Abstract
Background
Since Misumi et al. and Siewert proposed a new classification for carcinoma of the gastroesophageal junction (GEJ), few surgical studies using these criteria have been reported from Eastern countries. Siewert type II adenocarcinomas are managed using general rules for either gastric or esophageal cancer. We set out to determine whether type II adenocarcinoma is a distinct clinical entity requiring a more specific treatment plan.
Methods
Among 125 Japanese patients who underwent resection of adenocarcinoma of the GEJ (type I, 2; type II, 44; type III, 79), 101 who underwent R0 resections (type II, 40; type III, 61) were analyzed to evaluate surgical results and compare clinicopathologic factors.
Results
Barrett’s epithelium was recognized in two patients with type II adenocarcinoma. Type II differed significantly from type III in higher prevalence of Borrmann macroscopic type 2, more frequent lymph node metastasis (58% vs. 34%), higher metastatic rate to lower mediastinal lymph nodes (13%), increased risk of hepatic recurrence, and lower 5-year survival after R0 resection (67.4% vs. 87.1%).
Conclusions
Clinicopathologic differences were evident between type II and III adenocarcinomas. Siewert type II adenocarcinoma differs sufficiently to be considered a clinical entity distinct and independent from type III.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
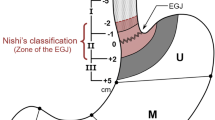
“Carcinoma of the cardia” is an ambiguous term, because the cardia is the border between the esophagus and the stomach. Consequently, the literature contains many discrepancies regarding classification of tumors in this location as well as surgical results.1–4 In 1978, Nishi et al. proposed a definition for carcinoma of the cardia as a tumor with a center within 2 cm of the anatomic gastroesophageal junction (GEJ) irrespective of histology.5 While this criterion has been used widely in Japan,6–8 it has not been accepted worldwide. Based on study of distribution of the cardiac glands, Misumi et al. defined carcinoma of the cardia in 1989 as a tumor with a center between 1 cm proximal and 2 cm distal to the anatomic GEJ.9 In 1996, Siewert et al. suggested a similar definition for carcinoma of the cardia, representing part of a more broadly defined group – carcinoma of the GEJ—denoting a tumor having a center within 5 cm of the anatomic GEJ.10 This classification was approved at a consensus conference during the Second International Gastric Cancer Congress held in Munich in April 1997. Subsequently, an increasing number of studies have used this classification.
In contrast to a decline in incidence of gastric carcinoma, Western countries have experienced a continuing rise in incidence of adenocarcinoma of the GEJ.11,12 However, no such increase has been reported in Japan,13 possibly reflecting etiologic and histologic differences in carcinoma of the GEJ between East and West. Despite much discussion concerning differences in gastric or esophageal carcinoma between these geographic regions, few of these disputes have extended to carcinoma of the cardia because of lack of an internationally used definition. In particular, few Eastern studies have used the Siewert classification.14–16
Because of its location directly at the gastroesophageal junction, type II adenocarcinoma may arise from Barrett’s epithelium (intestinal metaplasia of the esophagus), cardiac epithelium, or fundic epithelium. An important disparity exists among surgeons concerning type II adenocarcinomas, whereby some authors manage these lesions by general rules for gastric cancer14,17–20; while others use rules for esophageal cancer.21,22 For consistent management one needs to determine whether type II adenocarcinoma is a distinct clinical entity.
The aim of the present study was twofold: to evaluate surgical results in 101 adenocarcinomas of the GEJ, and to clarify clinicopathologic differences between type II and III adenocarcinomas.
PATIENTS AND METHODS
Patients
Carcinoma of the GEJ was defined as a tumor with a center between 5 cm oral and 5 cm aboral of the anatomic GEJ of the resected specimen, according to the Siewert classification. A tumor with a center more than 1 cm above the anatomic GEJ was classified as a type I cancer (carcinoma of the distal esophagus), whereas a lesion whose center was between 1 cm oral and 2 cm aboral of the anatomic GEJ was a type II cancer (true carcinoma of the cardia). Finally, a tumor with a center more than 2 cm below the anatomic GEJ was a type III cancer (subcardial gastric carcinoma).10 Medical records were reviewed for all patients with carcinoma of the esophagus or stomach who underwent resection between January 1987 and December 2003 at two institutions (First Department of Surgery, Nagoya University Hospital; and Department of Surgery, Japanese Red Cross Nagoya First Hospital). During this period, operative procedures were selected by two chief surgeons (Y.N. and T.H.), who followed the same surgical strategy for carcinoma of the GEJ and made no major change in its management during the study period specified. The diagnostic work-up in all patients included endoscopy with biopsy, barium swallow, ultrasonography of the abdomen, and computed tomography of the chest and abdomen.
