Abstract
Growth of invasive, opportunistic plants (i.e. lampenflora) in popular tour caves is a significant concern for land managers worldwide. Numerous chemicals at various concentrations have been utilized to remove phototrophic lampenflora colonizing artificially lit surfaces within these caves; however formulations, effectiveness, and impacts appear anecdotal and temporally limited. At Crystal Cave, Sequoia National Park, California, we study lampenflora and cave springtail (Tomocerus celsus) response to a single 0.05 ml/cm2 dose of 1.0% sodium hypochlorite, 0.5% sodium hypochlorite, and 15.0% hydrogen peroxide compared to no treatment over the course of one year. Additionally, we explore potential food web impacts resulting from invasive lampenflora in naturally oligotrophic caves by utilizing stable isotope analysis of T. celsus found on and off lampenflora. Time-effect decay models indicate 1.0 and 0.5% sodium hypochlorite effectively eliminate lampenflora in 11 and 21 days, respectively, while lampenflora decay projections exceed 600 days with 15.0% hydrogen peroxide treatment. Repeat surveys of T. celsus indicate a negative response to 1.0% sodium hypochlorite (P = 0.02), and the probability of observing T. celsus was inversely related to the effectiveness of each treatment. Further, T. celsus had similar diets regardless of their lampenflora association (P = 0.92). We conclude that treatments of sodium hypochlorite at or below 0.5% achieve management goals with limited impacts to the presence or diet of a common cave-adapted indicator species.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Globally, caves are an irreplaceable landscape feature valued for their unique archeological, geological, and biological resources (Gallao and Bichuette 2015; Hildreth-Werker and Werker 2006). Cave environments provide considerable insight into current conservation issues such as understanding deadly novel zoonotic pathogens (Lorch et al. 2013) or reconstructing past ecological and climatic conditions using well-preserved rodent middens, sediments, and speleothems (Betancourt et al. 1990; McCabe-Glynn et al. 2013; Mead et al. 2006; Mead and Phillips III 1981). For reasons such as these, caves are afforded special protection, such as the Federal Cave Protection Act of 1988. However, concurrent goals of facilitating cave recreation for public enjoyment may lead to the degradation of the unique cave resources land managers intend to protect. One of the most visible and prominent biological alterations in recreational tour caves is the introduction or proliferation of phototrophic organisms growing near light installations. Used to enhance visibility for esthetic value and tour safety, artificial cave lighting can inadvertently introduce invasive growth of algae, cyanobacteria, diatoms, ferns, lichens and moss, i.e. lampenflora (Cigna 2011; Mulec and Kosi 2009; Piano et al. 2015). The phototrophic biofilm associated with plants near cave lights can forever change the appearance of cave formations to a green or dirty brown appearance when enveloped by CaCO3 (Mulec and Kosi 2009). While shifts in community composition of native cave microorganisms and invertebrates have not been measured in relation to invasive lampenflora, it is a possible response given the prominence and diversity of colonized lampenflora (Castello 2014; Smith and Olson 2007) in an otherwise highly specialized and oligotrophic habitat (Mulec et al. 2008; Piano et al. 2015; Silva et al. 2015).
Fortunately, lampenflora growth in tour caves can be reduced or eliminated through specific management actions like limiting the duration that lights are used and replacing broad spectrum lights with narrow wavelength, low intensity light bulbs (Olson 2006). However, this cost restrictive method can still leave legacy lampenflora present long after photosynthesis-restricting light bulbs are installed. Active lampenflora cleaning methods are usually necessary and previous attempts have consisted of manual (e.g., scrubbing, scraping, and steam cleaning), and chemical approaches, e.g., sodium hypochlorite (NaClO), hydrogen peroxide (H2O2) (Cigna 2011; Gillieson 2011; Grobbelaar 2000; Mulec and Kosi 2009). Steam cleaning and other manual removal methods may damage formations and unintentionally disperse lampenflora microorganisms (Gillieson 2011; Mulec and Kosi 2009). Additionally, these cleaning techniques are labor intensive and may not be feasible for large restoration projects.
