Abstract
Inflammatory breast cancer (IBC) is the most aggressive form of locally advanced breast cancer. It can be diagnosed based on a clinical or pathologic basis. We evaluated the usefulness of 18F-fluorodeoxyglucose-positron emission tomography (FDG-PET) scans for diagnosing and staging IBC. We retrospectively reviewed the medical records of seven consecutive patients with IBC who underwent FDG-PET scanning for the initial staging. Four patients had follow-up PET scans after chemotherapy. All seven patients presented with diffuse breast enlargement, redness, and peau d’orange for 1 to 5 months’ duration. In addition, four patients had a palpable breast mass, and three had axillary lymph node enlargement. Mammography showed diffuse, increased parenchymal density and skin thickening in 85% and parenchymal distortion in 43%. There was no evidence of distant metastasis on computed tomography of the chest or abdomen. Pathologic examination of breast biopsy specimens showed infiltrating ductal carcinoma in six patients, and one had lobular carcinoma. All patients had prechemotherapy whole-body PET scans that showed diffuse FDG uptake in the breast with superimposed intense foci in the primary tumor. Furthermore, there was skin enhancement in 100%, axillary lymph node in 85%, and skeletal metastases in 14% of the patients, confirmed by bone scintigraphy. Postchemotherapy FDG-PET scans performed in four patients showed response in the primary tumor, axillary lymph nodes, and skeletal metastases. The FDG-PET scan is thus useful for displaying the pattern of FDG breast uptake that reflects the extent of the pathologic involvement in IBC (i.e., diffuse breast involvement and dermal lymphatic spread). It can also detect the presence of lymph node and skeletal metastases, demarcating the extent of the disease locally as well as distally.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Inflammatory breast cancer (IBC) is a diffuse form of infiltrative breast carcinoma. It tends to infiltrate widely throughout the breast substance, involving most of the lymphatics and producing acute swelling, redness, and tenderness of the breast. It is referred to clinically as inflammatory carcinoma.
Inflammatory breast cancer is a distinct clinicopathologic entity and accounts for 1% to 3% of all breast cancers [1, 2, 3]. It is classified as T4d tumor according to the TNM classification system [2, 3]. It is important to differentiate patients with true IBC from those with locally advanced breast cancer who have secondary skin involvement, as the latter group may have a better prognosis [4].
The diagnostic accuracy of 18F-fluorodeoxyglucose positron emission tomography (FDG-PET) imaging for palpable breast tumors is probably superior to other imaging techniques currently available, including magnetic resonance imaging, mammography, and sonography [5]. The high positive predictive value of PET imaging suggests a potential role as a staging procedure to identify the multicentricity of primary breast cancer, as well as lung, bone, and lymph node metastases using only one imaging procedure [5]. In this study we present our experience with FDG-PET scanning in seven patients with known IBC.
Materials and Methods
Seven patients with IBC who had undergone FDG-PET scanning were reviewed retrospectively. These cases were referred between 1995 and 2001 to the combined breast clinic at the King Faisal Specialist Hospital and Research Center, Riyadh, a tertiary hospital for cancer management. The patients’ characteristics, clinical presentation, TNM stage, chemotherapy, surgical intervention, pathologic assessment, and outcome were collected from the medical records. Radiologic investigations including mammography, abdominal ultrasonography (US), computed tomography (CT) of the chest and abdomen, bone scintigraphy, and FDG-PET scans were reviewed.
FDG Whole-body PET Scans
Patients were given injections of 370 MBq (10 mCi) 18F-FDG after a 12-hour overnight fast. Imaging was initiated 45 minutes after injection using an ECAT EXACT 47 scanner (Siemens, Hoffman, Estates, IL, USA). This three-ring system with a 16.2 cm field of view produces 47 contiguous image planes with plane spacing of 3.375 mm and in-plane resolution of 6.7 mm. A whole-body technique was used whereby images were obtained in sequential bed positions and joined in a single display. Whole-body images were obtained (8–10 minutes in each bed position) and processed, after which they were reconstructed using a 0.4 Hann filter and corrected for attenuation. Transmission scanning was performed immediately after emission using external rotating germanium-68 rod sources.