Record review identified 169 patients with a carcinoma of the GEJ (type I, 31; type II, 56; type III, 82). Among these tumors, 125 were adenocarcinomas of the GEJ (type I, 2; type II, 44; type III, 79). No patient underwent preoperative chemotherapy or radiotherapy. Twenty-two patients underwent R1 or R2 resection, and103 patients (type I, 2; type II, 40; type III, 61) underwent R0 resection. Because the aim of the study was to compare surgical and clinicopathologic differences between Siewert types, the two patients with type I adenocarcinomas – both associated with Barrett’s epithelium – were excluded from the study. Accordingly, 101 patients with type II or III adenocarcinoma of the GEJ who underwent R0 resection were included.
Macroscopic and Histologic Evaluation
Gross pathologic findings of carcinomas of the GEJ were classified in accordance with guidelines established by the Japanese Gastric Cancer Association.23 All resected specimens were examined with respect to histologic type and grade, associated Barrett’s epithelium, and pathologic tumor status (including mucosal T1a and submucosal T1b tumors), as well as pathologic nodal status according to the recommendations of the International Union Against Cancer.24 The TNM classification of stomach cancer was used,24,25 in which “M1lym” ordinarily is defined as distant lymph node involvement including retropancreatic, paraaortic, portal, retroperitoneal, or mesenteric lymph nodes. However, considering discrepancies in staging between esophageal and gastric cancers with regard to metastasis to lower mediastinal lymph nodes (such as lower thoracic paraesophageal and supradiaphragmatic nodes), involvement of these nodes was classified as a regional rather than distant metastasis in this study.
Surgical Procedures
After preoperative evaluation of the linear extent of esophageal invasion, depth of invasion, curability, and general condition of the patient, one of the following three approaches was selected: a right thoracotomy with laparotomy, a left thoracoabdominal approach,26 or an abdominal approach. If proximal tumor extension exceeded 5 cm beyond the GEJ, subtotal esophagectomy was performed. If proximal tumor extension was 2 –5 cm beyond the GEJ and tumor invasion was deeper than submucosal, the left thoracoabdominal approach was chosen to obtain a wide surgical field for lower mediastinal lymph node dissection. Under other circumstances, or if patient condition did not allow thoracotomy, an abdominal approach including lower mediastinal dissection through the esophageal hiatus was used. The esophagus was transected with a macroscopically clear margin of at least 2 cm. The type of gastrectomy performed was determined by the distal extent of the tumor and depth of invasion. If cancer invasion was confined to the upper part of the stomach and the mucosal layer, proximal gastrectomy was performed. Otherwise, total gastrectomy with D2 lymph node dissection was performed as defined by the Japanese rules for investigation and treatment of gastric cancer;23 in that instance, alimentary tract continuity was re-established with a Roux-en-Y esophagojejunostomy. If tumor extended proximally beyond the GEJ, dissection of lower mediastinal lymph nodes was performed even in the abdominal approach. If cancer invasion was beyond the submucosal layer, complete lymph node clearance in the splenic hilum and along the splenic artery was achieved by distal pancreatosplenectomy (until 1999, when this procedure was abandoned) or splenectomy.
Statistical Analysis
Data are expressed as frequencies or means (± standard deviation). Percentages were compared by the chi-squared test and means by Student’s t-test. Follow-up information through May 2005 was compiled for all survivors by correspondence with the patient, patient’s family, or physician. Survival data were analyzed with respect to Siewert category and various prognostic factors. The Kaplan-Meier method was used to estimate survival curves, and the generalized Wilcoxon method was used to evaluate differences between these curves.27,28 Survival rate calculations included patients who died of operative complications or deaths from any cause during follow-up.