Chemical methods such as the recommended solutions of 5 and 10% NaClO are very effective at killing lampenflora, rapidly improving cave esthetics with minimal effort (Iliopoulou-Georgoudaki et al. 1993). Although effective as an algicidal agent, NaClO treatments may harm non-target species, alter substrate, release harmful by-products, and fail to exterminate microflora in substrate cavities (Faimon et al. 2003; Iliopoulou-Georgoudaki et al. 1993; Mulec and Kosi 2009). H2O2 treatments are considered to be an environmentally friendly surrogate to NaClO. However, its effectiveness is inconclusive, since H2O2 treatments can result in immediate lampenflora recolonization, requiring frequent reapplication for successful eradication (Faimon et al. 2003; Olson 2006). H2O2 is also highly corrosive and may dissolve cave bedrock if not saturated with calcite prior to application (Faimon et al. 2003).
One popular tour cave with an ongoing lampenflora problem is Crystal Cave in Sequoia National Park, California. Crystal Cave has been operating with an artificial lighting system for 75 years (Despain 2003). As the only tour cave within the park, there is high recreational demand, resulting in nearly 60,000 visitors a year (Wightman 2015). While most broad spectrum lights in Crystal Cave were replaced with low spectrum LED lights in 2010, continued growth and/or presence of legacy lampenflora persists throughout the touring portion of the cave. In order to restore Crystal Cave to its historical oligotrophic condition, active lampenflora removal methods are needed that consider its cultural, esthetic, biological, and geological resources. While the current guidance favors corrective lighting over chemical treatment, there is little consideration to removing legacy lampenflora that exists after corrective lighting is installed (Cigna 2011; Faimon et al. 2003; Mulec and Kosi 2009; Olson 2006). Additionally, the recommended treatments and solutions of those chemical treatments are often not tested against each other in field conditions, and do not identify or measure the longer term environmental impacts and efficacy of treatment. Further, since lampenflora present a potential artificial food and nutrient source in a nutrient deprived system, it is unknown whether cave adapted fauna are using it to supplement or supplant their natural food sources. Therefore, the purpose of the present study was to 1) determine what commonly recommended chemical concentration effectively removes legacy lampenflora in Crystal Cave; 2) identify how treatments from each chemical affect the probability of observing a common cave invertebrate, the springtail (Tomocerus celsus); and 3) understand the food web dynamics of T. celsus inhabiting areas with and without lampenflora.
Methods
Study Site
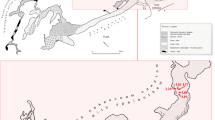
Crystal Cave is a popular tour cave in Sequoia National Park, CA, with 4.8 km of mapped passage, including 550 meters of paved trail within the cave (Fig. 1). The main entrance is located at 1386 m elevation, on a south facing slope. A subterranean creek, which is visible along some portions of the paved tour trail, connects the Yucca Creek and Cascade Creek watersheds and is responsible for cave development. Crystal Cave is located within coarsely crystalline, vertically bedded marble of the accreted Kings Terrane, in which a few marine fossils of Early Jurassic age have been identified (Moore 2000). Shallow marine sediments, including limestone, were uplifted beginning in the late Cenozoic and have since been subjected to metamorphism and erosion yielding the isolated metamorphic pendants, including the prominent northwest-southeast trending karst belts, that we observe today (Stock et al. 2004). Minimum age estimates for the formation of Crystal Cave range from 1.2 to 0.65 Ma based upon isotopic analysis of cave deposits (Despain and Stock 2005; Stock et al. 2005).
A map of Crystal Cave in Sequoia National Park, Tulare County, CA. The map inset displays the junction room in Crystal Cave, which is one of 9 rooms included in this study. This map depicts lampenflora locations and nearby lights. Also included are the experimental plots, which are grouped in blocks of 4 plots each. The experimental plots were visited 9 times over the course of one year and surveyed for lampenflora and cave springtails (Tomocerus celsus). Cave springtails were also collected from untreated lampenflora areas (green shapes without plots) and all areas of the cave without lampenflora (no green) for SIA
As a tour cave, Crystal Cave requires infrastructure such as paved trails, railings, and lights to facilitate public access. The 117 lights along the tour loop are used solely during the public touring season, which operates from approximately May through November. During the six month tour season, lights are turned on and off intermittently, totaling approximately 8 h a day, as visitors move between rooms. During the six months the cave is closed, the lights remain off at all times. (Wightman 2015).
