Abstract
The marine dinoflagellate Cochlodinium polykrikoides has spread worldwide and is responsible for harmful algal blooms. The chemical biocides, copper sulfate (CuSO4) and sodium hypochlorite (NaOCl), are known to be effective in removing bloom-forming or biofouling organisms. Here, we assessed the biocidal efficiency and toxicological properties of NaOCl and CuSO4 on the physiological and catalase responses of C. polykrikoides. The endpoints used were cell counts, pigment content, chlorophyll autofluorescence (CAF), and antioxidant catalase (CAT) activity. The test organism showed a dose-dependent decrease in growth rate against the algicides; 72-h median effective concentrations (EC50) were 0.584 and 0.633 mg L–1 for NaOCl and CuSO4, respectively. The decrease in pigment levels and CAF intensity showed that NaOCl and CuSO4 might affect the photosynthetic processes of the exposed cells. Furthermore, a considerable increase in CAT activity in the cells was detected, indicating that the algicides might generate reactive oxygen species, thereby markedly damaging the cells. These results suggest that the test algicides are very effective in removing C. polykrikoides by inducing cellular stress and inhibiting cell recovery at higher concentrations.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Harmful algal blooms (HABs) are significant and pose a growing major threat to public and ecosystem health, affecting fisheries and creating associated economic loss in developed and developing countries (Anderson 2007). Cochlodinium polykrikoides Margalef is a harmful dinoflagellate that is widely distributed in tropical and warm temperate waters throughout the world (Richlen et al. 2010). The species causes fish kills by producing massive amounts of mucous and depleting oxygen supplies (Kim et al. 2000). Consequently, blooms of this species lead to severe economic losses and environmental impacts, particularly in countries such as South Korea and Japan (Kim et al. 2004; Lee 2008). The causative organism spreads through oceanic regions and has recently expanded its geographic range to include Europe, India, Middle Eastern countries, and North America (Kudela and Gobler 2012). Several physical, chemical, and biological mitigation measures have been developed and used by several countries for controlling HABs (Kim 2006). Some chemicals (e.g., copper sulfate (CuSO4), hydrogen peroxide (H2O2), magnesium hydroxide, ozone, and sodium hypochlorite (NaOCl)) with algicidal properties have been used for the control and removal of HABs (Kim et al. 2000; Sengo and Anderson 2004; Qian et al. 2010).
Because they are very cost effective, both CuSO4 and NaOCl have long been used as biocides in the freshwater industry (Haas 1999; USEPA 2009). These chemicals can be used for cleaning swimming pools, on aquaculture farms, and even for removing HABs (Kim et al. 2007). In particular, NaOCl is a strong oxidizing biocide (Hilgren et al. 2007) and reacts with water to form hypochlorous acid (HOCl) (White 1999), which is a strong reactive oxygen species (ROS) (Halliwell 2006) that leads to damage in the physiological and biochemical activities of exposed organisms (Phe et al. 2005), whereas CuSO4 is a non-oxidizing biocide (Gant et al. 2007) that releases Cu(II) ions when dissolved in water. Although copper is considered an essential micronutrient for algal growth, high concentrations can lead to denaturation of nucleic acids, alteration of proteins, and permeabilization of the cell membrane (Verhoeven and Eloff 1979). Moreover, free and excess copper is very toxic because it generates ROS in the cells (Cervantes and Gutierrez-Corona 1994).
Even under optimal conditions, ROS such as superoxide radical (O2 –), H2O2, hydroxyl radical (OH−), and HOCl are generated as by-products of normal metabolism in different subcellular compartments, including chloroplasts, mitochondria, and plasma membranes that are linked to the electron transport systems (Asada 1999; Gómez et al. 2004; Trachootham et al. 2008). However, biotic or abiotic stress might produce an excessive concentration of ROS, resulting in oxidative damage in cells (Lushchak 2011). To mitigate and repair the damage initiated by ROS most organisms, including plants and algae, have developed complex antioxidant systems, such as low molecular weight compounds and antioxidant enzymes (Ogawa 2005). Catalase (CAT), a tetrameric heme-containing enzyme, can catalyze the breakdown of H2O2 to water and molecular oxygen. CAT, along with superoxide dismutase (SOD), represents the first line of defense against free radicals (Radocanović et al. 2010). Considering that algicides induce severe cellular stress to generate high amounts of ROS, the evaluation of antioxidant enzyme activity might provide insights into the mode of action of the biocide chemicals (Trivedi et al. 2012). Moreover, biochemical markers are considered to be sensitive indicators of environmental and subcellular stress under both laboratory and field conditions (Hinton and Lauren 1990).