RESULTS
Reasons for R1 or R2 resection in 22 of 123 patients who underwent resection for types II and III adenocarcinoma of the GEJ are presented in Table 1. The rate of R0 resection in type II (91%) was higher than that in type III (77%; P = 0.056). The leading cause for R1 or R2 resection in type III was peritoneal dissemination (15%). The oral margin was microscopically cancer-positive in 2 of 123 patients (1.6%). Mean ages of patients with type II or III tumors who underwent R0 resection were 62.9 (± 11.7) and 61.9 (± 11.4) years, respectively (P = 0.684). Male: female ratios were 2.6:1 and 3.1:1, respectively (P = 0.744).
Surgical Procedures
Procedure selection for the 101 patients who underwent R0 resection is summarized in Table 2. A left thoracoabdominal approach was indicated more frequently for type II (53%) than for type III (10%) disease. Total gastrectomy was performed for at least 90% of cases in both types of adenocarcinoma (90% in type II, 93% in type III). One patient with type III adenocarcinoma died within 30 days after surgery from acute enterocolitis caused by methicillin-resistant Staphylococcus aureus. The operative mortality rate was 1.0%, with no in-hospital deaths except for this patient.
Macroscopic Characteristics
Gross pathologic type differed significantly between the two regional types of adenocarcinoma (P = 0.019); Borrmann type 2 was more prevalent in type II (55%) than in type III (18%). Adenocarcinoma. Overall tumor extent did not differ between the two locations, but longitudinal extent of esophageal invasion was greater in type II (15 ± 13 mm) than in type III (3 ± 7 mm) disease (Table 3).
Histologic Characteristics
Barrett’s epithelium was present in two patients with type II adenocarcinoma. A difference in histologic grade fell slightly short of statistical significance (P = 0.054): well-differentiated adenocarcinoma was more frequent in type II (23%) patients, whereas poorly differentiated adenocarcinoma was more frequent in type III (49%) patients. Although no significant difference in distribution of pT category was apparent between types II and III, frequency of lymph node metastasis in type II (58%) was significantly higher than in type III (34%; P = 0.022). Analysis of frequency of lymph node metastasis by type within each pT category showed significantly higher frequency in pT2 type II tumors (74%) than in pT2 type III (26%; P = 0.006) (Fig. 1). Patients with type II disease presented at somewhat higher stages than those with type III, but the difference was not significant (Table 3).
Distribution of Lymph Node Metastases
Frequencies of metastasis to each lymph node location (positive cases / R0 resections) are summarized in Figure 2. In this study the lesser curvature, short gastric artery, and left gastroepiploic artery lymph nodes were classified as proximal gastric lymph nodes. The most frequently involved locations for both type II and III adenocarcinomas were paracardial and proximal gastric lymph nodes. Metastasis to the lower mediastinal lymph nodes was significantly more frequent in type II (13%) patients than in type III (2%) patients ( P = 0.024).
Metastatic Sites in First Relapse after R0 Resection
Relapse after 101 R0 resections was confirmed in 12 and 8 patients in the type II and III groups, respectively. Metastatic sites representing the first relapse are presented in Figure 3. The most frequent site of first recurrence in type II disease was the liver (6/12; 50%), whereas in type III adenocarcinoma , the most frequent site was the peritoneum (4/8; 50%). Hepatic recurrence was significantly more frequent in type II than in type III disease (P = 0.015). No anastomotic recurrence in the esophageal wall was observed.
Survival
The median duration of follow-up for survivors was 4.7 years. The 5-year survival for all 123 patients who underwent resections for type II and III adenocarcinoma was 66.9%, and that for 101 patients who underwent R0 resections was 78.4%. Cumulative survival curves for the 123 patients who underwent R0 to R2 resection for types II and III disease are presented in Figure 4; survival did not differ between type II and III groups (P = 0.739); however, overall 5-year survival for patients who underwent R0 resection of a type II adenocarcinoma (67.4%) was significantly lower than that following R0 resections for type III disease (87.1%, P = 0.042; Fig. 5).
