Abstract
Background
Minimally invasive parathyroidectomy (MIP) is now widely accepted where a single adenoma can be localized preoperatively. In our unit, MIP is offered once a parathyroid adenoma is localized with a sestamibi (MIBI) scan, with or without a concordant neck ultrasound. The aim of this study was to compare the accuracy of surgeon performed ultrasound (SUS) with radiologist performed ultrasound (RUS) in the localization of a parathyroid adenoma in MIBI-positive primary hyperparathyroidism (PHPT).
Patients and Methods
This is a prospective study of patients undergoing parathyroidectomy for sporadic primary hyperparathyroidism (PHPT) from April 2005 to October 2006 at the University of Sydney Endocrine Surgical Unit. Patients were then divided into those who underwent preoperative RUS or SUS.
Results
Two-hundred eighteen patients formed the study group. One hundred forty-eight (66%) patients had RUS and 87 (39%) had SUS. Overall, RUS correctly localized the parathyroid adenomas in 121 of 148 (82%) patients. Surgeon performed ultrasound correctly localized the abnormal parathyroid adenoma in 72 of 87 (83%) of cases. There was no significant difference in the proportion of patients with single gland disease, double adenomas, or hyperplasia correctly localized by SUS or RUS. Incorrect interpretation of ultrasound imaging was due to cystic degeneration in thyroid nodules, lymph nodes, retro-esophageal location of adenomas and ectopic and small parathyroid glands.
Conclusions
Surgeon performed ultrasound is a useful adjunctive tool to MIBI localization for facilitating MIP and when performed by experienced parathyroid surgeons, it can achieve accuracy rates equivalent to that of a dedicated parathyroid radiologist.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In the cases of primary hyperparathyroidism (PHPT) due to a single adenoma, minimally invasive parathyroidectomy (MIP) is now widely accepted as the procedure of first choice. Accurate preoperative localization, however, is the key to successful MIP. Primary localization techniques include technetium-99m sestamibi scanning (MIBI) and ultrasound (US) of the neck. The combination of MIBI and neck US has a 74–90% sensitivity in localizing a parathyroid adenoma while those of neck US and MIBI are 38–96% and 56–95% respectively [1–3].
First used in the 1970s [4–6], US localization of the parathyroid adenoma has several advantages. It is noninvasive, relatively inexpensive, and does not expose the patient to ionizing radiation. Ultrasound, however, has the disadvantage of being operator-dependent, with sensitivities ranging from 20% to 79% among sonographers, depending on skill, experience, and interest [2, 7]. In our institution, we have a dedicated parathyroid and thyroid radiologist, and the accuracy of radiologist performed neck US (RUS) is 88% [8]. We recognize, however, that not all practices will have a dedicated radiologist interested and skilled in performing neck US to localize parathyroid disease. Surgeon performed ultrasound (SUS) would therefore be a logical option. Notwithstanding the operator-dependency, there are many advantages to surgeons performing US to localize the parathyroid adenoma. Surgeons, familiar with the intricacies of neck anatomy are in a unique position to correlate US findings with operative experience, and are thus able to use SUS as an extension of the physical examination. There is also the convenience factor for the patient, eliminating the need for multiple visits to the surgeon and radiology practices, as well as cost-savings [9]. The sensitivity of SUS in localizing parathyroid adenomas has been reported to be 67%–87% [10–12]. The aim of the present study was to compare the accuracy of SUS with RUS in the localization of parathyroid adenoma in MIBI positive PHPT.
Materials and methods
A prospective study of consecutive patients undergoing surgery for PHPT at the University of Sydney Endocrine Surgical Unit from April 2005 to October 2006 was undertaken. All patients presenting with biochemically confirmed PHPT underwent a MIBI scan. The patients also underwent either a SUS by authors S.S. or M.S. or RUS by author B.B. If the patient had concordant MIBI and US, he or she underwent a MIP. If the MIBI was positive but the US was negative, the initial procedure planned was MIP with a lower threshold for conversion to open exploration. If, however, both investigations were non-localizing, the patient went directly to an open four-gland exploration.
Our unit protocol for management of PHPT is shown in Figure 1. Patients who had renal, familial, or incidental hyperparathyroidism who underwent concomitant thyroidectomy or had a negative preoperative MIBI scan were excluded from this study. Data collected included site of the parathyroid adenoma, procedure type, and surgical complications.