The effects of potential algicides on algal growth have been analyzed based on measuring the inhibition of algal growth, pigment content, and photosynthetic rate (Gregg and Hallegraef 2007; Song et al. 2010; Viriyatum 2013); however, the possible modes of action of biocides and biochemical responses of the target organisms have not been clearly explained. In this study, we evaluated the efficiency of the oxidizing biocide NaOCl and non-oxidizing biocide CuSO4 in controlling HABs by targeting the harmful dinoflagellate C. polykrikoides, with emphasis on changes in the physiological and antioxidant enzyme CAT activities caused by both the algicides.
Materials and methods
Cell culture, growth conditions and maintenance
Cochlodinium polykrikoides (CP-01) was obtained from the National Fisheries Research and Development Institute (NFRDI) of Korea, cultured in f/2 medium (Guillard and Ryther 1962), and maintained at 20 °C using a 12:12-h light/dark cycle with a photon flux density of 65 μmol photons m−2 s−1.
Preparation and treatment of the algicide
NaOCl was commercially obtained (Cat. No. 425044, Sigma-Aldrich Co., USA), and a stock solution (1,000 mg L−1) was prepared using autoclaved distilled water (ADW). The chlorine (Cl) concentrations in NaOCl were determined spectrophotometrically using diethyl-phenylenediamine (DPD) at 515 nm (APHA 1998). Similarly, CuSO4 (Cat. No. C1297, Sigma, USA) was commercially obtained, and a stock solution (1,000 mg L−1) was prepared using ADW. Copper concentrations from the dissolved CuSO4 were analyzed using a NexION 300X ICP-MS (PerkinElmer, USA).
For the algicide assay, we used exponential growth phase cultures (200 mL) with an initial cell density of 1,300 ± 0.5 × 104 cells mL−1 and individually treated with NaOCl (0.1, 0.5, 1.0, 2.0, and 3.0 mg L−1) and CuSO4 (0.5, 1.0, 2.0, 3.0, and 5.0 mg L−1) at nominal doses and considering the demand of the medium, as determined previously. Control cultures were maintained as per OECD guidelines (OECD 2011). Test doses also considered the concentrations observed in environmental discharges elsewhere (Calderon 2000; Watson and Yanong 2006). Samples were drawn after 0, 6, 12, 24 and 72 h of exposure to the algicides. The cultures were harvested and washed with filtered sterilized seawater prior to the assay.
Cell count and median effective concentration
Cell counts in each test flask were determined using a plankton-counting chamber (HMA-S6117, Matsunami Glass, Japan) and were plotted against the exposure times.
Percent inhibition (or survival percentage) and 72-h median effective concentration (EC50) were calculated, following the recommendation by the Organisation for Economic Co-operation and Development testing guidelines (OECD 2011). Percent inhibition was calculated based on the following equation:
where %I = percent inhibition in average specific growth rate, μC = mean value for μ in the control, and μT = mean value for growth rate in the treated samples.
The 72-h EC50 values were estimated using a sigmoidal dose–response curve and plotted using Origin version 8.5 (MicroCal Software Inc., USA) based on the sigmoidal four-parameter equation (Teisseyre and Mozrzymas 2006):
where a is the response value at 0 or minimum asymptote, b is the response value for infinite concentration or maximum asymptote, c is the midrange point, d is the steepness of the curve or the Hill slope, and x is the dilution coefficient.
Analysis of pigment and chlorophyll autofluorescence
Chlorophyll a (Chl a) and carotenoid (CAR) were measured by concentrating 10 mL of the culture at different time intervals. The pigments were extracted with 90 % acetone after overnight incubation in the dark. The supernatants extracted were measured using a DU730 Life Science UV/vis spectrophotometer (Beckman Coulter, Inc., USA). The Chl a and CAR concentrations were estimated according to Parsons et al. (1984).