Results
The clinical presentation, pathologic results, mammographic findings, and management of seven patients with IBC are summarized in Table 1. All patients were staged according to the TNM staging system (T, tumor; N, lymph nodes; M, metastasis), and the primary tumor was staged as T4d (specific for IBC). Infiltrating ductal carcinoma was the predominant pathologic type; only one patient had infiltrating lobular carcinoma (patient 3). The mean age was 46.4 years (range 38–53 years), and six patients were premenopausal. Clinical presentation included redness and peau d’orange in 100%, which appeared over a relatively short duration (1–5 months) associated with diffuse breast enlargement in 71% (5/7), a palpable mass in 57% (4/7), and breast tenderness in 43% (3/7).Bilateral mammography was performed in six patients and showed diffuse increased parenchymal density associated with diffuse increased skin thickening in 83% (5/6), parenchymal distortion and microcalcification in 33% (2/6), each and axillary lymph node involvement in 16% (1/6). The chest radiograph was normal in all patients. Bone scans showed skeletal metastases in 14% (1/6). CT and US of the abdomen excluded distant metastasis in five and two patients, respectively.
The characteristics of the 12 FDG-PET scans are summarized in Table 2. Examples of the breast appearance, lymph node involvement, and skeletal metastases are displayed in Figures 1, 2, 3, 4. Seven patients had undergone a total of 12 whole-body 18FDG-PET scans: 7 at their initial presentation, 3 on follow-up after completion of chemotherapy, one 2 follow-up PET scans during chemotherapy (in one patient after the second and fourth cycles).
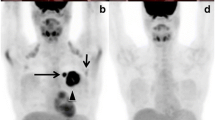
Patient 1. A. Initial 18-fluorodeoxyglucose positron emission tomography (FDG-PET) scan showing breast enlargement with diffuse breast uptake (primary tumor resected), prominent skin uptake, and multiple (two or three) axillary lymph nodes. B. Skeletal metastases were detected in the sternum, thoracic spine, and left humerus. C, D. Follow-up FDG-PET scans after the second cycle of chemotherapy showed no significant change in the breast uptake, but there was clearance of the skin and axillary, sternal, and thoracic spine uptake. E, F. Follow-up FDG-PET scans after the fourth cycle of chemotherapy showed clearance of the breast uptake (E) and subsequent decreased humeral uptake, which represented a late response (F).
Patient 2. A, B. Initial FDG-PET scan showing breast enlargement with diffuse breast uptake, multifocal primary tumor uptake, and prominent skin uptake (A) as well as multiple [2, 3] axillary lymph nodes (B). C, D. Follow-up FDG PET scan after the third cycle of chemotherapy showing decreased breast size, decreased breast and skin uptake, cleared tumor uptake (C), and cleared axillary uptake (D).
Patient 3. A, B. Initial FDG-PET scan showing breast enlargement with diffuse breast uptake, multifocal primary tumor uptake, and prominent skin uptake (A) but no axillary uptake (B). C, D. Follow-up FDG-PET scan after the fourth cycle of chemotherapy showing decreased breast size, decreased skin uptake, and clearance of the breast and tumor uptake.
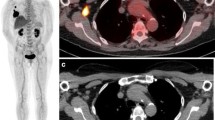
In all patients the initial PET scan showed an interesting pattern of abnormal FDG breast uptake with relative breast enlargement and diffuse increased uptake along with superimposed intense scattered foci and overlying skin enhancement (Fig. 1, 2, 3, 4A,B). Ipsilateral axillary lymph node involvement was present in 86% (6/7), and one patient (no. 4) had ipsilateral infraclavicular and supraclavicular lymph node involvement in continuity with the axillary lymph nodes (Fig. 4B). Another patient (no. 1) had multiple foci of increased FDG uptake that represented bone metastases involving the sternum, thoracic spine, and humerus (Fig. 1B).
