Abstract
Amiodarone-associated thyrotoxicosis (AAT) is often poorly tolerated owing to underlying cardiac disease, and it is frequently refractory to conventional medical treatment. The goal of this study was to describe the patient characteristics, management, and outcomes of all the patients treated surgically for AAT at a single institution. We conducted a retrospective chart review of all patients managed surgically for AAT (April 1985 through November 2002) at the Mayo Clinic in Rochester, Minnesota. Altogether, 29 men and 5 women, ages 39 to 85 years (median 60 years), treated with amiodarone for 3 to 108 months underwent near-total or total thyroidectomy. Frequent symptoms were worsening heart failure, fatigue, weight loss, and tremor. Altogether, 12 patients failed medical management of their AAT, and 21 received no preoperative medical therapy. One patient had been successfully managed medically but required definitive treatment. Common indications for operation were the need to remain on amiodarone, cardiac decompensation, medically refractory disease, and severe symptoms, both hyperthyroid and cardiac, necessitating prompt resolution. The median ± SD American Society of Anesthesiologists (ASA) classification (1 = healthy through 5 = moribund) was 3.00 ± 0.58. A total of 27 specimens had histology consistent with AAT. Complications included death (n = 3), rehospitalization (n = 3), symptomatic hypocalcemia (n = 2), pneumonia (n = 2), cervical hematoma (n = 1), prolonged ventilatory wean (n = 1), and stroke (n = 1); one patient developed hypotension, adult respiratory distress syndrome, and sepsis. Of the 31 surviving patients, 25 (80%) remained on amiodarone postoperatively. The median follow-up was 29 months, at which time all surviving patients were free of hyperthyroid symptoms. Thyroidectomy is an effective treatment for AAT but has a high incidence of perioperative morbidity and mortality. The cardiovascular co-morbidities and high operative risk in this group of patients may account for the increased complication rate.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Amiodarone is a potent, iodine-rich (37% by molecular weight) class III antiarrhythmic agent that was approved for use in the United States in 1986 [1]. It is indicated for the treatment of refractory ventricular and supraventricular arrhythmias. Amiodarone is often used in patients with refractory arrhythmias who have failed other classes of antiarrhythmic agents. It is a fat-soluble drug with a long half-life (107 days), which allows the effects to be seen months after discontinuation [2]. Conventional doses of 100 to 600 mg of amiodarone per day provide 37 to 222 mg of organic iodine, approximately 10% of which is deiodinized, resulting in a large expansion of the iodine pool [3].
In the setting of amiodarone-induced hyperthyroidism, the spectrum of hyperthyroidism ranges from a mild disorder alleviated by discontinuing the amiodarone to severe, life-threatening thyrotoxicosis [1]. Amiodarone-associated thyrotoxicosis (AAT) occurs in a patient population with underlying cardiac dysrhythmias that do not tolerate the cardiac effects of hyperthyroidism. Patients with AAT frequently experience recurrence of arrhythmias and worsening heart failure as well as the symptoms of hyperthyroidism. This combination results in severe symptomatology and can result in total cardiac decompensation.
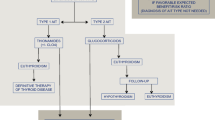
Two forms of AAT have been described. Type I occurs in patients with a preexisting adenomatous goiter and results from excessive hormone production secondary to iodine excess [4]. Type II is found in patients without preexisting thyroid disease [4, 5] and is thought to result from a chemical-induced thyroiditis, which causes follicular damage and fibrosis with release of preformed hormone [5]. Type I AAT is seen more commonly in iodine-deficient areas, whereas type II is more common in iodine-replete regions such as the United States. Type II AAT occurs in 2% of patients treated with amiodarone in the United States [1].