Chlorophyll autofluorescence (CAF) was measured using a fluorescent microscope (Axioskop, Carl Zeiss, Germany) at ×400 magnification. An ultraviolet dichroic (G365/395–488 nm) source was used for excitation, and emission was collected by setting the detection bandwidth between 630 and 750 nm. Digital image analysis was performed using ImageJ 1.29× (National Institutes of Health (NIH), USA). Mean fluorescence intensity (MFI) was expressed in terms of pixel gray value, ranging from 0 to 270. The reported MFI values indicate the average MFI values obtained from a minimum of 50 individual cells.
Analysis of catalase activity
CAT activity was measured according to Aebi (1984), which was based on H2O2 degradation by CAT in the samples. Five milliliters of the algal culture was centrifuged at 4,200 rpm for 10 min. Two milliliters of extraction buffer (1.0 M phosphate buffer) was added to the pellet. The cells were homogenized using a Teflon pestle (BelArt F19922-0001, Scienceware, USA) in ice, and then the tube was placed in a water bath at 40 °C for 5 min (modified from Soto et al. 2011) for extraction. The homogenate was centrifuged at 4,200 rpm for 10 min, and the supernatant was collected for the assay. Added to 100 μL of the supernatant were 1.6-mL 1.0 M phosphate buffer, 0.2-mL 0.3 % H2O2, and 3 mM EDTA, and the mixture was shaken well for 3 min. CAT activity was calculated using an extinction coefficient of 0.036 per millimolar per centimeter and calculated per cell. One unit of the enzyme was considered as the amount necessary to decompose 1.0 μL of H2O2 per minute at 25 °C. The absorbance of the supernatant was read at 240 nm in a DU730 Life Science UV/Vis spectrophotometer.
Statistical analysis
All data presented are mean values of triplicates. One-way analysis of variance (ANOVA) with post hoc Dunnett’s multiple comparison test using Graphpad InStat (Graphpad Software, Inc., USA) was used for comparisons between non-treated and treated cultures. P < 0.001 was accepted as significant. The correlation between MFI and Chl a was tested using Pearson’s correlation coefficient (R 2) and an Excel spreadsheet (Microsoft, USA).
Results and discussion
Stability of NaOCl and CuSO4
The concentration of NaOCl measured immediately after adding it to the algal suspension was 15–20 % less than the nominal doses (0.1, 0.5, 1.0, 2.0, and 3.0 mg L−1; Fig. 1). In addition, after 60 min of exposure, the available total residual oxidants (TROs) were from ~10 to 15 % for 2.0 and 3.0 mg L−1, respectively, whereas they were below detectable limit (BDL; >0.01 mg L−1) at lower concentrations. A possible reason is that NaOCl is highly reactive and volatile; it reacts with the inorganic and organic substances present in the medium (Jegatheesan et al. 2009). Therefore, the TRO level or free (chlorine) Cl2 concentration is usually BDL after 1–3 h of exposure (Abdul-Baki 1974). However, Cl2 damages cell membranes immediately (within 15 min) after addition, making the cell unable to regrow (Phe et al. 2005; Patil and Jagadeesan 2011). These merits (i.e., acute effects with little residues left in water) allow the use of Cl2 as a potential algicide for the control of HAB in the environment.
CuSO4 is a non-oxidizing biocide that forms Cu(II) ions, which in turn, form several complexes with the organic and inorganic compounds present in the medium. In addition, CuSO4 is highly soluble in water and is strongly bioaccumulative in cells (García-Villada et al. 2004). In the present study, the measured concentrations of Cu(II) did not show much change from the nominal concentrations (Table 1), suggesting that it persists in the environment (Raman and Cook 1990).
Biocide efficiency and toxic effects
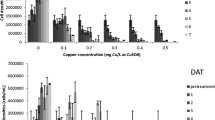
The individual biocidal efficiency of NaOCl and CuSO4 against C. polykrikoides was quantified using percent survivability with the increase in NaOCl and CuSO4 doses (Fig. 2). Percent reduction in cell counts was significant (P < 0.001) over all time intervals for each algicide exposure. Similarly, Chl a and carotenoid levels decreased significantly (P < 0.001) after 6- and 72-h exposures (Fig. 3). Hence, the results clearly show that NaOCl and CuSO4 have detrimental, biocidal effects on the exposed C. polykrikoides cells.
