures is being investigated to try and exploit the “antidiabetic” effects of gastrointestinal surgery.
Insulin sensitivity
Much of the non-weight-loss effect of bariatric surgery has explored the incretin pancreatic-stimulating effect. Perhaps more important is the effect on insulin sensitivity. A New Zealand group has described early improvements in insulin sensitivity within 6 days of RYGB surgery [66]. Weight loss has a profound effect on improving insulin sensitivity and reversing the metabolic and inflammatory cascade (Fig. 1). A recent study compared banding and gastric-enteral anastomosis procedures in C57Bl6 mice that were fed a high-fat diet. Intestinal gluconeogenesis increased after the diversionary procedure, but not after gastric banding. This enhanced intestinal gluconeogenesis is sensed in the portal vein and signaled centrally to reduce food intake and improve hepatic insulin sensitivity. Interestingly, this effect was abolished by portal vein denervation in GLUT-2 knockout mice, suggesting signaling pathways for the effect on food intake and insulin sensitivity. Another animal model has confirmed that a combination of an incretin effect and improved insulin sensitivity may be dually responsible for the improved glucose tolerance [67].
Hypoglycemia
There have now been a number of reports of severe hypoglycemia in patients following RYGB [68–71]. The exact pancreatic pathology has been debated, but it is clear that it is a serious concern for some patients. It has been proposed that this may be an example of “too much of a good thing” [72]. Dietary changes have been used to try and treat the hypoglycemia. One group has suggested that hypoglycemia occurs in patients who have lost gastric restriction and that revisional surgery may help; others have resorted to subtotal pancreatectomy to treat patients with a serious intractable problem [68–71].
It should be recognized that diversionary procedures have a down side, with increased invasiveness, higher perioperative morbidity and mortality, and greater long-term nutritional concerns than less invasive, adjustable, and easily reversible procedures such as the laparoscopic adjustable gastric band [73, 74]. Choice of procedure requires a careful risk-benefit analysis.
American Diabetes Association—Standards of Medical Care—2009
Until very recently there has been little interest in bariatric surgery as a therapy for type-2 diabetes, but in its most recent “Standards of Medical Care in Diabetes - 2009” the American Diabetes Association has indicated a positive, but cautioned, approach [75]. ADA key evidence-based recommendations are:
-
Bariatric surgery should be considered for adults with BMI ≥ 35 kg/m2 and type-2 diabetes, especially if the diabetes is difficult to control with lifestyle and pharmacologic therapy.
-
Although small trials have shown glycemic benefits for bariatric surgery in patients with type-2 diabetes and BMI of 30-35 kg/m2, there is currently insufficient evidence to generally recommend surgery in those with BMI < 35 outside a research protocol.
-
The long-term benefits, cost effectiveness, and risks of bariatric surgery in individuals with type-2 diabetes should be studied in well-designed randomized controlled trials with optimal medical and lifestyle therapy as the comparator.
Conclusion
There is clear evidence that bariatric surgery is associated with a 50–85% diabetes remission rate in the severely obese, that early intervention is more likely to provide remission [76], and that death related to type-2 diabetes and overall mortality is reduced [29, 45, 77].
There is an enormous burden in those currently eligible for surgery (BMI > 35), yet we know few receive surgical therapy [78]. Current guidelines provide information about when bariatric surgery could be considered; however, when will bariatric surgery be a standard of care for the recently diagnosed type-2 diabetic at BMI = 40 when they are not reaching weight loss and key diabetes targets of glycemic control, dyslipidemia, and hypertension within a year of diagnosis? [79]. Let’s move to where healthcare providers have high-quality data and consensus regarding those who will clearly benefit from surgery.
The future of bariatric and metabolic surgery and the discoveries that have been made are very exciting. Novel therapies are bound to emerge, but we need to move forward ethically and scientifically with properly designed and conducted studies in both animal models and humans.