Five postchemotherapy FDG-PET scans were available for four patients (the first patient had two follow-up scans). All four patients had evidence of tumor response reflected by decreased breast size in 80% (4/5), clearance of the diffuse FDG breast uptake and focal tumor uptake in 40% (2/5), and decreased axillary uptake in 80% (4/5) (Figs. 1, 2, 3C,D). The first patient had no significant change in breast uptake (she had had an incisional biopsy at the referring center 6 weeks prior to the PET scan), but she did exhibit clearance of the skin, axillary, sternal, and thoracic spine uptake after the second cycle of chemotherapy (Fig. 1C,D). Interestingly, after the fourth cycle, the FDG-PET scan showed clearance of the breast uptake and a further decrease in the humeral uptake, which likely represented a late response (Fig. 1E,F) [6]. One patient (no. 3) had generalized, prominent FDG uptake in the muscle and bone marrow, which was most likely related to the chemotherapy (Fig. 3D).
Four patients were treated with neoadjuvant chemotherapy followed by modified radical mastectomy and regional radiotherapy, as they had no distant metastases on initial presentation. Histopathologic examination of the mastectomy specimens showed ductal carcinoma in situ of less than 1 cm with one of three lymph nodes containing malignant cells (patient 2), multifocal invasive lobular carcinoma with 7 of 16 lymph nodes positive for malignancy (patient 3), no residual malignancy in the breast or axillary lymph nodes (patient 5), and a 7 cm invasive ductal carcinoma with all nine dissected lymph nodes positive for metastases (patient 7) (Table 1).
Three patients are alive and disease-free for 43, 24, and 4 months, respectively. One patient (no. 7) developed widespread distant metastases after completion of therapy and died of her disease within 21 months. Three patients received palliative chemotherapy for metastatic disease involving the bone, skin nodules, and supraclavicular lymph nodes, respectively. One was lost to follow-up, and two have been alive for 17 and 45 months, respectively.
Discussion
We evaluated the appearance of FDG-PET scans in seven patients with IBC. The pattern of FDG breast uptake correlates with the extent of the pathologic breast involvement. The relative breast enlargement along with diffuse, increased FDG breast uptake with superimposed scattered foci of intense FDG uptake reflects the diffuse nature of tumor involvement in IBC. In addition, the enhanced FDG skin uptake was likely related to the dermal lymphatic spread, which is a pathognomonic histologic feature of IBC but not necessary for the diagnosis [2, 3]. Furthermore, whole-body images detected the presence of axillary and supraclavicular lymph nodes as well as skeletal metastases. Mapping the lymphatic system in patient 4, who had no evidence of distant metastasis, by detecting ipsilateral supraclavicular lymph node involvement in a continuum with the involved axillary lymph nodes further supports the observation that infraclavicular and supraclavicular lymph nodes are part of a continuum in the regional lymph node drainage of the breast rather than representing distant metastases [7].
The follow-up PET scan was useful for assessing the response to chemotherapy in the primary tumor as evidenced by the decreased FDG breast and skin uptake in 40%, whereas lymph node metastases showed a higher response (80%). It was hypothesized that the response of metastatic breast cancer in axillary lymph nodes may be of even greater prognostic importance than the response of the tumor that remains in the breast [8]. In the case of skeletal metastases, PET scans monitored the stages of chemotherapy response in the various lesions. Interestingly, PET scans acquired after the fourth cycle of chemotherapy reflected the response more accurately than did scans acquired after the second cycle. This observation of a late response with uptake in the primary tumor or metastatic foci might be related to the heterogeneity of breast cancer. As in our patient, if PET scans are done only early in the course of chemotherapy, late responders may be missed unless multiple follow-up scans are acquired [6].