As for numerical comparisons, 72-h EC50 was calculated using growth rates, which were 0.584 mg L−1 for NaOCl and 0.633 mg L−1 for CuSO4. In addition, the minimal effective concentration was estimated by determining EC5, EC10, and EC20 values (Table 2), which represent the minimal algicide concentration that evokes an effect on the test species. The estimates show that C. polykrikoides was highly sensitive to exposure to algicidal agents. The present data are in accordance with those detected from the dinoflagellate Prorocentrum minimum (Guo and Ki 2012; Ebenezer and Ki 2013). Previous reports and our findings suggest that the two tested algicides are highly efficient in controlling dinoflagellate bloom (Han et al. 2001; Jeong et al. 2002; Oliveira-Filho et al. 2004).
Effect of algicides on CAF levels
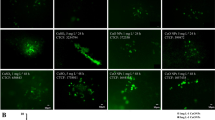
Microalgae exhibit autofluorescence properties resulting from the presence of photosynthetic pigments (Trampe et al. 2011); therefore, measuring CAF has been reported to be a rapid, sensitive method by which the physiological status of microalgae can be assessed (Nancharaiah et al. 2007). In the present study, CAF as MFI per cell decreased significantly (P < 0.05) with the increase in exposure time and algicide dose (Fig. 4). A positive correlation was observed between MFI and Chl a levels for both NaOCl (R 2 = 0.964, P < 0.01) and CuSO4 (R 2 = 0.935, P < 0.01), respectively. After 6- and 12-h exposures, the MFI for 1.0, 2.0, and 3.0 mg L−1 NaOCl decreased significantly (P < 0.05) by approximately 25–46 % compared to that for the control; however, no significant decrease was observed for low doses (0.1 and 0.5 mg L−1). Subsequently, after 24- and 72-h exposures, the percent reduction at 0.1 and 0.5 mg L−1 NaOCl was between 28 and 60 % compared to that for the control (Fig. 4), and no fluorescence was observed for the high doses (1.0–3.0 mg L−1).
As for CuSO4, after 6- and 12-h exposures, the MFI for 2.0, 3.0, and 5.0 mg L−1 showed a significant decrease (P < 0.05) by approximately 10–34 % compared to that for the control (Fig. 4), although the low doses (0.5 and 1.0 mg L−1) showed no significant decrease. However, after 24 and 72 h of exposure, the percent reduction for 0.5 and 1.0 mg L−1 CuSO4 was between 35 and 67 % of that of the control. A previous study by Ebenezer and Ki (2013) showed that the oxidative biocide Cl2, for which the test doses ranged from 1.0 to 3.0 mg L−1, showed a marked reduction of MFI of P. minimum. In addition, Ma et al. (2011) reported that 0.2 mg L−1 of Cl2 completely suppressed the photosynthetic activity of the diatom Phaeodactylum tricornutum. Moreover, CuSO4 showed a significant reduction of CAF in the freshwater microalgae Pseudokirchneriella subcapitata and Chlorella sp. at 0.05–0.25 mg L−1, after a 72-h exposure (Stauber et al. 2002). The bleaching property of NaOCl can affect pigments, especially carotene and proteins related to it (Albrich et al. 1981), whereas CuSO4 interferes with carbon fixation, affecting the photosynthetic apparatus (Stiborová et al. 1986). NaOCl and CuSO4 have been widely used as algicides; however, the mode of toxicity of each chemical is different. Nevertheless, NaOCl and CuSO4 might affect the photosynthetic efficiency of the test dinoflagellates, most likely damaging their photosystems (Oukarroum et al. 2012; Ebenezer and Ki 2013).
Effect of algicides on antioxidant CAT activity
In general, the amount of ROS generated is equal to the amount eliminated in robust cells; however, when the cell undergoes oxidative stress, the ROS concentration within the cell is more than the amount eliminated, which leads to the destruction of cellular mechanisms (Lushchak 2011). The algicides NaOCl and CuSO4 are known to induce ROS production in chemical-exposed cells (Letelier et al. 2005; Halliwell 2006). The deleterious effects of free radicals produced because of ROS generation can be controlled by specific antioxidant systems (Lushchak 2011). CAT is an antioxidant enzyme that decomposes H2O2 to protect cells from ROS damage (Radocanović et al. 2010). In the present study, considerable variations in CAT activity were observed depending on algicide dose and exposure time (Fig. 5). For cells exposed to NaOCl, the CAT activity increased significantly (P < 0.001) by up to seven times at lower doses (0.1 and 0.5 mg L−1) compared to that in the control. An increased activity after 6 h of exposure to NaOCl was observed, but the activity remained stable even after 72 h of exposure. Moreover, higher doses (1.0–3.0 mg L−1) did not show any significant change in CAT activity compared to that for the control. A similar trend was observed in SOD and reduced glutathione (GSH) activities (unpublished data). The increased CAT activity at lower doses could be due to the enzyme’s involvement in degrading ROS; however, at higher doses, increased ROS concentrations could lead to inactivation of antioxidant enzymes (Shao et al. 2008). For this possible reason, the higher doses in the present study might have inactivated the CAT activity. Previous reports also state that low levels (0.05–0.13 mg L−1 NaOCl) induce CAT protein radical formation (Bonini et al. 2007). Similarly, higher levels of HOCl have the ability to destroy CAT (Mahawar et al. 2011), which explains why no CAT activity was observed at high NaOCl doses in the present study.