Treatment with amiodarone can induce hyperthyroidism [1, 5, 6, 7, 8, 9, 10, 11, 12] or hypothyroidism [11, 12, 13, 14, 15]. Hypothyroidism is managed with thyroid hormone replacement. AAT is notoriously refractory to medical management [1, 7, 8, 9, 10, 16, 17]. Patients with AAT demonstrate low radioactive iodine uptake, thereby precluding treatment with radioactive iodine (131I) [1, 15]. This is thought to be secondary to the large iodine pool caused by amiodarone administration [1]. Medical management of AAT typically involves discontinuation of amiodarone and treatment with an antithyroid medication such as propylthiouracil, carbimazole, potassium perchlorate, methimazole, steroids, or a cholecystographic agent [1, 4, 6, 8, 10, 15, 16, 17, 18, 19, 20]. Discontinuation of amiodarone alone may result in resolution of thyrotoxic symptoms in some patients, but because of the long half-life this can take several weeks to months to occur [1, 7, 9, 15, 16, 18]. Many patients with severe dysrhythmias are placed on amiodarone as a last resort, having failed therapy with other antiarrhythmic agents; and in these patients discontinuing the amiodarone may be undesirable or unacceptable [18]. Furthermore, stopping amiodarone in a thyrotoxic patient with a history of malignant, refractory arrhythmias may not be a safe option and may lead to worsening of the patient’s cardiac condition [1, 17, 18].
Patients with worsening arrhythmias often cannot tolerate the state of hyperthyroidism and therefore require prompt resolution of their thyrotoxic status. There are a few small series in the literature describing surgical management of AAT [1, 7, 8, 9, 10, 21], and in this study we describe our experience with it.
Materials and Methods
The records of all patients treated surgically for AAT at our institution were reviewed. Patient demographics, duration of amiodarone treatment, thyrotoxic symptoms, management of AAT, indications for thyroidectomy, and factors that may affect outcome were recorded. The diagnosis of AAT was based on the presence of hyperthyroid symptoms, suppression of thyroid-stimulating hormone (TSH), elevation of serum thyroid hormone levels and a negative antithyroid or thyroid-stimulating antibody titer, or any combination of these conditions occurring in the setting of amiodarone therapy. Cardiac status was evaluated using the ejection fraction (EF), when available, documented by echocardiography or angiography. American Society of Anesthesiology (ASA) status was noted. Preoperative and postoperative thyroid function tests were obtained. Perioperative events were recorded, including the operating time, operation performed, operative mortality, length of hospital stay, and complications. Postoperative complications were determined to be those that occurred during the 30-day period following thyroidectomy. The last comprehensive medical examination or evaluation by a cardiologist, endocrinologist, or surgeon at our institution determined follow-up. The last visit or communication with someone at our institution determined survival. Data were evaluated to determine any factors influencing outcome.
Results
Between April 1985 and November 2002 a total of 34 patients underwent total or near-total thyroidectomy at our institution. Patient characteristics and clinical data are shown in Table 1. Four patients had a history of thyroid disorders: Two had known goiters, one initially experienced amiodarone-induced hypothyroidism, and one had a history of benign thyroid nodules. The median duration of amiodarone treatment upon diagnosis of AAT was 36 months (range 3–108 months). Most patients (74%) were treated for 20 to 50 months.
Preoperative symptoms included worsening arrhythmia/congestive heart failure (53%), weight loss (47%), tremor (38%), fatigue (32%), heat intolerance (21%), weakness (18%), palpitations (15%), diarrhea (15%), and insomnia (6%). Operative indications are listed in Table 2. Fifteen patients had more than one indication. The most common indication was the inability or unwillingness by the patient’s cardiologist to stop amiodarone. Most of these patients (95%) had severe, refractory arrhythmias and had failed treatment with multiple other antiarrhythmic agents in the past.
The median operating time was 110 minutes (range 56–170 minutes). 19 patients (56%) underwent near-total thyroidectomy, and 15 patients (44%) underwent total thyroidectomy. The operation performed was based on the surgeon’s preference. All 34 patients had general endotracheal anesthesia. Eighteen patients (53%) received perioperative β-blockade. No patient experienced thyroid storm perioperatively. The median weight of the thyroid glands was 24.5 g (range 12.3–174.0 g). The median hospital stay was 3 days (range 1–35 days).
The perioperative complications are listed in Table 3. Complications were included if they occurred within 30 days of the procedure. Ten patients experienced at least one postoperative complication, for a morbidity rate of 29%. Three patients required rehospitalization. The first was admitted for pneumonia and dehydration and the second for symptomatic hypocalcemia; the third patient developed atrial fibrillation on the night of discharge but spontaneously converted back to normal sinus rhythm after an intravenous bolus of amiodarone. There were no recurrent laryngeal nerve injuries in this series, and no patients experienced permanent hypocalcemia. There were three deaths, for an operative mortality rate of 9%.