DISCUSSION
In this study surgical outcomes and clinicopathologic factors were compared between Siewert type II and III adenocarcinomas of the GEJ. No significant difference was seen between types II and III for distributions of age, gender, tumor length, or pT, whereas significant differences were evident for gross pathologic type, frequency and distribution of lymph node metastasis, recurrence site, and overall survival after R0 resection.
A continuing rise in incidence of adenocarcinoma of the GEJ has been noted in Western countries.11,12 Siewert et al. reported frequencies of associated Barrett’s epithelium in type I and II adenocarcinoma of 77.9% and 9.8%, respectively.17 Nakamura et al. accumulated 1263 cases of type I and II adenocarcinoma from 98 Japanese institutions, reporting respective frequencies of associated Barrett’s epithelium of 33% and 4.7% in type I and II adenocarcinomas.15 In our series, prevalence of associated Barrett’s epithelium was 4.5% (2/44) in type II adenocarcinomas. No increase in incidence of adenocarcinoma of the GEJ has been detected in Japan,13 whereas numbers of type I adenocarcinomas and prevalence of associated Barrett’s epithelium in types I and II adenocarcinoma are both lower in Japan than in the West. Okabayashi et al. reported that occurrence of early carcinoma of the cardia (defined according to the Nishi classification) had no relationship to obesity, smoking, ethanol consumption, Barrett’s esophagus, or gastroesophageal reflux disease in Japan.8 Thus, etiologic differences between the West and Japan may exist for carcinoma of the GEJ.
Our study disclosed a significantly higher frequency of lymph node metastasis in type II adenocarcinoma than in type III disease, especially involving the pT2 subgroup. These results were compatible with those of other studies.6,7 In addition, our study showed a difference in the distribution of lymph node metastases between type II and III adenocarcinoma. Although metastasis to paracardial and proximal gastric regions was common in both types II and III, a considerable metastatic rate to lower mediastinal nodes (13%) was observed in type II adenocarcinoma. The metastatic rate to the lower mediastinal lymph nodes in type II adenocarcinoma has been reported as 5%–15.6%.15–17,29 By lymphography, Aikou et al. showed lymphatic pathways extending from the gastric cardia to the mediastinal lymph nodes.30 Our results were consistent with their lymphographic findings. Accordingly, dissection of the lower mediastinal lymph nodes is important for adequate removal of type II cancers.
Our study detected significant differences in relapse between type II and III adenocarcinoma, which is also in agreement with other studies.7,31 Although increased risk of hepatic recurrence in type II disease may reflect predominance of well-differentiated adenocarcinoma and high frequency of lymph node metastasis, it is clinically important. Nonetheless, as presented in Figures 4 and 5, survival after Ro to R2 resection between types II and III adenocarcinoma did not differ, but survival after R0 resection for type II disease was significantly lower than for type III. Hepatic recurrence was a main cause of death for patients in the type II group. Certain adjuvant therapies accompanying surgery have been reported to be effective in decreasing hepatic metastasis from colorectal cancer.32 If further investigations could identify a high-risk group for hepatic recurrence in carcinoma of the GEJ, individualized prophylactic therapy might be indicated to reduce this risk in selected patients.
Type II tumors are staged in the manner of gastric cancer by some authors,14,17–20 but more like esophageal tumors by others.21,22 The celiac axis node is considered a distant metastasis according to the TNM staging system for esophageal cancer, but it represents regional metastasis for gastric cancer. Similarly, metastasis to the lower mediastinal nodes is considered to extend beyond regional for gastric cancer, but is classified regional for esophageal cancer. Definition of “distant” nodal metastasis therefore should be considered in assessing type II adenocarcinomas. The metastatic rate to the celiac axis nodes in type II adenocarcinoma was reported to be 7.0% –12%.15,17,29 In our study, this rate was 8.0%, and median survival time for these patients was 1.6 years. The metastatic rate to lower mediastinal lymph nodes was 13%, and the median survival time for these patients was 2.0 years. These data suggest that while metastasis to these nodes is not rare, outcome for patients with type II adenocarcinomas involving these two node groups is not as poor as one might expect. Therefore, we believe that both the celiac axis and the lower mediastinal lymph nodes should be classified as regional nodes with respect to type II adenocarcinoma.