As for CuSO4, the CAT activity showed a dose-dependent increase after 6 h of exposure and the trend remained the same even after 12 h of exposure (Fig. 5b). However, after 24 and 72 h of exposure, CAT activity showed a marginal increase at lower concentrations (0.5 and 1.0 mg L−1) compared to that of the control, although no significant change in activity was observed at higher doses (2.0, 3.0, and 5.0 mg L−1). It is reported that Cu(II) ions produce H2O2 and superoxide radical through a Haber–Weiss reaction (Santo et al. 2008; Letelier et al. 2005). As CAT is involved in the dissipation of H2O2, an increase in CAT activity after 6 and 12 h of exposure, thus, is explainable; however, at higher concentrations, H2O2 forms a complex with Cu(II) that leads to a break in the DNA, resulting in cell death (Li and Trush 1993). Moreover, Cu(II) ions at concentrations around 1.0 mg L−1 have been found to induce oxidative stress and damage to DNA in the marine dinoflagellate P. minimum (Guo et al. unpublished). In the present study, we could not observe CAT activity after 24 and 72 h of exposure to CuSO4 because of a decrease in the viable cell numbers. The pathways leading to cell death when exposed to the algicides as recorded in the present study is explained using a schematic diagram (Fig. 6).
In conclusion, it is obvious that both NaOCl and CuSO4 are algicidal agents that are highly effective in reducing the C. polykrikoides blooms, as revealed by a marked reduction in growth rate and Chl a levels in the test species. It can also be noted that the Cochlodinium cell count and Chl a levels in C. polykrikoides did not recover following exposure to higher doses of the algicides. From our data, it is clear that NaOCl causes immediate damage to the target cell; hence, the use of this algicide at the wake of a bloom could prevent the bloom from expanding. On the other hand, CuSO4 showed a dose-dependent decrease in cell counts. Copper-based algicides are one of the approved algicides for use in swimming pools and on aquaculture farms according to the World Health Organization (WHO); however, their persistence in the marine environment must be taken in to account. Both algicides induced ROS production and were successful in inactivating antioxidant enzyme activity at higher concentrations; however, these chemicals can also be a threat to non-target organisms, and as such, their presence and concentrations in the marine environment must be continuously monitored.