Death 1
A 77-year-old man had paroxysmal atrial fibrillation and an EF of 45%. He had been treated with amiodarone for 34 months, having failed numerous other antiarrhythmic agents in the past. He presented with a 4-week history of tremor, weight loss, and heat sensitivity. He received no medical treatment for AAT. His cardiologist thought he would not tolerate cessation of amiodarone. During the preoperative evaluation he was noted to have a critical stenosis of his left carotid artery. He underwent concomitant left carotid endarterectomy and total thyroidectomy. Postoperatively he was noted to have a large left cerebral infarct, and he expired on postoperative day 3.
Death 2
A 61-year-old man on chronic hemodialysis with atrial fibrillation and an EF of 15% had been treated with amiodarone for 48 months. He developed AAT and was experiencing acutely worsening shortness of breath and orthopnea. The patient required immediate resolution of his hyperthyroidism and did not undergo a trial of medical treatment. He underwent simultaneous total thyroidectomy and placement of a dialysis catheter. On postoperative day 3 he developed hypotension, bradycardia, respiratory failure, and electromechanical dissociation. He expired on postoperative day 5.
Death 3
A 54-year-old man with an EF of 10% was awaiting heart transplantation. He had been treated with amiodarone for 24 months for recurrent ventricular fibrillation. He developed AAT and was successfully treated with propylthiouracil (PTU) for 26 weeks, achieving euthyroidism and good symptom control. The patient needed to discontinue PTU secondary to interactions with antirejection medications if he was to receive a cardiac transplant. Total thyroidectomy was therefore undertaken for definitive treatment of his hyperthyroidism. The surgical procedure was technically uncomplicated, and the thyroid was safely removed with minimal blood loss. The patient was hemodynamically stable throughout the operation but experienced sudden cardiac arrest at the conclusion of the procedure and expired due to cardiac failure on the night of the operation.
Patients who developed a postoperative complication were compared to those who were complication-free to determine if any factors might predict a negative outcome (Table 4). Six of ten patients (60%) with a complication were on perioperative β-blockers, whereas only 53% of the total group were taking β-blockers at the time of operation. Sixteen thyroidectomies were performed between January 1999 and November 2002, and seven patients (44%) experienced a complication.
Available preoperative and postoperative laboratory values for all 34 patients are shown in Table 5. The median ± SD follow-up was 29 ± 51 months. Of the 31 surviving patients, 25 (80%) continued to take amiodarone. The thyrotoxic symptoms resolved in all 31 surviving patients. The actual 6-month survival was 86%, with a median ± SD survival of 19 ± 51 months. Long-term survival was dictated by each patient’s underlying cardiac disease, not by thyrotoxicosis.
Discussion
Amiodarone-associated thyrotoxicosis is an uncommon condition that develops in about 1.5% to 3.0% of patients taking the drug [1, 4, 11, 12]. Patients with AAT have a history of arrhythmias that are often severe and refractory to other antiarrhythmic agents. These patients have preexisting cardiac dysfunction and often do not tolerate a state of hyperthyroidism, so they require timely resolution of their hyperthyroidism. This study represents the largest reported series of AAT patients managed surgically.
Amiodarone-associated thyrotoxicosis can be managed medically or surgically, and many series have reported on both forms of treatment [1, 6, 7, 8, 9, 10, 13, 16, 17, 18, 19, 20, 21]. Thyroidectomy results in an immediate reversal of hyperthyroidism, with rapid resolution of symptoms [1, 7, 8, 9, 10, 21]. Patients with AAT are not optimal surgical candidates because of preexisting cardiac disease and their hyperthyroidism. These factors combine to place this population at higher risk for perioperative morbidity and mortality. Medical therapy generally requires discontinuation of amiodarone, but it may take weeks to achieve a euthyroid state [6, 9, 16, 17, 18, 19, 20]. Occasionally, patients who were successfully treated medically for AAT experience a relapse, requiring further medical treatment [16, 17, 18]. Hyperthyroid patients with arrhythmias are also at high risk for mortality and may succumb during medical therapy [17].
In this study, 13 patients (38%) had undergone medical therapy for AAT with intent to cure before proceeding to thyroidectomy. Ten patients were treated with PTU only, two with methimazole, and one with both PTU and potassium perchlorate. Of the 13 patients treated medically for AAT, 12 had unsuccessful results. One patient was successfully treated with PTU for 26 weeks while remaining on amiodarone. This patient later required thyroidectomy because there was the potential for dangerous interactions of the PTU with antirejection medications if he were to receive a cardiac transplant.