Experimental Design
Lampenflora mapping
In March 2015, using a georeferenced Crystal Cave basemap in the Arc Collector application (ESRI Inc.), the perimeter of all lampenflora areas were mapped along the touring portion of the cave (Fig. 1). These 92 unique lampenflora areas mapped were all associated with nearby lights, and likely consisted of lampenflora supported by decades of using artificial light sources. For this study, lampenflora species composition was not recorded, although prior research indicates the visible green-tinted biofilm typically consists of algae, diatoms, and cyanobacteria (Cennamo et al. 2012; Piano et al. 2015).
Lampenflora treatments
Twenty lampenflora study blocks were randomly selected from the 92 mapped lampenflora locations to measure the efficacy of four chemicals/concentration treatments—1.0% NaClO, 0.5% NaClO, 15% H202, and no treatment. To mitigate the environmental impacts related to NaClO, we chose concentrations one order of magnitude below recommended concentrations (Iliopoulou-Georgoudaki et al. 1993; Mulec and Kosi 2009), and for H202, we chose the concentration found effective at removing lampenflora in a previous study (Faimon et al. 2003). Each of the 20 study blocks contained four circular plots (A = 345 cm2), The 80 plots received one of the four treatments, randomly shuffled so that no common neighbor pattern existed. Edge effects among plots within a block were eliminated by ensuring that the treatment runoff and overspray from one plot did not enter a nearby plot. On April 22, 2015 each plot was dosed evenly with 0.05 ml/cm2 of prepared treatment using a calibrated spray bottle. Treatments were applied through a tube (L = 35 cm, A = 345 cm2) that enforced consistent spray distances and reduced spray drift. NaClO concentrations were derived using the active ingredient of bleach (Clorox, Inc.), and the H202 concentration was derived from 30% reagent grade H202 (Fisher BioReagents BP2633). All solutions were diluted using deionized water.
Treatment efficacy and effects
The effectiveness of each treatment on lampenflora removal was determined by placing a gridded circular plot reader (A = 345 cm2) over each plot and visually counting the number of grid cells out of 64 that contained green biofilm.
To investigate the impact of each treatment on the cave invertebrate community, we elected to survey for a common indicator species in each plot. To determine which indicator species would be chosen for this goal, we first conducted opportunistic terrestrial invertebrate searches in March 2015. These pre-project surveys were limited to the 92 lampenflora areas, and compared well with National Park Service time-constrained invertebrate surveys from 2003–2004, which revealed that cave springtails (Tomocerus celsus) were the most abundant identifiable species in Crystal Cave (NPS 2005). Once T. celsus was established as an indicator species, we conducted targeted searches in the study area, constrained by the perimeter of the circular plot, and completed along with the lampenflora surveys.
Prior to the April 22, 2015 plot treatments, all 80 plots were visited to conduct a survey of lampenflora and T. celsus. Each plot was then visited 9 more times after treatments over the course of a year to conduct repeated lampenflora and T. celsus surveys. These repeated surveys were completed on days 7, 14, 21, 68, 98, 126, 152, 211, and 371 after treatment.
Stable isotope analysis
Since T. celsus were found to be the most common invertebrate during pre-project surveys, and since many species of Collembola are feeding generalists (Hopkin 1997), it suggested there might be either a habitat or diet association with the lampenflora. Therefore, stable isotope analysis (SIA) was used to examine whether T. celsus found on lampenflora had a diet that differed from those found elsewhere in the cave. SIA is a commonly used technique for determining diet composition by measuring the naturally occurring differences in isotopic ratios (eg., C13:C12 or N15:N14) in the tissue of food sources, which are transmitted in predictable ways to the organisms which consume them (Peterson and Fry 1987; Vander Zanden and Vadeboncoeur 2002). C13 and N15 were selected for analysis because they have been employed successfully in previous cave studies and are among the most commonly analyzed elements in SIA of food webs. This method has been used to identify niche partitioning and trophic level in cave dwelling invertebrates (Hutchins et al. 2014) and springtails (Endlweber et al. 2009; Scheu and Folger 2004).