References
Abdul-Baki AA (1974) Pitfalls in using sodium hypochlorite as a seed disinfectant in 14C incorporation studies. Plant Physiol 53:768–771
Aebi H (1984) Catalase in vitro. Methods Enzymol 105:121–126
Albrich JM, Mccarthy CA, Hurst JK (1981) Biological reactivity of hypochlorous acid: implications for microbicidal mechanisms of leukocyte myeloperoxidase. Proc Natl Acad Sci U S A 78:210–214
American Public Health Association (APHA) (1998) Standard methods for the examination of water and wastewater, 20th edn. American Public Health Association, Washington, DC
Anderson DM (2007) The ecology and oceanography of harmful algal blooms: multidisciplinary approaches to research and management. IOC Technical Series 74, UNESCO 2007, IOC/2007/TS/74. http://lib.ruppin.ac.il/multimedia_michmoret/Anderson_BruunMemorialLecture2005.pdf. Accessed 29 Jan 2014
Asada K (1999) The water-water cycle in chloroplasts: scavenging of active oxygens and dissipation of excess photons. Annu Rev Plant Physiol Plant Mol Biol 50:601–639
Bonini MG, Siraki AG, Bhattacharjee S, Mason RP (2007) Glutathione-induced radical formation on lactoperoxidase does not correlate with the enzyme's peroxidase activity. Free Radic Biol Med 42:985–992
Calderon RL (2000) The epidemiology of chemical contaminants of drinking water. Food Chem Toxicol 38:513–520
Cervantes C, Gutierrez-Corona F (1994) Copper resistance mechanisms in bacteria and fungi. FEMS Microbiol Rev 14:121–137
Ebenezer V, Ki J-S (2013) Physiological and biochemical responses of the marine dinoflagellate Prorocentrum minimum exposed to the oxidizing biocide chlorine. Ecotoxicol Environ Saf 92:129–134
Gant VA, Wren MWD, Rollins MSM, Jeanes A, Hickok SS, Hall TJ (2007) Three novel highly charged copper-based biocides: safety and efficacy against healthcare-associated organisms. J Antimicrob Chemother 62:294–299
García-Villada L, Rico M, Altamirano M, Sánchez-Martín L, Lopez-Rodas V, Costas E (2004) Occurrence of copper resistant mutants in the toxic cyanobacterium Microcystis aeruginosa: characterization and future implications in the use of copper sulphate as an algaecide. Water Res 38:2207–2213
Gómez JM, Jiménez A, Olmos E, Sevilla F (2004) Location and effects of long-term NaCl stress on superoxide dismutase and ascorbate peroxidase isoenzymes of pea (Pisum sativum cv. Puget) chloroplasts. J Exp Bot 55:119–130
Gregg MD, Hallegraef GM (2007) Efficacy of three commercially available ballast water biocides against vegetative microalgae, dinoflagellate cysts and bacteria. Harmful Algae 6:567–584
Guillard RRL, Ryther JH (1962) Studies of marine plankton diatoms. I. Cyclotella nana Hustedt and Detonula covervacea (Cleve) Gran. Can J Microbiol 8:229–239
Guo R, Ki JS (2012) Differential transcription of heat shock protein 90 (HSP90) in the dinoflagellate Prorocentrum minimum by copper and endocrine-disrupting chemicals. Ecotoxicology 21:1448–1457
Halliwell B (2006) Reactive species and antioxidants. Redox biology is a fundamental theme of aerobic life. Plant Physiol 141:312–322
Han FX, Hargreaves JA, Kingery WL, Huggett DB, Schlenk DK (2001) Accumulation, distribution, and toxicity of copper in sediments of catfish ponds receiving periodic copper sulfate applications. J Environ Qual 30:912–919
Haas CN (1999) Disinfection. In: Letterman RD (ed) Water quality and treatment a handbook of community water supplies. American Water Works Association 5th edn. McGraw-Hill, New York, pp 14.1–14.60.
Hilgren J, Swanson KM, Diez-Gonzalez F, Cords B (2007) Inactivation of Bacillus anthracis spores by liquid biocides in the presence of food residue. Appl Environ Microbiol 73:6370–7
Hinton DE, Lauren DJ (1990) Liver structural alterations accompanying chronic toxicity in fishes potential bio-markers of exposure. In: McCarthy JF, Shugart LR (eds) Biomarkers of environmental contamination. Lewis Publishers, Boca Raton, pp 17–57
Jegatheesan V, Kim S-H, Joo CK, Baoyu G (2009) Evaluating the effects of granular and membrane filtrations on chlorine demand in drinking water. J Environ Sci 21:23–29