FDG-PET scanning has been shown to be useful for screening and staging infiltrating ductal carcinoma, especially in mammogram findings that are difficult to interpret, such as a dense breast [9] postoperatively [9] and after augmentation mammoplasty [10]. It has also been helpful for differentiating a benign from a malignant breast mass [9]. FDG-PET scans display both tumor anatomy and metabolism [11]. FDG uptake in tumors is reasonably well correlated with the number of viable cancer cells present in the tumor, although it may also be related to the tumor proliferation rate [5, 11]. The histopathologic response of breast cancer to chemotherapy could be predicted by FDG-PET scans with an accuracy of 88% and 91% after the first and second courses of chemotherapy, respectively [12]. Furthermore, FDG-PET scans after a single pulse of chemotherapy were able to predict a complete pathologic response with a sensitivity of 90% and a specificity of 74% [8]. They can differentiate responders from nonresponders early in the course of therapy [6, 8, 12]. Monitoring chemohormonal therapy of breast cancer using FDG-PET scanning has been reported [6, 8, 9, 12, 13]. However, small lesions (< 1 cm) may be missed, as in two of our patients, representing a false-negative scan.
Inflammatory breast cancer is a clinicopathologic entity characterized by diffuse brawny induration, erythema, and edema (peau d’orange) of the breast skin with a raised edge reminiscent of erysipelas [3]. The skin is thickened to 2 to 8 mm (average 4 mm). Dermal lymphatic invasion may be present but is not necessary for diagnosing true IBC [2, 3]; it could have prognostic significance, however [3]. Usually, it is not necessary to obtain a skin biopsy to establish the diagnosis of IBC when a patient presents with characteristic clinical findings, as skin biopsies are negative in as many as 50% of patients [14]. An underlying palpable mass is present in 30% to 60%, and palpable axillary lymph nodes are present in 50% to75% of cases [2, 4]. There is no consistent histopathologic type of breast carcinoma associated with IBC: The histology ranges from infiltrating ductal to medullary [3]. IBC is associated with a unique clinical syndrome that includes a fulminant evolution and extremely poor survival [2, 15]. Distant metastases are found in about 10% to 36% at presentation [2, 4].
Conclusions
Whole-body FDG-PET scans can characterize the extent of the pathologic involvement in inflammatory breast carcinoma. It can also map the lymphatic system, detect the presence of skeletal metastases, and monitor the stages of the response of the primary tumor and the lymphatic and skeletal metastases to chemotherapy.
Currently, the role of PET in breast cancer patients is complementary vis-à-vis other imaging modalities that are less expensive and more widely available; in other words, PET does not replace these well established modalities, particularly mammography. However, it can be helpful in equivocal cases or in the presence of negative radiologic studies in patients with a high clinical probability of metastases. For example, for staging locally advanced breast cancer, PET scanning has been shown to be cost-effective by detecting unexpected sites of metastases, thereby altering patient management. Furthermore, PET scans are extremely useful for detecting functional changes earlier than anatomic studies. However, more comparative studies are needed to define the exact role of PET in a well established clinical pathway.
Résumé.