T. celsus were captured opportunistically from untreated lampenflora areas and areas without lampenflora in nine rooms throughout the cave. All samples were placed in aluminum drying tins within 4 h of being collected and dried for 12–24 h in a drying oven at 55 °C. Samples were then ground using a glass mortar and pestle and placed in tin capsules. To obtain a sufficient amount of tissue for SIA, we consolidated several individual T. celsus into one sample based on similar collection location (i.e. cave room, microhabitat) and lampenflora presence. The combined samples were then analyzed for the ratio of C13 (δ 13 C) and N15 (δ 15 196 N) by the University of California, Davis Stable Isotope Facility using an isotope ratio mass spectrometer.
Statistical Analysis
Lampenflora counts from each survey visit were used to determine the dose-response relationship with each one-time treatment over the course of a year. To complete this, we chose to compare the exponential decay model curves among each treatment type. Within R Statistical Software (R Core Team 2015), we used a three-parameter exponential decay model with a mean function and a non-zero lower limit defined because complete decay of lampenflora was not achieved with any treatment at any time. The dose-response exponential decay model (drm function in the drc package) tested how the average number of cells containing lampenflora changed over time (days) and among the four treatments (C. Ritz 2005). The estimated decay (ED) command was then used to determine the time after treatment at which 90% of the lampenflora decay occurred. Briefly, the ED command allows the user to define a point on the fitted exponential decay curve for each treatment, and return the time at which that value is achieved. For this study, we chose 90% as a biologically relevant dose since the primary project goal was to find the most effective chemical agent to completely remove lampenflora. We then used selectivity indices (SI command in drc package), which compares the relative differences of the exponential decay curve at an ED value of interest (Knezevic et al. 2007). Again, 90% (i.e. ED90) lampenflora decay was chosen for the comparison among dose-response curves so we could statistically test the differences between treatment types when our goal of near lampenflora removal would be achieved (Knezevic et al. 2007).
A generalized linear mixed effects model (glmer) was used to model the probability T. celsus was observed in each treatment plot over time (Bates et al. 2014). This mixed-effects logistic regression model was appropriate to utilize given that our response variable was binary, and we had a traditional blocked study design that resulted in nested, hierarchal data. Further, the glmer is a logistic regression model that accounts for random variation in a data set. Therefore, the experimental block was identified as the random effect to account for the block specific sources of random variation likely from unmeasured environmental variables (i.e. substrate, microhabitat, and microclimate) that are known to influence cave communities (Piano et al. 2015). The presence of T. celsus (Yes = 1, N = 0) was the response variable, and the treatment type and the time since treatment (d) were the fixed effects. The days since treatment were rescaled to the center to reduce the model eigenvalue.
For the SIA, paired t-tests were run to test group similarity. The t-test was run for each isotopic signature (δ13C and δ15N) found in T. celsus collected on and off lampenflora. Statistical significance was determined at P ≤ 0.05.
Results
Treatment Efficacy
The modeled estimated effective dose at which 90% (ED90) of the lampenflora is controlled with 1% NaClO is 11.3 ( ± 4.8) days, followed by 0.5% NaClO at 20.7 ( ± 8.4) days (Fig. 2). The ED90 for 15% H202 is 6.1 × 103 ( ± 23.0) days, and 8.0 × 10101 ( ± 23.0) days for the untreated plots (Fig. 2). There were no significant differences between the relative potencies at ED90 between both NaClO treatments (P = 0.44). However, relative ED90 comparisons of all other treatment combinations were significantly different from each other (P < 0.05; Table 1).
Treatment Effects
The probability of observing a T. celsus on an experimental plot was low ( < 15%) across all treatment types and study duration. There was a negative relationship between T. celsus observations and the days after treatment, but this effect was not significant (estimate and standard error; est = 0.15, se = 0.13, P = 0.24; Fig. 3). The probability of observing T. celsus was highest in the H2O2 plots during the first year after treatments. This was not significantly different from the plots receiving no treatment (est = −0.05, se = 0.32, P = 0.87; Fig. 3) and 0.5% NaClO (est = −0.60, se = 0.35, P = 0.09; Fig. 3), but significantly higher from those receiving 1.0% NaClO (est = −0.85, se = 0.37, P = 0.02; Fig. 3).