Jeong HJ, Kim HR, Kim KI, Kim KY, Park KH, Kim ST, Yoo YD, Song JY, Kim JS, Seong KA, Yih WH, Pae SJ, Lee CH, Huh MD, Lee SH (2002) NaOCl produced by electrolysis of natural seawater as a potential method to control marine red tide dinoflagellates. Phycologia 41:643–656
Kim CK, Lee SG, Kim HG (2000) Biochemical responses of fish exposed to a harmful dinoflagellate Cochlodinium polykrikoides. J Exp Mar Biol Ecol 254:131–141
Kim DI, Matsuyama Y, Nagasoe S, Yamaguchi M, Yoon YH, Oshima Y, Imada N, Honjo T (2004) Effects of temperature, salinity and irradiance on the growth of the harmful red tide dinoflagellate Cochlodinium polykrikoides Margalef (Dinophyceae). J Plankton Res 26:61–66
Kim HG (2006) Mitigation and controls of HABs. Ecological Studies 189:327–338
Kim J-G, Kim B, Lee C-G (2007) Alga-lytic activity of Pseudomonas fluorescens against the red tide causing marine alga Heterosigma akashiwo (Raphidophyceae). Biol Control 41:296–303
Kudela RM, Gobler CJ (2012) Harmful dinoflagellate blooms caused by Cochlodinium sp.: global expansion and ecological strategies facilitating bloom formation. Harmful Algae 14:71–86
Lee YS (2008) Utilization of various nitrogen, phosphorus, and selenium compounds by Cochlodinium polykrikoides. J Environ Biol 29:799–804
Letelier ME, Lepe AM, Faundez M, Salazar J, Marin R, Aracena P, Speisky H (2005) Possible mechanisms underlying copper-induced damage in biological membranes leading to cellular toxicity. Chem Biol Interact 151:71–82
Li Y, Trush MA (1993) DNA damage resulting from the oxidation of hydroquinone by copper: role for a Cu(II)/Cu(I)) redox cycle and reactive oxygen generation. Carcinogenesis 14:1303–1311
Lushchak VI (2011) Environmentally induced oxidative stress in aquatic animals. Aquat Toxicol 101:13–30
Ma Z, Gao K, Li W, Xu Z, Lin H, Zheng Y (2011) Impacts of chlorination and heat shocks on growth, pigments and photosynthesis of Phaeodactylum tricornutum (Bacillariophyceae). J Exp Mar Biol Ecol 397:214–219
Mahawar M, Tran V, Sharp JS, Maier RJ (2011) Synergistic roles of Helicobacter pylori methionine sulfoxide reductase and GroEL in repairing oxidant-damaged catalase. J Biol Chem 286:19159–19169
Nancharaiah YV, Rajadurai M, Venugopalan VP (2007) Single cell level microalgal ecotoxicity assessment by confocal laser scanning microscopy and digital image analysis. Environ Sci Technol 41:2617–2621
Ogawa K (2005) Glutathione - associated regulation of plant growth and stress responses. Antioxid Redox Signal 7:973–981
Oliveira-Filho ECD, Lopes RM, Paumgartten FJER (2004) Comparative study on the susceptibility of freshwater species to copper-based pesticides. Chemosphere 56:369–374
Organisation for Economic Cooperation and Development (OECD) (2011) OECD guidelines for the testing of chemicals, Test no. 201: freshwater algal and cyanobacteria growth inhibition test. OECD Publications, Paris
Oukarroum A, Perreault F, Popovic R (2012) Interactive effects of temperature and copper on photosystem II photochemistry in Chlorella vulgaris. J Photochem Photobiol B 110:9–14
Parsons TR, Maita Y, Lalli CM (1984) A manual of chemical and biological methods for seawater analysis. Pergamon Press, Oxford, p 84
Patil JS, Jagadeesan V (2011) Effect of chlorination on the development of marine biofilms dominated by diatoms. Biofouling 27:241–254
Phe MH, Dossot M, Guilloteau H, Block JC (2005) Nucleic acid fluorochromes and flow cytometry prove useful in assessing the effect of chlorination on drinking water bacteria. Water Res 39:3618–3628
Qian H, Yu S, Sun Z, Xie X, Liu W, Fu Z (2010) Effects of copper sulfate, hydrogen peroxide and N-phenyl-2-naphthylamine on oxidative stress and the expression of genes involved photosynthesis and microcystin disposition in Microcystis aeruginosa. Aquat Toxicol 99:405–12
Radocanović TB, Borković-Mitić SS, Perendija BR, Despotović SG, Pavlović SZ, Cakić PD, Saičić ZS (2010) Superoxide dismutase and catalase activities in the liver and muscle of barbel (Barbus barbus) and its intestinal parasite (Pomphoryinchus laevis) from the Danube river, Serbia. Arch Biol Sci 62:97–105