Le cancer du sein en poussée évolutive (PEV) inflammatoire est la forme la plus agressive du cancer du sein localement avancé. Le diagnostic est soit clinique soit anatomopathologique. Nous avons évalué l’utilité du PET scan au fluorodésoxyglucose 18 (FDG-PET) pour le diagnostic et le staging du cancer du sein en PEV. Nous avons revu rétrospectivement les dossiers médicaux de sept patientes consécutives porteuses de PEV qui ont eu un FDG-PET dans leur bilan initial. Quatre patientes ont eu un FDG-PET après chimiothérapie. Toutes les patientes avaient un sein globalement augmenté de volume, rouge et siège d’un phénomène de peau d’orange pendant 1-5 mois. Quatre patientes avaient en plus une masse palpable et trois avaient une augmentation de volume d’adénopathies axillaires. A la mammographie, on a vu une augmentation diffuse de la densité parenchymateuse et un épaississement de la peau chez 85% des patientes et une distorsion du parenchyme chez 43%. Il n’y avait aucune évidence de métastases à distance sur la tomodensitométrie du thorax ou de l’abdomen. L’examen anatomopathologique de la biopsie du sein a montré un carcinome canalaire infiltrant chez 6 patientes et un carcinome de type lobulaire chez l’autre. Toutes les patientes avaient un FDG-PET du corps entier qui montrait une prise du produit diffuse au niveau du sein avec des foyers intenses surajoutés au niveau de la tumeur primitive. En plus on a trouvé une prise de produit au niveau de la peau chez 100% des patientes, des adénopathies axillaires chez 85% et des métastases osseuses chez 14% des patientes, confirmés par la scintigraphie osseuse. Le FDG-PET post-chimiothérapie a montré une réponse positive au niveau de la tumeur primitive, des adénopathies axillaires et des métastases osseuses. Le FDG PET scan est utile en cas de PEV car il reflète l’intensité pathologique de la maladie, notamment au niveau du sein et au niveau des lymphatiques de la peau. Il peut également être utile pour détecter la présence de ganglions lymphatiques et des métastases osseuses afin de définir l’étendue de la maladie localement et à distance.
Resumen.
El cáncer inflamatorio de mama (IBC) constituye la forma localmente más agresiva del cáncer avanzado de mama. Su diagnóstico se fundamenta tanto en los hallazgos clínicos como anatomopatológicos. Valoramos la eficacia de la tomografía con positrones previa administración de 18-fluordeoxiglucosa (FDG-PET) en el diagnóstico y estadificación del IBC. Se analizan retrospectivamente las historias clínicas de 7 pacientes con IBC en los que se efectuó, en estadios iniciales una FDG-PET; en cuatro se realizó un seguimiento con tomografía PET tras quimioterapia. Los 7 pacientes presentaban aumento difuso del tamaño de la mama, enrojecimiento y piel de naranja desde hacía 1 a 5 meses. A la palpación, en 4 pacientes, se constató tumor mamario y en 3, ganglios linfáticos axilares. La mamografía mostraba un aumento difuso de la densidad del parénquima mamario y engrosamiento de la piel en el 85% de los casos; en el 43% se observaron deformidades del parénquima. La tomografía axial computarizada (CT) no evidenció metástasis a distancia ni en tórax ni en abdomen. La biopsia reveló en 6 pacientes un carcinoma ductal infiltrante y en 1 caso cáncer de tipo lobular. Todos los pacientes fueron tratados preoperatoriamente con quimioterapia; la tomografía con PET mostró una difusa hipercaptación de FDG en la mama, con focos de mayor intensidad en el tumor primario; hipercaptación en la piel en el 100% de los casos, en las adenopatías linfáticas en el 85% y en el 14% en metástasis óseas que fueron confirmadas mediante escintigrafía ósea. La FDG-PET post quimioterapia realizada en 4 pacientes confirmó una regresión tanto del tumor primario como de los ganglios metastásicos y de las metástasis óseas. La tomografía FDG-PET es útil en el diagnóstico de los cánceres inflamatorios de mama pues permite evaluar su extensión no sólo en el parénquima mamario sino también el grado de infiltración linfática de la piel; además, detecta las metástasis ganglionares y óseas. Por consiguiente, dicha exploración permite valorar tanto la afectación local como la producida a distancia.