The results of SUS were considered to be correct if the adenoma was localized to the correct side and the patient was cured at 6-month follow-up. A Terason® 2000 portable US machine with a 7.5–12 MHz linear array transducer was used. The patient was placed in the supine position with the neck extended, and the neck was examined as described by Solorzano et al. Briefly, scans were performed from the supraclavicular to submental region bilaterally with transverse and longitudinal views. To assist identification of parathyroid tissue in the thymus or below the sternal notch, the transducer was angled inferiorly above the clavicles [13]. The depth of focus was set at 2 cm from the skin. However, when the MIBI suggested a descended superior parathyroid adenoma—a type C gland on the M.D. Anderson classification [14]—the depth of focus was increased to 3.5 cm. Abnormal parathyroid glands appeared at the appropriate anatomic locations as enlarged, homogeneously hypoechoic structures, either oval or triangular shaped with sharp borders and doppler showing peripheral blood flow [15]. The presence or absence of a lesion and the site of the lesion were recorded. The US was performed preoperatively in the surgeons’ rooms on the initial visit and again on the operating table to facilitate incision placement.
Minimally invasive parathyroidectomy was performed using the lateral focused mini-incision technique as previously described [16]. In brief, under general anesthesia or controlled regional anesthesia, the patient is placed in the supine position with neck extension. For a superior parathyroid adenoma, a 2-cm skin incision is placed 1 cm superior to the neck crease line, lateral to the medial margin of the sternomastoid muscle, whereas a 2-cm skin incision is placed 1 cm inferior to the neck crease line and medial to the medial margin of the sternomastoid muscle for an inferior parathyroid adenoma. The sternomastoid muscle is retracted laterally while the strap muscles are retracted medially. The space medial to the common carotid artery and posterior to the thyroid gland is then dissected from pretracheal fascia to prevertebral fascia [16]. If there was no evidence of an abnormal gland on MIP, the procedure was then converted to an open exploration. Serum intact parathyroid hormone levels were measured for each patient prior to incision and 30 min after parathyroid excision. These results, however, were used purely to aid with informed discharge of the patient and the results were not acted upon intraoperatively [17]. Cure was defined as normocalcemia at 6 months postoperation.
Statistical analysis was performed using the Stata 9 statistical software package (College Station, Texas, USA). Categorical data were analyzed with Fisher’s exact test. Continuous variables were compared with the Student’s t-test. Statistical significance was defined as a two-sided p value < 0.05.
Results
In the period April 2005 to October 2006, 448 patients underwent parathyroid surgery within our unit. After exclusion of patients as outlined previously, 218 patients with a positive MIBI were included in the study. The demographic data of the 218 patients in the study group are given in Table 1. The main indications for parathyroidectomy in the study group are listed in Table 2. One-hundred ninety-seven (90%) patients underwent MIP, 12 (6%) were converted from MIP to open parathyroidectomy, and 9 (4%) had an open procedure. Of the 9 patients who had open exploration, 2 patients had had total thyroidectomy previously, 5 patients had MIBI which showed multiple foci, and the remaining 2 patients had multiple abnormal glands seen on US. Surgeon performed ultrasound was performed in 87 patients, and RUS was performed in 148 (17 patients had both SUS and RUS).
Overall, RUS correctly localized the parathyroid adenomas in 121 of 148 (82%) patients, and SUS correctly localized the abnormal parathyroid adenoma in 72 of 87 (83%) of cases. There was no difference in accuracy between the two surgeons, with one at 83.1% and the other at 82.1%. Thirty eight of the 218 (17%) patients had an incorrect US (Table 3). The mean and median weights of the parathyroid glands that were missed are 603.4 mg and 296 mg, respectively, compared to 1,056.8 mg and 570 mg, respectively, for parathyroid glands that were correctly identified. Table 4 correlates pathological findings and mean and median weights of the parathyroid gland with accuracy of the US. When the parathyroid glands were grouped into those < 500 mg and ≥ 500 mg in weight, ultrasound was not found to be more sensitive than sestamibi in identifying the smaller glands. For glands < 500 mg, the accuracy of ultrasound and sestamibi was 75.2% and 83.2%, respectively, compared to 86.7% and 88.5%, respectively, for glands ≥ 500 mg.
One-hundred ninety-five patients (89%) in the study group were ultimately shown to have single gland disease and were cured. SUS was performed in 78 patients and RUS in 123 patients. In this group the sensitivities of RUS and SUS in correctly localizing the abnormal gland were equivalent, being 89.3% and 89.9%, respectively. The test characteristics of RUS and SUS in single gland disease are shown in Table 5. There was no significant difference in the proportion of patients with single gland disease correctly localized by SUS or RUS (p = 0.89).