Generalized linear mixed effects model results depicting the probability of observing a cave springtail (Tomocerus celsus) before and after chemical treatments at Crystal Cave in Sequoia National Park, CA. The x-axis represents the period of monitoring after treatments. The short vertical tick marks on the x-axis represent actual observation days
Stable Isotope Analysis
The isotopic signatures of T. celsus collected on and off lampenflora showed no significant difference in mean δ13C (p = 0.92) or δ15N (p = 0.98; Table 2).
Discussion
Despite ostensibly conclusive guidance with recommendations for removing and reducing invasive lampenflora in caves, the present study is the first to apply empirical methods that compare the effectiveness and biological impact of two common treatments at relevant active ingredient mixtures. To the best of our knowledge, previously endorsed NaClO treatment concentrations were derived arbitrarily, and detrimental effects of its use for lampenflora control are theoretical and anecdotal (Faimon et al. 2003; Iliopoulou-Georgoudaki et al. 1993). Further, the recommended treatment concentrations of 5 and 10% NaClO appear incorrect and excessive since this would be akin to treating lampenflora with undiluted or even a more concentrated concentration of Clorox, Inc. Regular Bleach (5.25% NaClO). The most widely recommended 15% H2O2 treatment concentration was derived based on a study of two locations within a cave in the Czech Republic (Faimon et al. 2003). The study areas of unknown size were treated with successive concentrations of H2O2, starting with 5% H2O2 and ending with multiple treatments of a 15% concentration (Faimon et al. 2003). It is unknown how successive treatments of strengthening H2O2 concentrations contributed to the lampenflora removal, and the authors assessed success through digital photographs without providing details on how lampenflora die-off was quantified. While these studies are the foundation of lampenflora removal guidelines, many important details remain unanswered. The current study addressed these limitations by isolating the treatment effect on lampenflora surveys through repeat surveys. The biological effect was also investigated using a common indicator species, T. celsus.
Effective Treatment
Both NaClO treatments were effective at removing lampenflora, with the model-ED reached at 11 and 21 days for 1.0 and 0.5% NaClO concentrations, respectively. The success of NaClO is even more notable since the concentrations were at an order of magnitude lower than the recommended guidance. It’s likely that NaClO concentrations less than 0.5% would still be effective at reducing cave lampenflora, albeit with longer decay rates. In vitro, NaClO can reach effective algicidal concentrations at 4 orders of magnitude below the 0.5% NaClO concentration the current study used (Ebenezer et al. 2014).
The recommended concentration of 15% H2O2 was ineffective at reducing lampenflora growth, as the ED was projected to be approximately 2 years after treatment. The current guidance recommends 3 successive H2O2 treatments of increasing concentration for successful removal. This was not adhered to in the present study since this guidance failed to describe the spatial, temporal, and other essential application parameters for achieving lampenflora removal (Faimon et al. 2003). The present study held the application rates and frequency constant across all treatment types and found that H202 plots responded similarly to the untreated controls.
Impact of Treatments
At 0.5−1.0% NaClO, acute mortality of most organisms within a treated area are expected, given that the concentration of NaClO far exceeds the concentration at which 50% (EC50) of all plant and animals documented in the Environmental Protection Agencies ECOTOX database perish (E.P.A. 2016). It is likely that endemic microorganisms, some of which play an active role in cave formation, are also impacted (Barton and Northup 2007). Given its acute toxicity and potential to release harmful byproducts in the cave environment (Faimon et al. 2003), reducing the NaClO concentration while retaining its lampenflora control properties should be attempted. The ultimate cost associated with reduced concentrations of NaClO may be the time at which lampenflora decay is achieved and acute mortality can be mitigated by manually removing invertebrates prior to treatments.
Following the current H2O2 application guidance of frequent treatments could increase the likelihood of direct mortality events of preferential cave organisms. In addition, multiple H2O2 treatments would also increase the occupational risk since it was the only treatment that resulted in skin irritations of those conducting the experiment despite the use of DuPont Tyvek suits and respirators.