Patients with AAT can present with end-stage cardiac failure requiring immediate treatment. In this situation surgical management is often recommended because of the rapid resolution of the hyperthyroidism. Other patients have mild symptoms and minimal TSH suppression. If the patient is able to discontinue amiodarone and tolerate the hyperthyroid symptoms for several weeks, medical management is often attempted with minimal morbidity and good success. Unfortunately, most AAT cases do not fall into either of these two categories.
During our study period (1985–2002), more than 60 patients were diagnosed with AAT at the Mayo clinic. Most of those not proceeding to operation had minor symptoms and did not require amiodarone to control their arrhythmia. Amiodarone was discontinued in those cases, and another, appropriate antiarrhythmic agent was instituted. An antithyroid medication may or may not have been started, with the patient’s symptoms resolving over a period of weeks to months. Our study represents a select group of patients with a complex and difficult problem. Most of the patients in this series could not stop taking amiodarone because it was the only antiarrhythmic that had controlled their arrhythmia in the past: They had been previously treated with other antiarrhythmic agents that had proved ineffective. This series also includes many patients who required immediate resolution of the hyperthyroid state. Many patients were in end-stage cardiac failure and would not tolerate a course of medical therapy that lasted several weeks to months.
Most of the patients undergoing thyroidectomy in this series were, in fact, poor surgical candidates. Each had a history of cardiac dysrhythmias, and many were in severe heart failure and highly symptomatic. This poor state of health is difficult to convey objectively. The median ASA score in this series was 3.0 (mean 3.2). The median EF in this series was 45% (range 10–66%). Ten patients (30%) had an EF of less than 30%, and three had an EF of less than 20%. The EF, however, does not take into account diastolic dysfunction, cardiac symptoms, functional status, or propensity for fatal arrhythmias. Most patients in this series were not ideal surgical candidates because of poor cardiac function, illustrated by the two patients who died postoperatively from cardiac causes. We believe the underlying cardiac disease accounts for many of the complications noted in the series.
One of the three deaths resulted from a massive cerebral infarction. This patient had undergone simultaneous thyroidectomy and carotid endarterectomy. Death likely resulted from the endarterectomy portion of the procedure. This case epitomizes the poor baseline health status of this particular group of patients.
The postoperative complication rate in this series was much higher than the rates reported for thyroidectomy performed for any other indication [22, 23]. The overall complication rate in our series was 29%, with 10 patients experiencing at least one complication. This rate is much higher than the 7.6% to 14.0% reported for thyroidectomy in the literature [22, 23]. In two series of thyroidectomies, the most common complication was hypocalcemia, with incidences of 6.2% and 7.8%, respectively [22, 23]. This is in line with our 6% rate. The rate of other surgical complications, such as wound infection and recurrent nerve injury, was also in line with those of other reported series [22, 23]. The mortality rate in our series was 9% compared to 0% and 0.2%, respectively [22, 23]. The difference in mortality rates is most likely due to the inherent cardiac dysfunction present in the patient population affected by AAT.
We studied several preoperative factors in an attempt to identify patients at risk for postoperative complications. Patients experiencing complications tended to be younger than those not experiencing complications (57.0 ± 8.5 vs. 61.0 ± 11.0 years) and had a higher EF (55.0% ± 21.0% vs. 37.5% ± 14.0%). The mean duration of amiodarone treatment in both groups was 36 months. Only 2 of the 10 patients with complications were taking amiodarone for ventricular arrhythmias. We believe that the lack of preoperative predictors of postoperative morbidity and mortality is a reflection of the preexisting cardiac co-morbidities and poor functional status inherent in this group of patients.
Several interventions were tried throughout the course of this series to decrease the morbidity associated with the operation. Seventeen patients were given either a short course (< 2 weeks) of antithyroid therapy (n = 4) or were initially treated only with antithyroid medications (n = 13). The complication rate in this group of patients was 35% which is similar to that noted for patients not receiving antithyroid therapy (65%). Altogether, 18 patients were β-blocked perioperatively, and 6 of them (33%) experienced a complication. Moreover, 6 of the 10 patients who had a complication (60%) were β-blocked at operation. It is difficult to determine whether β-blockade contributed to negative patient outcomes or was simply a marker of poor cardiac performance status.