Whether a left thoracoabdominal approach offers a greater survival benefit than a transabdominal approach remains an unsettled issue, despite much debate. However, the answer may depend in large part on the precise location of the carcinoma, the frequency of lymph node metastasis to the lower mediastinum, likely postoperative morbidity, prognostic advantage of specific procedures in specific situations, and extent of tumor-free proximal margins. Some surgeons have postulated that a long, macroscopically clear proximal margin is required to ensure a microscopically negative margin for resection of a GEJ carcinoma.9,33–36 However, other studies and our own results do not support this view in type II adenocarcinoma.14,17 Lack of need for a particularly long proximal margin may be specific to type II adenocarcinomas, which commonly show Borrmann type 2 macroscopic morphology and well-differentiated adenocarcinoma by histology.8,14,16,20,37 Our finding that a microscopically positive proximal margin was very rare (1.6%:2/123) appears to validate procedure selection criteria at our institutions.
In conclusion, this study in Japanese patients disclosed considerable differences between type II and III adenocarcinomas of the GEJ. Discrimination between type II and III has clinical importance because of significant differences in frequency and distribution of lymph node metastasis, recurrence sites, and overall survival. Type II adenocarcinoma can be categorized as a distinct clinical entity independent of type III adenocarcinoma. Additional work is needed to further define the staging system for carcinoma of the cardia, which should greatly facilitate international comparisons of etiologic, biologic, and therapeutic factors.
References
Clark GW, Peters JH, Ireland AP, et al. Nodal metastasis and sites of recurrence after en bloc esophagectomy for adenocarcinoma. Ann Thorac Surg 1994;58:646–653
Wright CD, Mathisen DJ, Wain JC, et al. Evolution of treatment strategies for adenocarcinoma of the esophagus and gastroesophageal junction. Ann Thorac Surg 1994;58:1574–1578
Millikan KW, Silverstein J, Hart V, et al. A 15-year review of esophagectomy for carcinoma of the esophagus and cardia. Arch Surg 1995;130:617–624
Tachimori Y, Kato H, Watanabe H, et al. Difference between carcinoma of the lower esophagus and the cardia. World J Surg 1996;20:507–510
Nishi M, Nomura H, Kajisa T, et al. Surgical problem of carcinoma in the esophagogastric junction (in Japanese with English abstract). Stomach and Intestine 1978;13:1497–1507
Nakane Y, Okamura S, Boku T, et al. Prognostic differences of adenocarcinoma arising from the cardia and the upper third of the stomach. Am Surg 1993;59:423–429
Ohno S, Tomisaki S, Oiwa H, et al. Clinicopathologic characteristics and outcome of adenocarcinoma of the human gastric cardia in comparison with carcinoma of other regions of the stomach. J Am Coll Surg 1995;180:577–582
Okabayashi T, Gotoda T, Kondo H, et al. Early carcinoma of the gastric cardia in Japan: is it different from that in the West? Cancer 2000;89:2555–559
Misumi A, Murakami A, Harada K, et al. Definition of carcinoma of the gastric cardia. Langenbecks Arch Chir 1989;374:221–226
Siewert JR, Stein HJ. Carcinoma of the cardia: carcinoma of the gastroesophageal junction―classification, pathology and extent of resection. Dis Esophagus 1996;9:173–182
Powell J, McConkey CC. Increasing incidence of adenocarcinoma of the gastric cardia and adjacent sites. Br J Cancer 1990;62:440–443
Devesa SS, Blot WJ, Fraumeni JF Jr. Changing patterns in the incidence of esophageal and gastric carcinoma in the United States. Cancer 1998;83:2049–2053
Ozawa S, Ando N, Kitagawa Y, et al. Does incidence of carcinoma of the esophagogastric junction increase (in Japanese with English abstract)? Nippon Geka Gakkai Zasshi 1998;99:542–546
Kodera Y, Yamamura Y, Shimizu Y, et al. Adenocarcinoma of the gastroesophageal junction in Japan: relevance of Siewert’s classification applied to 177 cases resected at a single institution. J Am Coll Surg 1999;189:594–601