References
JL Glass RC Frazee (1995) ArticleTitleInflammatory breast cancer Am. Surg. 61 121–124 Occurrence Handle1:STN:280:ByqC28%2Fjs1E%3D
MJ Lopez KA Porter (1996) ArticleTitleInflammatory breast cancer: special problems in breast cancer therapy Surg. Clin. North Am. 76 411–429 Occurrence Handle1:STN:280:BymC1M3gt10%3D
SR Chittoor SM Swain (1998) Locally advanced breast carcinoma: role of medical of oncology KI Bland EM Copeland (Eds) The Breast, Comprehensive Management of Benign and Malignant Diseases EditionNumber2nd edition Saunders Philadelphia 1277–1298
Fleming RYD, Singletary SE (1999) Inflammatory Breast Cancer, M. D Anderson Solid Tumor Oncology Series, Springer, New York, pp 294–305
N Avril J Dose F Jônicke et al. (1996) ArticleTitleMetabolic characterization of breast tumors with positron emission tomography using F-18 fluorodeoxyglucose J. Clin. Oncol. 14 1848–1857 Occurrence Handle1:STN:280:BymB2cznsVc%3D
T Jansson JE Westlin H Ahlström et al. (1995) ArticleTitlePositron emission tomography studies in patients with locally advanced and/or metastatic breast cancer: a method for early therapy evaluation? J. Clin. Oncol. 13 1470–1477 Occurrence Handle1:STN:280:ByqB2s7islY%3D
RA Brito V Valero AU Buzdar et al. (2001) ArticleTitleLong term results of combined modality therapy for locally advanced breast cancer with ipsilateral supraclavicular metastases: the University of Texas M. D. Anderson Cancer Center experience J. Clin. Oncol. 19 628–633 Occurrence Handle1:CAS:528:DC%2BD3MXhtlGrtrg%3D
IC Smith AE Welch AW Hutcheon et al. (2000) ArticleTitlePositron emission tomography using [18F]-fluorodeoxy-d-glucose to predict the pathologic response of breast cancer to primary chemotherapy J. Clin. Oncol. 18 1676–1688 Occurrence Handle1:STN:280:DC%2BD3c3islyjsg%3D%3D
FL Flanagan F Dehdashti BA Siegel (1998) ArticleTitlePET in breast cancer Semin. Nucl. Med. 28 290–302 Occurrence Handle1:STN:280:DyaK1M%2Fhs1Kiug%3D%3D
RL Wahl MA Helvie AE Chang et al. (1994) ArticleTitleDetection of breast mass after augmentation mammoplasty using 18-fluorodeoxyglucose-PET J. Nucl. Med. 35 872 Occurrence Handle1:STN:280:ByuB3s7ivVY%3D
RL Wahl K Zasadny M Helvie et al. (1993) ArticleTitleMetabolic monitoring of breast cancer chemotherapy using positron emission tomography: initial evaluation J. Clin. Oncol. 11 2101–2111 Occurrence Handle1:STN:280:ByuD2crotFc%3D
M Schelling N Avril J Nôhrig et al. (2000) ArticleTitlePositron emission tomography using [18F]fluorodeoxyglucose for monitoring primary chemotherapy in breast cancer J. Clin. Oncol. 18 1689–1695 Occurrence Handle1:STN:280:DC%2BD3c3islyjsw%3D%3D
F Dehdashti F Flanagan JE Moretimer et al. (1999) ArticleTitlePositron emission tomography assessment of “metabolic flare” to predict response of metastatic breast cancer to antiestrogen therapy Eur. J. Nucl. Med. 26 51–56 Occurrence Handle1:CAS:528:DyaK1cXnvFSis70%3D
Rosen PP (1997) Unusual Clinical Presentations of Carcinoma: Rosen’s Breast Pathology, Lippincott-Raven, Philadelphia, pp 567–595
AA Tardivon J Viala AC Rudelli et al. (1997) ArticleTitleMammographic patterns of inflammatory breast carcinoma: a retrospective study of 92 cases Eur. J. Radiol. 24 124–130 Occurrence Handle1:STN:280:ByiB2M7jslY%3D Occurrence Handle9097054
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Baslaim, M., Bakheet, S., Bakheet, R. et al. 18-Fluorodeoxyglucose-Positron Emission Tomography in Inflammatory Breast Cancer . World J. Surg. 27, 1099–1104 (2003). https://doi.org/10.1007/s00268-003-6893-z
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00268-003-6893-z