References
Zimmet P, Alberti KG, Shaw J (2001) Global and societal implications of the diabetes epidemic. Nature 414:782–787
National Institutes of Health (1980) Successful diet and exercise therapy is conducted in Vermont for “diabesity”. JAMA 243:519–520
Astrup A, Finer N (2000) Redefining type 2 diabetes: ‘diabesity’ or ‘obesity dependent diabetes mellitus’? Obes Rev 1:57–59
Colditz GA, Willett WC, Rotnitzky A et al (1995) Weight gain as a risk factor for clinical diabetes mellitus in women. Ann Intern Med 122:481–486
Chan JM, Rimm EB, Colditz GA et al (1994) Obesity, fat distribution, and weight gain as risk factors for clinical diabetes in men. Diabetes Care 17:961–969
Leibson CL, Williamson DF, Melton LJ III et al (2001) Temporal trends in BMI among adults with diabetes. Diabetes Care 24:1584–1589
Gregg EW, Cheng YJ, Narayan KM et al (2007) The relative contributions of different levels of overweight and obesity to the increased prevalence of diabetes in the United States: 1976–2004. Prev Med 45:348–352
American Diabetes Association (2008) Economic costs of diabetes in the U.S. in 2007 (2008) Diabetes Care 31:596-615
National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) (2004) National Diabetes Statistics: Complications of diabetes in the United States. Available at http://diabetes.niddk.nih.gov/DM/PUBS/statistics/#complications. Accessed 26 April 2009
Lazar MA (2005) How obesity causes diabetes: not a tall tale. Science 307:373–375
Kershaw EE, Flier JS (2004) Adipose tissue as an endocrine organ. J Clin Endocrinol Metab 89:2548–2556
Boden G (2001) Free fatty acids—the link between obesity and insulin resistance. Endocr Pract 7:44–51
Ruan H, Lodish HF (2004) Regulation of insulin sensitivity by adipose tissue-derived hormones and inflammatory cytokines. Curr Opin Lipidol 15:297–302
Boden G, Shulman GI (2002) Free fatty acids in obesity and type 2 diabetes: defining their role in the development of insulin resistance and beta-cell dysfunction. Eur J Clin Invest 32(Suppl 3):14–23
Lu JY, Huang KC, Chang LC et al (2008) Adiponectin: a biomarker of obesity-induced insulin resistance in adipose tissue and beyond. J Biomed Sci 15:565–576
Rhodes CJ (2005) Type 2 diabetes—a matter of beta-cell life and death? Science 307:380–384
Weir GC, Bonner-Weir S (2004) Five stages of evolving beta-cell dysfunction during progression to diabetes. Diabetes 53(Suppl 3):S16–S21
Wajchenberg BL (2007) Beta-cell failure in diabetes and preservation by clinical treatment. Endocr Rev 28:187–218
Guillausseau PJ, Meas T, Virally M et al (2008) Abnormalities in insulin secretion in type 2 diabetes mellitus. Diabetes Metab 34(Suppl 2):S43–S48
U.K. Prospective Diabetes Study Group (1995) U.K. prospective diabetes study 16. Overview of 6 years’ therapy of type II diabetes: a progressive disease. U.K. Prospective Diabetes Study Group. Diabetes 44:1249–1258
U.K. Prospective Diabetes Study Group (1998) Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). UK Prospective Diabetes Study (UKPDS) Group. Lancet 352:837–853
U.K. Prospective Diabetes Study Group (1999) Quality of life in type 2 diabetic patients is affected by complications but not by intensive policies to improve blood glucose or blood pressure control (UKPDS 37). U.K. Prospective Diabetes Study Group. Diabetes Care 22:1125–1136
U.K. Prospective Diabetes Study Group (1998) Tight blood pressure control and risk of macrovascular and microvascular complications in type 2 diabetes: (UKPDS 38). U.K. Prospective Diabetes Study Group [see comments]. BMJ 317:703–713 Erratum. BMJ 1999 Jan 2; 318(7175):29
Williamson DF, Thompson TJ, Thun M et al (2000) Intentional weight loss and mortality among overweight individuals with diabetes. Diabetes Care 23:1499–1504
Knowler WC, Barrett-Connor E, Fowler SE et al (2002) Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med 346:393–403
Tuomilehto J, Lindstrom J, Eriksson JG et al (2001) Prevention of type 2 diabetes mellitus by changes in lifestyle among subjects with impaired glucose tolerance. N Engl J Med 344:1343–1350
Pi-Sunyer X, Blackburn G, Brancati FL et al (2007) Reduction in weight and cardiovascular disease risk factors in individuals with type 2 diabetes: one-year results of the look AHEAD trial. Diabetes Care 30:1374–1383
Eriksson KF, Lindgarde F (1991) Prevention of type 2 (non-insulin-dependent) diabetes mellitus by diet and physical exercise. The 6-year Malmo feasibility study. Diabetologia 34:891–898