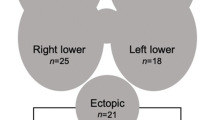
Nine patients (4%) in the study group were shown to have double adenomas.
In patients with double parathyroid adenomas proven after successful surgery, the sensitivity of RUS was 37.5%, and SUS was unable to detect any cases of double adenomas preoperatively. The test characteristics of RUS and SUS in the setting of double adenoma are shown in Table 6. There was no significant difference in the proportion of patients with double adenoma correctly localized by SUS or RUS (p = 0.3).
Fourteen patients (6%) in the study group had hyperplasia proven at final histopathological review. The sensitivity of RUS and SUS for correctly identifying hyperplastic glands was 11.1% and 16.7%, respectively. The test characteristics of RUS and SUS in the setting of hyperplasia are shown in Table 7. There was no significant difference in the proportion of patients with hyperplasia correctly localized by SUS or RUS (p = 0.76).
Of those with multiglandular disease, the size of the glands ranged from 11 to 1,670 mg. Five of the 23 (21.7%) patients with multiglandular disease had glands under 200 mg, which would be difficult to visualize on ultrasound.
Because of our unit protocol, sestamibi scan was always performed prior to ultrasound. Eighty percent (176/220) of patients had concordant US and sestamibi studies. Sestamibi and ultrasound were discordant in 7.7% (17/220) of cases. However, these differences usually occurred when the adenoma was not seen on ultrasound. Of the discordant studies, sestamibi was correct 76.5% of the time, and ultrasound was correct in only 23.5% of cases. Sestamibi correctly localized the side of the adenoma in 85.9% (189/220), and ultrasound overall, RUS, and SUS correctly localized the side of the adenoma in 81.8% (180/220), 81.8% (121/148), and 82.8% (72/87) of cases, respectively. Localization of the adenoma to each quadrant of the neck was not assessed.
Parathyroid surgery was successful in 214 of 218 patients, giving a cure rate of 98%, defined as a normal serum calcium level at 6 months postoperation. Our complications include permanent hypoparathyroidism in 1 (0.5%) patient, temporary RLN in 3 (1.4%) patients, and permanent recurrent laryngeal nerve palsy in 1 (0.5%). Wound hematoma developed in 3 (1.4%) patients, one of whom required surgical drainage, and wound infection occurred in 2 (0.9%) patients.
Discussion
It is our unit protocol that all patients with biochemically proven primary hyperparathyroidism undergo a MIBI scan. Only patients with a positive MIBI scan go on to have an US prior to undergoing MIP. We do not advocate patients’ undergoing MIP with US as the only preoperative localization study because work done in our unit has demonstrated that the size of a parathyroid gland does not correlate with function[18]. Using US localization alone, it is therefore possible to remove an enlarged nonfunctioning parathyroid gland and miss the smaller pathological gland.
In the present study, we found that the sensitivity of SUS in localizing single gland disease was 89.9%. This sensitivity was as good as that of RUS and compares favorably with reports of SUS for localizing parathyroid adenomas in the literature [10–12]. Van Husen et al. studied 53 patients who underwent parathyroid surgery with SUS. Twenty-six of these patients also had RUS. They found that SUS was much better than RUS at lateralizing the adenoma to the correct side, with sensitivities of 82% and 42%, respectively [10]. Among 226 patients, Solorzano et al. reported a sensitivity of 76% for SUS in identifying an abnormal parathyroid gland [11], and Steward et al. noted a sensitivity of 91% in 97 patients [12]. The high sensitivity of SUS is thought to be associated with the surgeon’s familiarity with neck anatomy. Because SUS can be performed with sensitivity comparable to RUS, it is helpful for units without specialized radiologists.
SUS is advantageous because it allows the surgeon to directly visualize the parathyroid adenoma in the preoperative setting and correlate it with the thyroid and other landmarks in the neck. For surgeons in their initial experience with MIP, it facilitates placement of the neck incision, thereby increasing the ease of the operation and allowing a small 2-cm incision to be made [19]. SUS also has advantages for the patient, in that it is more convenient, making it unnecessary to attend multiple appointments, as well as more economical. Surgeons also readily learn to localize the parathyroid adenoma with neck US as this is familiar territory. Milas et al. surveyed residents and fellows and found that 20 US were required to gain confidence with parathyroid adenoma localization [9].