T. celsus was selected as an indicator species since it is a common and widespread representative of the terrestrial invertebrate community in Crystal Cave. Collembolan springtails have been used prominently as indicators of ecosystems (Greenslade 2007), including for landscape disturbances such as prescribed fire, metal pollution in soil, and in situ pesticide effects (Bargmann et al. 2016; Frampton 1997; Kim and An 2014). For the present study, repeat T. celsus observations were modeled to determine the probability of observing an individual before, and up to a year, after treatments. To help isolate the treatment effect, a generalized linear mixed effect model was able to account for random variation from unique microhabitat and microclimates unmeasured in each experimental block. While the probability of observing T. celsus remained low and declining throughout the year for all treatment types, the 1% NaClO treatment had the greatest negative effect on observing T. celsus, followed by 0.5% NaClO. The effect of 1.0% NaClO was statistically significant from H2O2 and the untreated plots, but this effect was also present during the initial surveys prior to the treatments. Nonetheless, the probability of observing T. celsus was inversely related to the success of treatment in reducing lampenflora.
It is unknown exactly why T. celsus observations were lower for the NaClO treatments, but it could be due to lingering effects from direct mortality during treatments, persistent toxicity from NaClO degradation by-products (Faimon et al. 2003), or a shift in habitat and diet if T. celsus were preferentially consuming the biofilm constituents within the lampenflora. To address the latter issue, SIA was deployed. The nitrogen and carbon isotopic signatures of T. celsus found on lampenflora and those found in other locations in the cave were highly similar, indicating that the two populations likely do not have significantly different diets. SIA therefore provided no indication that lampenflora are acting as an important or preferential food resource for T. celsus.
Conclusion
Caves and other karst features play critical but largely unheralded roles in modern society. Not only do they host distinct and locally endemic organisms and beautiful speleothems, it is estimated that as much as 40% of the United States population receives drinking water from karst aquifers (Quinlan and Ewers 1989). For all of their importance, karst environments are extremely vulnerable to anthropogenic impacts (Green et al. 2006). Accordingly, land managers play an important role in educating the public about these critical resources. Utilizing the allure of the mysterious underground environment to attract visitors, important conservation messages can be conveyed to a receptive public. This comes at a significant cost to heavily visited tour caves that are subjected to high levels of anthropogenic inputs—from shed hair and skin, to diseases such as White-Nose Syndrome that has devastated bat populations (Lorch et al. 2013), to infrastructure “improvements” such as artificial lighting (Cigna 2011). In all cases, land managers must weigh potential impacts and benefits.
Having acknowledged the impacts of artificial light sources in Crystal Cave, this study was conducted to identify effective lampenflora treatment options. Concentrations of 0.5 and 1% NaClO were both found to be effective at removing lampenflora in a relatively short period of time, while H2O2 had limited effect. Our results suggest that the ubiquitous T. celsus were impacted to a small degree by NaClO treatments although the mechanisms of action remain uncertain. Finally, there was no evidence indicating a lampenflora-induced alteration of the subterranean food chain.
Before treatment, managers should weigh potential impacts against species abundance and the ratio of impacted to unimpacted cave area. In this study, invertebrate surveys found that highly abundant species tended to inhabit lampenflora areas, treated areas around artificial light sources represented a small fraction of the potential habitat, and SIA indicated that the most common species, T. celsus, did not rely on a diet closely linked with lampenflora. Furthermore, the treated areas had high air-exchange rates and receive abundant inputs of ground water allowing for the natural removal of contaminants. While low intensity lights are clearly needed to abate aggressive lampenflora growth, future studies should explore the minimum concentration of NaClO needed to achieve effective removal of legacy lampenflora and determine what, if any, routine maintenance treatments are needed to inhibit recolonization.