MacDonald KG Jr, Long SD, Swanson MS et al (1997) The gastric bypass operation reduces the progression and mortality of non-insulin-dependent diabetes mellitus. J Gastrointest Surg 1:213–220
Norris SL, Zhang X, Avenell A et al (2004) Long-term effectiveness of lifestyle and behavioral weight loss interventions in adults with type 2 diabetes: a meta-analysis. Am J Med 117:762–774
Khan MA, St. Peter JV, Breen GA et al (2000) Diabetes disease stage predicts weight loss outcomes with long-term appetite suppressants. Obes Res 8:43–48
Zimmet P, Shaw J, Alberti KG (2003) Preventing type 2 diabetes and the dysmetabolic syndrome in the real world: a realistic view. Diabet Med 20:693–702
Wing RR, Marcus MD, Epstein LH et al (1987) Type II diabetic subjects lose less weight than their overweight nondiabetic spouses. Diabetes Care 10:563–566
Pories WJ, Swanson MS, MacDonald KG et al (1995) Who would have thought it? An operation proves to be the most effective therapy for adult-onset diabetes mellitus. Ann Surg 222:339–350; discussion 350–352
Pories WJ, MacDonald KG Jr, Morgan EJ et al (1992) Surgical treatment of obesity and its effect on diabetes: 10-y follow-up. Am J Clin Nutr 55:582S–585S
Pories WJ, Albrecht RJ (2001) Etiology of type II diabetes mellitus: role of the foregut. World J Surg 25:527–531
Buchwald H, Avidor Y, Braunwald E et al (2004) Bariatric surgery: a systematic review and meta-analysis. JAMA 292:1724–1737
Vetter ML, Cardillo S, Rickels MR et al (2009) Narrative review: effect of bariatric surgery on type 2 diabetes mellitus. Ann Intern Med 150:94–103
Higgins JPT, Green S (eds) (2008) Cochrane handbook for systematic reviews of interventions ver. 5.0.1 [updated September 2008]. The Cochrane Collaboration, 2008. Available at www.cochrane-handbook.org. Accessed 4 February 2009
Pontiroli AE, Pizzocri P, Librenti MC et al (2002) Laparoscopic adjustable gastric banding for the treatment of morbid (grade 3) obesity and its metabolic complications: a three-year study. J Clin Endocrinol Metab 87:3555–3561
Sjostrom L, Lindroos AK, Peltonen M et al (2004) Lifestyle, diabetes, and cardiovascular risk factors 10 years after bariatric surgery. N Engl J Med 351:2683–2693
Dixon JB, O’Brien PE, Playfair J et al (2008) Adjustable gastric banding and conventional therapy for type 2 diabetes: a randomized controlled trial. JAMA 299:316–323
Sjostrom CD, Lissner L, Wedel H et al (1999) Reduction in incidence of diabetes, hypertension and lipid disturbances after intentional weight loss induced by bariatric surgery: the SOS Intervention Study. Obes Res 7:477–484
Torgerson JS (2003) Swedish obese subjects—where are we now? Int J Obes 27:19
Sjostrom L, Narbro K, Sjostrom CD et al (2007) Effects of bariatric surgery on mortality in Swedish obese subjects. N Engl J Med 357:741–752
Keating CL, Dixon JB, Moodie ML et al (2009) Cost-efficacy of surgically induced weight loss for the management of type 2 diabetes: randomised controlled trial. Diabetes Care 32:580–584
Keating CL, Dixon JB, Moodie ML et al (2009) Cost-effectiveness of surgically induced weight loss for the management of type 2 diabetes: modelled lifetime analysis. Diabetes Care 32:567–574
Dixon JB, O’Brien P (2002) Health outcomes of severely obese type 2 diabetic subjects 1 year after laparoscopic adjustable gastric banding. Diabetes Care 25:358–363
Dixon JB, Dixon AF, O’Brien PE (2003) Improvements in insulin sensitivity and beta-cell function (HOMA) with weight loss in the severely obese. Diabet Med 20:127–134
Schauer PR, Burguera B, Ikramuddin S et al (2003) Effect of laparoscopic Roux-en Y gastric bypass on type 2 diabetes mellitus. Ann Surg 238:467–485
Clauson P, Linnarsson R, Gottsater A et al (1994) Relationships between diabetes duration, metabolic control and beta-cell function in a representative population of type 2 diabetic patients in Sweden. Diabet Med 11:794–801
Polonsky KS, Gumbiner B, Ostrega D et al (1994) Alterations in immunoreactive proinsulin and insulin clearance induced by weight loss in NIDDM. Diabetes 43:871–877
Gumbiner B, Van Cauter E, Beltz WF et al (1996) Abnormalities of insulin pulsatility and glucose oscillations during meals in obese noninsulin-dependent diabetic patients: effects of weight reduction. J Clin Endocrinol Metab 81:2061–2068
Sugerman HJ, Wolfe LG, Sica DA (2003) Diabetes and hypertension in severe obesity and effects of gastric bypass-induced weight loss. Ann Surg 237:751–756; discussion 757–758