Raman RK, Cook BC (1990) Guidelines for applying copper sulphate as an algicide: Lake Laomi field study, vol 217. Illinois State Water Survey, Springfield, pp 785–2800
Richlen MA, Morton SL, Jamali EA, Rajan A, Anderson DA (2010) The catastrophic 2008-2009 red tide in the Arabian Gulf region, with observations on the identification and phylogeny of fish-killing dinoflagellate Cochlodinium polykrikoides. Harmful Algae 9:163–172
Santo CE, Taudte N, Nies DH, Grass G (2008) Contribution of copper ion resistance to survival of Escherichia coli on metallic copper surfaces. Appl Environ Microbiol 74:977–986
Sengo MR, Anderson DM (2004) Controlling harmful algal blooms through clay flocculation. J Eukaryot Microbiol 51:169–172
Shao H-B, Chu L-Y, Lu Z-H, Kang C-M (2008) Primary antioxidant free radical scavenging and redox signaling pathways in higher plant cells. Int J Biol Sci 4:8–14
Song Y-C, Sivakumar S, Woo J-H, Ko S-J, Hwang E-J, Jo Q (2010) Removal of Cochlodinium polykrikoides by dredged sediment: a field study. Harmful Algae 9:227–232
Soto P, Gaete H, Hidalgo ME (2011) Assessment of catalase activity, lipid peroxidation, chlorophyll-a and growth rate in the freshwater green algae Pseudokirchneriella subcapitata exposed to copper and zinc. Lat Am J Aquat Res 39:280–285
Stauber JL, Franklin NM, Adams MS (2002) Applications of flow cytometry to ecotoxicity testing using microalgae. Trends Biotechnol 20:141–143
Stiborová M, Doubravová M, Březinova A, Friedrich A (1986) Effect of heavy metal ions on growth and biochemical characteristics of photosynthesis of barley (Hordeum vulgare L.). Photosynthetica 20:418–425
Teisseyre A, Mozrzymas JW (2006) The inhibitory effect of copper ions on lymphocyte KVI.3 potassium channels. J Physiol Pharmacol 57:301–314
Trachootham D, Lu W, Ogasawara MA, Nilsa RD, Huang P (2008) Redox regulation of cell survival. Antioxid Redox Signal 10:1343–1374
Trampe E, Kolbowski J, Schreiber U, Kühl M (2011) Rapid assessment of different oxygenic phototrophs and single-cell photosynthesis with multicolour variable chlorophyll fluorescence imaging. Mar Biol 158:1667–1675
Trivedi MH, Sangai NP, Renuka A (2012) Assessment of toxicity of copper sulphate pentahydrate on oxidative stress indicators on liver of gold fish (Carassius auratus). Bull Environ Pharmacol Life Sci 1:52–57
U.S. Environmental Protection Agency (USEPA) (2009) Reregistration eligibility decision (RED) for coppers. U.S. Environmental Protection Agency, Washington, DC. http://www.epa.gov/oppsrrd1/REDs/copper_red_amend.pdf. Accessed 29 Jan 2014
Verhoeven RL, Eloff JN (1979) Effect of lethal concentrations of copper on the ultrastructure and growth of Microcystis. Proc Electron Microsc Soc South Afr 9:161–162
Viriyatum R (2013) Effectiveness of coated, controlled-release copper sulfate as an algicide for phytoplankton control in ponds. PhD dissertation, Auburn University, Auburn, Alabama. http://hdl.handle.net/10415/3585. Accessed 29 Jan 2014
Watson CA, Yanong RPE (2006) Use of copper in freshwater aquaculture and farm ponds. University of Florida, Gainesville. FL. http://edis.ifas.ufl.edu/FA008. Accessed 29 Jan 2014
White GC (1999) Handbook of chlorination and alternative disinfectants, 4th edn. John Wiley and Sons Inc., New York, pp 212–287
Acknowledgements
This work was supported by a National Research Foundation of Korea (NRF) grant funded by the Korea government (nos. 2012-0001741, 2013-044476), a grant from the National Fisheries Research and Development (NFRDI) funded to J.-S. Ki, and a 2014 Research Grant from Sangmyung University.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary Fig. 1
Variation in chlorophyll autoflourescence in C. polykrikoides after 6 and 72 h exposure to algicides NaOCl (upper panel) and CuSO4 (lower panel). Scale = 20 μm. (PPT 491 kb)
Rights and permissions
About this article
Cite this article
Ebenezer, V., Lim, W.A. & Ki, JS. Effects of the algicides CuSO4 and NaOCl on various physiological parameters in the harmful dinoflagellate Cochlodinium polykrikoides . J Appl Phycol 26, 2357–2365 (2014). https://doi.org/10.1007/s10811-014-0267-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10811-014-0267-9