References
Bargmann T, Heegaard E, Hatteland BA, Chipperfield JD, Grytnes JA (2016) Species trait selection along a prescribed fire chronosequence. Insect Conserv Diver 9(5):446–455
Barton HA, Northup DE (2007) Geomicrobiology in cave environments: past, current and future perspectives. J Cave Karst Stud 69(1):163–178
Bates D, Mächler M, Bolker B, Walker S (2014) Fitting linear mixed-effects models using lme4. arXiv preprint arXiv:14065823
Betancourt JL, Van Devender TR, Martin PS (1990) Packrat middens: the last 40,000 years of biotic change. University of Arizona Press, Tucson, AZ
Ritz C, Streibig JC (2005) Bioassay analysis using R. J Stat Softw. 12(5):1–22.
Castello M (2014) Species diversity of bryophytes and ferns of lampenflora in grotta gigante (ne italy)/vrstna raznolikost mahov in praproti lampenflore v veliki jami v briscikih (sv italija). Acta Cardiol 43(1):185
Cennamo P, Marzano C, Ciniglia C, Pinto G, Cappelletti P, Caputo P, Pollio A (2012) A survey of the algal flora of anthropogenic caves of Campi Flegrei (Naples, Italy) archeological district. J Cave Karst Stud 74(3):243–250
Cigna A (2011) The problem of lampenflora in show caves. In Proc. 6th ISCA Congress, SNC of Slovak Republic, Slovak Caves Administration, pp 201-205
Despain J (2003) Hidden beneath the mountains: the caves of Sequoia and Kings Canyon national parks. Cave Books, Dayton, OH
Despain JD, Stock GM (2005) Geomorphic history of Crystal Cave, Southern Sierra Nevada, California. J Cave Karst Stud 67(2):92–102
Environmental Progection Agency (EPA) (2016) The ECOTOXicology knowledgebase. https://cfpub.epa.gov/ecotox/. Accessed 22 Aug 2016
Ebenezer V, Lim WA, Ki J-S (2014) Effects of the algicides CuSO4 and NaOCl on various physiological parameters in the harmful dinoflagellate Cochlodinium polykrikoides. J Appl Phycol 26(6):2357–2365
Endlweber K, Ruess L, Scheu S (2009) Collembola switch diet in presence of plant roots thereby functioning as herbivores. Soil Biol Biochem 41(6):1151–1154
Faimon J, Štelcl J, Kubešová S, Zimák J (2003) Environmentally acceptable effect of hydrogen peroxide on cave “lamp-flora”, calcite speleothems and limestones. Environ Pollut 122(3):417–422
The Federal Cave Protection Act (1988) vol 16 U.S.C. 4301
Frampton GK (1997) The potential of Collembola as indicators of pesticide usage: evidence and methods from the UK arable ecosystem. Pedobiologia 41(1):179–184
Gallao JE, Bichuette ME (2015) Taxonomic distinctness and conservation of a new high biodiversity subterranean area in Brazil. An Acad Bras Ciênc 87(1):209–217
Gillieson DS (2011) Management of caves. In: van Beynen PE (ed) Karst management. Springer, New York, pp 141–158
Green RT, Painter SL, Sun A, Worthington SR (2006) Groundwater contamination in karst terranes. Water Air Soil Pollut: Focus 6(1–2):157–170
Greenslade P (2007) The potential of Collembola to act as indicators of landscape stress in Australia. Anim Prod Sci 47(4):424–434
Grobbelaar JU (2000) Lithophytic algae: A major threat to the karst formation of show caves. J Appl Phycol 12(3–5):309–315
Hildreth-Werker V, Werker JC (2006) Cave conservation and restoration. National Speleological Society, Huntsville, AL
Hopkin SP (1997) Biology of the springtails:(Insecta: Collembola). Oxford University Press, Oxford, UK
Hutchins BT, Schwartz BF, Nowlin WH (2014) Morphological and trophic specialization in a subterranean amphipod assemblage. Freshwater Biol 59(12):2447–2461
Iliopoulou-Georgoudaki J, Pantazidou A, Theoulakis P (1993) An assessment of cleaning photoautotrophic microflora: the case of Perama cave, Ioannina Greece. Mémoires de Biospéologie 20:117–120
Kim SW, An Y-J (2014) Jumping behavior of the springtail Folsomia candida as a novel soil quality indicator in metal-contaminated soils. Ecol Indic 38:67–71