Performing an US before the patient undergoes MIP is beneficial as it enables the identification and evaluation of any concomitant thyroid disease prior to surgery. One of the concerns of surgeons who continue to argue for open parathyroidectomy is that incidental thyroid malignancy is missed. We know that there is an increased incidence of thyroid cancer associated with primary hyperparathyroidism [20]. SUS allows for evaluation of coincidentally detected thyroid nodules with fine needle aspiration (FNA) prior to surgery and in those in whom an atypical FNA is returned, we would offer a combination open hemithyroidectomy and parathyroidectomy.
The rate of multiglandular disease in this study was 11% (23/218), which lies within the range reported in the literature [1, 2, 10, 21–25]. As previously reported, preoperative localization is less accurate in multiglandular disease when compared to single gland disease [8]. In contrast to localizing single gland disease, in this study, SUS did not localize any double adenomas correctly, and it was negative in 1 of 6 patients with hyperplasia. Three of 8 RUS which predicted double adenomas were correct, and 1 of 9 patients with hyperplasia had a negative RUS. Of the 9 patients with double adenomas, 7 had RUS, 1 had SUS, and 1 had both. Three of the 8 RUS and none of the SUS were correct. Of the 14 patients with hyperplasia, 8 had RUS, 5 had SUS, and 1 had both. One RUS and 1 SUS reported a negative study. Therefore, in this study, RUS was correct in 24% of cases of multiglandular disease, and SUS was correct in 13%. This is in contrast to Solorzano et al., who reported a 22% rate of identification of multiglandular disease by SUS [11]. Overall, although RUS was better at localizing double adenomas and hyperplasia than SUS, we found that, in our hands, both SUS and RUS were very poor at localizing these 2 groups. Although there was no statistical difference between RUS and SUS in localizing multiglandular disease, there was a trend toward better localization by RUS compared to SUS.
Incorrect interpretation of US imaging was due to cystic degeneration in thyroid nodules, lymph nodes, ectopic locations of the adenomas such as intrathyroidal, retro-esophageal or within the carotid sheath, and small parathyroid glands. We now know that a positive MIBI scan demonstrating a descended superior adenoma on an oblique view—M.D. Andersen C gland—will not be seen on US. To us, a negative US would therefore confirm an M.D. Andersen C gland’s retroesophageal location. Consequently, during the operation, we would dissect straight down to the prevertebral fascia from a lateral approach to remove this adenoma.
This study has shown that SUS is as good as RUS in localizing single gland disease in patients with sporadic primary hyperparathyroidism. We acknowledge, however, that there are limitations to this study. First, the ultrasonographers, both radiologist and surgeons, were not blinded to the results of the sestamibi scan. Second, US is operator dependent and results at one institution may vary from another, as will the interobserver reliability. Finally, all three ultrasonographers did not perform US on the same patient to determine if there were any differences between surgeons and radiologist in terms of sensitivity. Nevertheless, we feel that the results of this study are valid as they are concordant with those from other studies [10–12].
Conclusions
SUS is a useful adjunctive tool to sestamibi localization for facilitating MIP. Concomitant thyroid pathology can be identified and investigated appropriately. Anatomical localization facilitates skin incision placement by the lateral mini-incision technique. SUS, when performed by experienced parathyroid surgeons, can achieve accuracy rates equivalent to that of a dedicated parathyroid radiologist.