Nakamura T, Ide H, Eguchi R, et al. Adenocarcinoma of the esophagogastric junction: a summary of responses to a questionnaire on adenocarcinoma of the esophagus and the esophagogastric junction in Japan. Dis Esophagus 2002;15:219–225
Ichikura T, Ogawa T, Kawabata T, et al. Is adenocarcinoma of the gastric cardia a distinct entity independent of subcardial carcinoma? World J Surg 2003;27:334–338
Siewert JR, Feith M, Werner M, et al. Adenocarcinoma of the esophagogastric junction: results of surgical therapy based on anatomical / topographic classification in 1,002 consecutive patients. Ann Surg 2000;232:353–361
Mariette C, Castel B, Toursel H, et al. Surgical management of and long-term survival after adenocarcinoma of the cardia. Br J Surg 2002;89:1156–1163
Ito H, Clancy TE, Osteen RT, et al. Adenocarcinoma of the gastric cardia: what is the optimal surgical approach? J Am Coll Surg. 2004;199:880–886
Fein M, Fuchs KH, Ritter MP, et al. Application of the new classification for cancer of the cardia. Surgery 1998;124:707–713
Peracchia A, Bonavina L, Incarbone R, et al. Results of surgical therapy in patients with adenocarcinoma of the esophagus and cardia. Gastric Cancer 1999;2:89–94
Hulscher JB, Van Sandick JW, Offerhaus GJ, et al. Prospective analysis of the diagnostic yield of extended en bloc resection for adenocarcinoma of the esophagus or gastric cardia. Br J Surg 2001;88:715–719
Japanese Gastric Cancer Association. Japanese classification of gastric carcinoma, 2nd English edition. Gastric Cancer 1998;1:10–24
Sobin LH, Wittekind C, International Union Against Cancer. (UICC), eds, TNM Classification of Malignant Tumors. 5th ed. New York: John Wiley & Sons, Inc., 1997
Fleming ID, American Joint Committee on Cancer Classification (AJCC), eds, AJCC Cancer Staging Manual. Philadelphia: Lippincott Williams & Wilkins, 1997
Akiyama H, Miyazono H, Tsurumaru M, et al. Thoracoabdominal approach for carcinoma of the cardia of the stomach. Am J Surg 1979;137:345–349
Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc 1958;53:457–481
Gehan EA. A generalized Wilcoxon test for comparing arbitrarily singly-censored samples. Biometrika 1965;52:203–224
Dresner SM, Lamb PJ, Bennett MK, et al. The pattern of metastatic lymph node dissemination from adenocarcinoma of the esophagogastric junction. Surgery 2001;129:103–109
Aikou T, Shimazu H. Difference in main lymphatic pathways from the lower esophagus and gastric cardia. Jpn J Surg 1989;19:290–295
De Manzoni G, Pedrazzani C, Pasini F, et al. Pattern of recurrence after surgery in adenocarcinoma of the gastroesophageal junction. Eur J Surg Oncol 2003;29:506–510
Sadahiro S, Suzuki T, Ishikawa K, et al. Prophylactic hepatic arterial infusion chemotherapy for the prevention of liver metastasis in patients with colon carcinoma: a randomized control trial. Cancer 2004;100:590–597
Sons HU, Borchard F. Cancer of the distal esophagus and cardia. Incidence, tumorous infiltration, and metastatic spread. Ann Surg 1986;203:188–195
Husemann B. Cardia carcinoma considered as a distinct clinical entity. Br J Surg 1989;76:136–139
Stipa S, Di Giorgio A, Ferri M. Surgical treatment of adenocarcinoma of the cardia. Surgery 1992;111:386–393
Mattioli S, Di Simone MP, Ferruzzi L, et al. Surgical therapy for adenocarcinoma of the cardia: modalities of recurrence and extension of resection. Dis Esophagus 2001;14:104–109
Heidl G, Langhans P, Mellin W, et al. Adenocarcinomas of esophagus and cardia in comparison with gastric carcinoma. J Cancer Res Clin Oncol 1993;120:95–99
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yuasa, N., Miyake, H., Yamada, T. et al. Clinicopathologic Comparison of Siewert Type II and III Adenocarcinomas of the Gastroesophageal Junction. World J. Surg. 30, 364–371 (2006). https://doi.org/10.1007/s00268-005-0434-x
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00268-005-0434-x