We also find it interesting that the last 16 patients (47%) experienced 70% of the complications. This could be explained by more widespread use of, and indications for, amiodarone, which could lead to AAT developing in a debilitated patient population. This group was also more likely to undergo a trial of medical therapy (70%) than patients treated earlier in the series (33%).
Conclusions
Thyroidectomy is an efficacious therapy for the rare patient with AAT. The operative morbidity and mortality rates are considerably higher in this complex group of patients than in patients undergoing thyroidectomy for other indications. This is likely due to preexisting cardiac co-morbidities.
Résumé
La thyrotoxicose associée à l’amiodarone (Amiodarone-Associated Thyrotoxicosis ou AAT) est souvent mal tolérée en raison de la maladie cardiaque sous-jacente; elle est fréquemment réfractaire au traitement médical conventionnel. Le but de cette étude a été de décrire les caractéristiques des patients, la prise en charge et l’évolution de tous les patients traités chirurgicalement pour AAT dans une seule institution. Il s’agit d’une revue rétrospective des dossiers de tous les patients traités chirurgicalement pour AAT (avril 1985 à nov 2002) à la Clinique Mayo, Rochester, Minnesota, USA. 29 hommes et cinq femmes, âgés entre 39 et 85 ans (médiane 60) traités par l’amiodarone pendant entre 3 et 108 mois ont eu une thyroïdectomie totale ou presque totale. Les symptômes les plus fréquents ont été une aggravation de l’insuffisance cardiaque, la fatigue, la perte pondérale et le tremblement. Pour 12 patients, le traitement médical de l’AAT a été considéré comme un échec alors que 21 n’ont reçu aucun traitement médical au préalable. Un patient a été traité médicalement avec succès mais a ensuite nécessité un traitement définitif. Les indications les plus fréquentes de l’intervention ont été le besoin de rester sous traitement par l’amiodarone, la décompensation cardiaque, la non-réponse médicale et la sévérité des symptômes, à la fois de l’hyperthyroïdie et cardiaques, nécessitant une résolution prompte. La classification ASA (médiane ± SD) (1 = sain à 5 = moribond) a été de 3.00 ± 0.58. Vingt et un prélèvements étaient consistants avec le diagnostic d’AAT. Les complications observées ont été le décès (n = 3), la re-hospitalisation (n = 3), une hypocalcémie symptomatique (n = 2), l’infection pulmonaire (n = 2), un hématome cervical (n = 1), la difficulté de sevrage ventilatoire (n = 1), un accident vasculaire (n = 1), et un patient qui a développé une hypotension, SDRA et sepsis. Vingt-cinq des 31 patients en vie (80%) sont restés sous amiodarone en postopératoire. La médiane de suivi a été de 29 mois: aucun des patients survivants n’avait de symptômes du type hyperthyroïdie. La thyroïdectomie est un traitement efficace de l’AAT mais la morbidité et la mortalité périopératoires sont élevées. Les co-morbidités cardiovasculaires et le haut risque opératoire dans ce groupe de patients peuvent expliquer le taux élevé de complications.