Knezevic SZ, Streibig JC, Ritz C (2007) Utilizing R software package for dose-response studies: the concept and data analysis. Weed Technol 21(3):840–848
Lorch JM, Muller LK, Russell RE, O’Connor M, Lindner DL, Blehert DS (2013) Distribution and environmental persistence of the causative agent of white-nose syndrome, geomyces destructans, in bat hibernacula of the eastern United States. Appl Environ Microbiol 79(4):1293–1301
McCabe-Glynn S, Johnson KR, Strong C, Berkelhammer M, Sinha A, Cheng H, Edwards RL (2013) Variable North Pacific influence on drought in southwestern North America since AD 854. Nat Geosci 6(8):617–621
Mead JI, McGinnis TW, Keeley JE (2006) A Mid-Holocene Fauna from Bear Den Cave, Sequoia National Park, California. Bull South Calif Acad Sci 105(2):43–58
Mead JI, Phillips III AM (1981) The late Pleistocene and Holocene fauna and flora of Vulture Cave. The Southwestern Naturalist, Grand Canyon, Arizona, pp 257–288
Moore JG (2000) Exploring the highest Sierra. Stanford University Press, Palo Alto, CA
Mulec J, Kosi G (2009) Lampenflora algae and methods of growth control. J Cave Karst Stud 71(2):109–115
Mulec J, Kosi G, Vrhovšek D (2008) Characterization of cave aerophytic algal communities and effects of irradiance levels on production of pigments. J Cave Karst Stud 70(1):3–12
National Park Service (2005) SEKI Cave diversity inventory. Unpublished raw data
Olson R (2006) Control of lamp flora in developed caves. Cave conservation and restoration. National Speleological Society, Huntsville, pp 343–348
Peterson BJ, Fry B (1987) Stable isotopes in ecosystem studies. Annu Rev Ecol Syst 18(1):293–320
Piano E, Bona F, Falasco E, La Morgia V, Badino G, Isaia M (2015) Environmental drivers of phototrophic biofilms in an Alpine show cave (SW-Italian Alps). Sci Total Environ 536:1007–1018
Quinlan JF, Ewers RO (1989) Subsurface drainage in the Mammoth Cave area. In: White WB, White EL (ed) Karst Hydrology. Springer, New York, pp 65–103
Scheu S, Folger M (2004) Single and mixed diets in Collembola: effects on reproduction and stable isotope fractionation. Funct Ecol 18(1):94–102
Silva MS, Martins RP, Ferreira RL (2015) Cave conservation priority index to adopt a rapid protection strategy: a case study in Brazilian Atlantic rain forest. Environ Manage 55(2):279–295
Smith T, Olson R (2007) A taxonomic survey of lamp flora (Algae and Cyanobacteria) in electrically lit passages within Mammoth Cave National Park, Kentucky. Int J Speleol 36(2):6
Stock GM, Anderson RS, Finkel RC (2004) Pace of landscape evolution in the Sierra Nevada, California, revealed by cosmogenic dating of cave sediments. Geology 32(3):193–196
Stock GM, Anderson RS, Finkel RC (2005) Rates of erosion and topographic evolution of the Sierra Nevada, California, inferred from cosmogenic 26Al and 10Be concentrations. Earth Surf Proc Land 30(8):985–1006
R Core Team (2015) R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria
Vander Zanden MJ, Vadeboncoeur Y (2002) Fishes as integrators of benthic and pelagic food webs in lakes. Ecology 83(8):2152–2161
Wightman K (2015) Crystal cave report. Sequioa Parks Conservancy, Three Rivers, CA
Acknowledgements
The authors thank J. Watkins, and A. Esperanza of Sequoia and Kings Canyon National Parks, and K. Wightman and the entire Crystal Cave support staff of the Sequoia Parks Conservancy for supporting the project in many ways. This project was funded by the U.S. National Park Service.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no competing interest.
Rights and permissions
About this article
Cite this article
Meyer, E., Seale, L.D., Permar, B. et al. The Effect of Chemical Treatments on Lampenflora and a Collembola Indicator Species at a Popular Tour Cave in California, USA. Environmental Management 59, 1034–1042 (2017). https://doi.org/10.1007/s00267-017-0842-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00267-017-0842-3