References
Lumachi F, Ermani M, Basso S et al. (2001) Localization of parathyroid tumours in the minimally invasive era: which technique should be chosen? Population-based analysis of 253 patients undergoing parathyroidectomy and factors affecting parathyroid gland detection. Endocr Relat Cancer 8:63–69
Purcell GP, Dirbas FM, Jeffrey RB et al. (1999) Parathyroid localization with high-resolution ultrasound and technetium Tc 99 m sestamibi. Arch Surg 134:824–828; discussion 828–830
Geatti O, Shapiro B, Orsolon PG et al. (1994) Localization of parathyroid enlargement: experience with technetium-99 m methoxyisobutylisonitrile and thallium-201 scintigraphy, ultrasonography and computed tomography. Eur J Nucl Med 21:17–22
Bambach CP, Riley JW, Picker RH et al. (1978) Preoperative parathyroid identification by ultrasonic scan. Med J Aust 2:227–229
Edis AJ, Evans TC Jr. (1979) High-resolution, real-time ultrasonography in the preoperative location of parathyroid tumors. Pilot study. N Engl J Med 301:532–534
Barraclough BH, Reeve TS, Duffy PJ et al. (1981) The localization of parathyroid tissue by ultrasound scanning prior to surgery in patients with hyperparathyroidism. World J Surg 5:91–95
Lloyd MN, Lees WR, Milroy EJ (1990) Pre-operative localisation in primary hyperparathyroidism. Clin Radiol 41:239–243
Yeh MW, Barraclough BM, Sidhu SB et al. (2006) Two hundred consecutive parathyroid ultrasound studies by a single clinician: the impact of experience. Endocr Pract 12:257–263
Milas M, Stephen A, Berber E et al. (2004) Ultrasonography for the endocrine surgeon: a valuable clinical tool that enhances diagnostic and therapeutic outcomes. Surgery 138:1193–1200; discussion 1200–1191
Van Husen R, Kim LT (2004) Accuracy of surgeon-performed ultrasound in parathyroid localization. World J Surg 28:1122–1126
Solorzano CC, Carneiro-Pla DM, Irvin GL 3rd (2006) Surgeon-performed ultrasonography as the initial and only localizing study in sporadic primary hyperparathyroidism. J Am Coll Surg 202:18–24
Steward DL, Danielson GP, Afman CE et al. (2006) Parathyroid adenoma localization: surgeon-performed ultrasound versus sestamibi. Laryngoscope 116:1380–1384
Solorzano CC, Lee TM, Ramirez MC, et al. (2005) Surgeon-performed ultrasound improves localization of abnormal parathyroid glands. Am Surg 71:557–562; discussion 562–553
Rodgers SE, Hunter GJ, Hamberg LM et al. (2006) Improved preoperative planning for directed parathyroidectomy with 4-dimensional computed tomography. Surgery 140:932–940; discussion 940–931
Barraclough BM, Barraclough BH (2000) Ultrasound of the thyroid and parathyroid glands. World J Surg 24:158–165
Agarwal G, Barraclough BH, Reeve TS et al. (2002) Minimally invasive parathyroidectomy using the “focused” lateral approach. II. Surgical technique. Aust N Z J Surg 72:147–151
Stalberg P, Sidhu S, Sywak M et al. (2006) Intraoperative parathyroid hormone measurement during minimally invasive parathyroidectomy: does it “value-add” to decision-making? J Am Coll Surg 203:1–6
Mun HC, Conigrave A, Wilkinson M et al. (2005) Surgery for hyperparathyroidism: does morphology or function matter most? Surgery 138:1111–1120; discussion 1120
Soon PS, Yeh MW, Sywak MS et al. (2007) Minimally invasive parathyroidectomy using the lateral focused miniincision approach: is there a learning curve for surgeons experienced in the open procedure? J Am Coll Surg 204:91–95
Sidhu S, Campbell P (2000) Thyroid pathology associated with primary hyperparathyroidism. Aust N Z J Surg 70:285–287
Molinari AS, Irvin GL 3rd, Deriso GT et al. (1996) Incidence of multiglandular disease in primary hyperparathyroidism determined by parathyroid hormone secretion. Surgery 120:934–936; discussion 936–937
Attie JN, Bock G, Auguste LJ (2001) Multiple parathyroid adenomas: report of thirty-three cases. Surgery 108:1014–1019; discussion 1019–1020
Gauger PG, Agarwal G, England BG et al. (2001) Intraoperative parathyroid hormone monitoring fails to detect double parathyroid adenomas: a 2-institution experience. Surgery 130:1005–1010
Haciyanli M, Lal G, Morita E et al. (2003) Accuracy of preoperative localization studies and intraoperative parathyroid hormone assay in patients with primary hyperparathyroidism and double adenoma. J Am Coll Surg 197:739–746
Berczi C, Mezosi E, Galuska L et al. (2002) Technetium-99m-sestamibi/pertechnetate subtraction scintigraphy vs ultrasonography for preoperative localization in primary hyperparathyroidism. Eur Radiol 12:605–609
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Soon, P.S.H., Delbridge, L.W., Sywak, M.S. et al. Surgeon Performed Ultrasound Facilitates Minimally Invasive Parathyroidectomy by the Focused Lateral Mini-incision Approach. World J Surg 32, 766–771 (2008). https://doi.org/10.1007/s00268-007-9436-1
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00268-007-9436-1