Taylor R (2008) Pathogenesis of type 2 diabetes: tracing the reverse route from cure to cause. Diabetologia 51:1781–1789
Rubino F (2008) Is type 2 diabetes an operable intestinal disease? A provocative yet reasonable hypothesis. Diabetes Care 31(Suppl 2):S290–S296
Cohen RV, Schiavon CA, Pinheiro JS et al (2007) Duodenal-jejunal bypass for the treatment of type 2 diabetes in patients with body mass index of 22–34 kg/m2: a report of 2 cases. Surg Obes Relat Dis 3:195–197
le Roux CW, Aylwin SJ, Batterham RL et al (2006) Gut hormone profiles following bariatric surgery favor an anorectic state, facilitate weight loss, and improve metabolic parameters. Ann Surg 243:108–114
Holdstock C, Zethelius B, Sundbom M et al (2008) Postprandial changes in gut regulatory peptides in gastric bypass patients. Int J Obes (Lond) 32:1640–1646
Laferrere B, Teixeira J, McGinty J et al (2008) Effect of weight loss by gastric bypass surgery versus hypocaloric diet on glucose and incretin levels in patients with type 2 diabetes. J Clin Endocrinol Metab 93:2479–2485
Wang TT, Hu SY, Gao HD et al (2008) Ileal transposition controls diabetes as well as modified duodenal jejunal bypass with better lipid lowering in a nonobese rat model of type II diabetes by increasing GLP-1. Ann Surg 247:968–975
Strader AD, Vahl TP, Jandacek RJ et al (2005) Weight loss through ileal transposition is accompanied by increased ileal hormone secretion and synthesis in rats. Am J Physiol Endocrinol Metab 288:E447–E453
Rubino F, Forgione A, Cummings DE et al (2006) The mechanism of diabetes control after gastrointestinal bypass surgery reveals a role of the proximal small intestine in the pathophysiology of type 2 diabetes. Ann Surg 244:741–749
Gersin KS, Keller JE, Stefanidis D et al (2007) Duodenal-jejunal bypass sleeve: a totally endoscopic device for the treatment of morbid obesity. Surg Innov 14:275–278
Rodriguez-Grunert L, Galvao Neto MP, Alamo M et al (2008) First human experience with endoscopically delivered and retrieved duodenal-jejunal bypass sleeve. Surg Obes Relat Dis 4:55–59
Wickremesekera K, Miller G, Naotunne TD et al (2005) Loss of insulin resistance after Roux-en-Y gastric bypass surgery: a time course study. Obes Surg 15:474–481
Meirelles K, Ahmed T, Culnan DM et al (2009) Mechanisms of glucose homeostasis after Roux-en-Y gastric bypass surgery in the obese, insulin-resistant Zucker rat. Ann Surg 249:277–285
Service GJ, Thompson GB, Service FJ et al (2005) Hyperinsulinemic hypoglycemia with nesidioblastosis after gastric-bypass surgery. N Engl J Med 353:249–254
Z’Graggen K, Guweidhi A, Steffen R et al (2008) Severe recurrent hypoglycemia after gastric bypass surgery. Obes Surg 18:981–988
Bantle JP, Ikramuddin S, Kellogg TA et al (2007) Hyperinsulinemic hypoglycemia developing late after gastric bypass. Obes Surg 17:592–594
Clancy TE, Moore FD Jr, Zinner MJ (2006) Post-gastric bypass hyperinsulinism with nesidioblastosis: subtotal or total pancreatectomy may be needed to prevent recurrent hypoglycemia. J Gastrointest Surg 10:1116–1119
Cummings DE (2005) Gastric bypass and nesidioblastosis—too much of a good thing for islets? N Engl J Med 353:300–302
Chapman A, Kiroff G, Game P et al (2004) Laparoscopic adjustable gastric banding in the treatment of obesity: a systematic review. Surgery 135:326–351
Gasteyger C, Suter M, Gaillard RC et al (2008) Nutritional deficiencies after Roux-en-Y gastric bypass for morbid obesity often cannot be prevented by standard multivitamin supplementation. Am J Clin Nutr 87:1128–1133
American Diabetes Association (2009) Standards of medical care in diabetes—2009. Diabetes Care 32(Suppl 1):S13–S61
Dixon JB, Pories WJ, O’Brien PE et al (2005) Surgery as an effective early intervention for diabesity: why the reluctance? Diabetes Care 28:472–474
Adams TD, Gress RE, Smith SC et al (2007) Long-term mortality after gastric bypass surgery. N Engl J Med 357:753–761
Sturm R (2007) Increases in morbid obesity in the USA: 2000–2005. Public health 121:492–496
Dixon JB (2008) Referral for a bariatric surgical consultation: it is time to set a standard of care. Obes Surg
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Dixon, J.B. Obesity and Diabetes: The Impact of Bariatric Surgery on Type-2 Diabetes. World J Surg 33, 2014–2021 (2009). https://doi.org/10.1007/s00268-009-0062-y
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00268-009-0062-y