Resumen
La tirotoxicosis asociada con la amiodarona (TAA) con frecuencia es mal tolerada por razón de la enfermedad cardiaca subyacente, la cual generalmente es refractaria al tratamiento médico convencional. El objetivo del presente estudio fue describir las características del paciente, el manejo y los resultados en todos los pacientes sometidos a tratamiento quirúrgico por TAA en una sola institución. Se hizo una revisión retrospectiva de las historias clínicas de todos los pacientes tratados quirúrgicamente por TAA (abril 1985 a noviembre 2002) en la Clínica Mayo, Rochester, Minnesota. Veintinueve hombres y cinco mujeres con edades entre 39 y 85 años (media 60) tratados con amiodarona por 3 a 108 meses, fueron sometidos a tiroidectomía casi total o tiroidectomía total. Los síntomas más frecuentes fueron falla cardiaca progresiva, fatiga, pérdida de peso y temblor. En doce pacientes falló el tratamiento médico de la TAA, en tanto que 21 no recibieron tratamiento médico preoperatorio. Un paciente ha sido manejado médicamente en forma exitosa pero requirió tratamiento definitivo. Las indicaciones comunes para operación fueron la necesidad de permanecer bajo tratamiento con amiodarona, descompensación cardiaca, enfermedad refractaria al tratamiento médico y síntomas severos, tanto de hipertiroidismo como cardiacos, que requerían pronta resolución. La clasificación ASA media ± DE (1 = saludable hasta 5 = moribundo), fue 3.00 ± 0.58. Veintisiete especímenes fueron histológicamente consistentes con TAA. Las complicaciones incluyeron muerte (n = 3), rehospitalización (n = 3), hipercalcemia sintomática (n = 2), neumonía (n = 2), hematoma cervical (n = 1), prolongado retiro del ventilador (n = 1), apoplejía (n = 1), y un paciente que desarrolló hipotensión, SDRA y sepsis; 25 de 31 pacientes sobrevivientes (80%) permanecieron bajo amiodarona en el postoperatorio. La media de seguimiento fue 29 meses, tiempo en el cual la totalidad de los sobrevivientes permanecía asintomática. La tiroidectomía representa un tratamiento efectivo en la TAA pero se acompaña de una elevada incidencia de morbilidad y mortalidad perioperatorias. Las comorbilidades cardiovasculares y el alto riesgo operatorio en este grupo de pacientes pueden explicar la alta tasa de complicaciones.
References
MD Brennan JA Heerden Particlevan JA Carney (1987) ArticleTitleAmiodarone-associated thyrotoxicosis (AAT): experience with surgical management Surgery 102 1062–1067 Occurrence Handle1:STN:280:BieD2s7itFA%3D Occurrence Handle3686345
DP Zipes EN Prystowsky JS Heger (1984) ArticleTitleAmiodarone: electrophysiologic actions, pharmacokinetics and clinical effects J. Am. Coll. Cardiol. 3 1059–1071 Occurrence Handle1:CAS:528:DyaL2cXhvVGit7o%3D Occurrence Handle6368644
RH Rao R McCready GS Spathis (1986) ArticleTitleIodine kinetic studies during amiodarone treatment J. Clin. Endocrinol. Metab. 63 563–568
GH Daniels (2001) ArticleTitleAmiodarone-induced thyrotoxicosis J. Clin. Endocrinol. Metab. 86 3–8 Occurrence Handle10.1210/jc.86.1.3 Occurrence Handle1:CAS:528:DC%2BD3MXhtVGguro%3D Occurrence Handle11231968
MD Brennan DZ Erickson JA Carney et al. (1995) ArticleTitleNongoitrous (type I) amiodarone-associated thyrotoxicosis: evidence of follicular disruption in vitro and in vivo Thyroid 5 177–183 Occurrence Handle1:STN:280:BymD1c3nsVw%3D Occurrence Handle7580265
IJ Chopra K Baber (2001) ArticleTitleUse of oral cholecystographic agents in the treatment of amiodarone-induced hyperthyroidism J. Clin. Endocrinol. Metab. 86 4707–4710 Occurrence Handle10.1210/jc.86.10.4707 Occurrence Handle1:CAS:528:DC%2BD3MXptVarsrg%3D Occurrence Handle11600529
S Claxton SN Sinha S Donovan et al. (2000) ArticleTitleRefractory amiodarone-associated thyrotoxicosis: an indication for thyroidectomy Aust. N. Z. J. Surg. 70 174–178 Occurrence Handle10.1046/j.1440-1622.2000.01780.x Occurrence Handle1:STN:280:DC%2BD3c3is12ltw%3D%3D Occurrence Handle10765898
AP Farwell SL Abend SKS Huang et al. (1990) ArticleTitleThyroidectomy for amiodarone-induced thyrotoxicosis J. A. M. A. 11 1526–1528
E Hamoir M Meurisse T Defechereux et al. (1998) ArticleTitleSurgical management of amiodarone-associated thyrotoxicosis: too risky or too effective? World J. Surg. 22 537–543 Occurrence Handle1:STN:280:DyaK1c3mtVaitA%3D%3D Occurrence Handle9597925
DC Mulligan CR McHenry W Kinney et al. (1993) ArticleTitleAmiodarone-induced thyrotoxicosis: clinical presentation and expanded indications for thyroidectomy Surgery 114 1114–1119 Occurrence Handle1:STN:280:ByuD2sfisFI%3D Occurrence Handle8256216
K Nademanee RW Piwonka BN Singh et al. (1989) ArticleTitleAmiodarone and thyroid function Prog. Cardiovasc. Dis. 31 427–437 Occurrence Handle10.1016/0033-0620(89)90017-0 Occurrence Handle1:CAS:528:DyaL1MXktFOjs7c%3D Occurrence Handle2652189
A Lombardi E Martino LE Braverman (1990) ArticleTitleAmiodarone and the thyroid Thyroid Today 13 1–7
E Martino F Aghini-Lombardi S Mariotti et al. (1987) ArticleTitleAmiodarone iodine-induced hypothyroidism: risk factors and followup in 28 cases Clin. Endocrinol. (Oxf.) 26 227–237 Occurrence Handle1:STN:280:BieD38josVY%3D
E Martino L Bartalena F Bogazzi et al. (2001) ArticleTitleThe effects of amiodarone on the thyroid Endocr. Rev. 22 240–254 Occurrence Handle10.1210/er.22.2.240 Occurrence Handle1:CAS:528:DC%2BD3MXjsFSntLo%3D Occurrence Handle11294826
CM Newman A Price DW Davies et al. (1998) ArticleTitleAmiodarone and the thyroid: a practical guide to the management of thyroid dysfunction induced by amiodarone therapy Heart 79 121–127 Occurrence Handle1:STN:280:DyaK1c7pvFWjsQ%3D%3D Occurrence Handle9538302
L Bartalena S Brogioni L Grasso et al. (1996) ArticleTitleTreatment of amiodarone-induced thyrotoxicosis, a difficult challenge: results of a prospective study J. Clin. Endocrinol. Metab. 81 2930–2933 Occurrence Handle10.1210/jc.81.8.2930 Occurrence Handle1:CAS:528:DyaK28XkvFWrtbw%3D Occurrence Handle8768854
SE Eaton HA Euinton CM Newman et al. (2002) ArticleTitleClinical experience of amiodarone-induced thyrotoxicosis over a 3-year period: role of colour-flow doppler sonography Clin. Endocrinol. (Oxf.) 56 33–38 Occurrence Handle10.1046/j.0300-0664.2001.01457.x Occurrence Handle1:CAS:528:DC%2BD38XitVShsL4%3D
F Osman JA Franklyn MC Sheppard et al. (2002) ArticleTitleSuccessful treatment of amiodarone-induced thyrotoxicosis Circulation 105 1275–1277 Occurrence Handle1:CAS:528:DC%2BD38XjtFals7o%3D Occurrence Handle11901034
E Martino F Aghini-Lombardi S Mariotti et al. (1986) ArticleTitleTreatment of amiodarone associated thyrotoxicosis by simultaneous administration of potassium perchlorate and methimazole J. Endocrinol. Invest. 9 201–207 Occurrence Handle1:STN:280:BiiD3cbmtlI%3D Occurrence Handle3020113
LJ Reichert HA Rooy Particlede (1989) ArticleTitleTreatment of amiodarone induced hyperthyroidism with potassium perchlorate and methimazole during amiodarone treatment B. M. J. 298 1547–1548 Occurrence Handle1:STN:280:BiaA3sngsV0%3D
F Bogazzi P Miccoli P Berti et al. (2002) ArticleTitlePreparation with iopanoic acid rapidly controls thyrotoxicosis in patients with amiodarone-induced thyrotoxicosis before thyroidectomy Surgery 132 1114–1118 Occurrence Handle10.1067/msy.2002.128561 Occurrence Handle12490863
N Bhattacharyya MP Fried (2002) ArticleTitleAssessment of the morbidity and complications of total thyroidectomy Arch. Otolaryngol. Head Neck Surg. 128 389–392 Occurrence Handle11926912
R Bellantone CP Lombardi M Bossola et al. (2002) ArticleTitleTotal thyroidectomy for management of benign thyroid disease: review of 526 cases World J. Surg. 26 1468–1471 Occurrence Handle10.1007/s00268-002-6426-1 Occurrence Handle12360381
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Houghton, S., Farley, D., Brennan, M. et al. Surgical Management of Amiodarone-associated Thyrotoxicosis: Mayo Clinic Experience. World J. Surg. 28, 1083–1087 (2004). https://doi.org/10.1007/s00268-004-7599-6
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00268-004-7599-6