Abstract
Despite their prevalence in both developed and developing countries, there have been surprisingly few field assessments of the ecological effectiveness of protected areas. This study aimed to assess the effectiveness of a key protected area in eastern Madagascar, Ranomafana National Park (RNP). We established paired 100 × 4-m vegetation transects (400 m2) within RNP and in remnant forests in the park’s peripheral zone. In each 400-m2 plot, all woody stems >1.5 cm in diameter at breast height were measured and identified to species. All species were also identified as native or non-native. We identified utilitarian species within all transects and they were sorted into use category. We calculated plot-level taxonomic biodiversity and functional diversity of utilitarian species; the latter was calculated by clustering the multivariate distances between species based on their utilitarian traits, and all metrics were tested using paired t-tests. Our results showed that there was significantly higher biodiversity inside RNP than in remnant forests and this pattern was consistent across all diversity metrics examined. Forests not located within the park’s boundary had significantly higher non-native species than within RNP. There was no statistically significant difference in functional diversity of utilitarian species inside RNP vs. remnant forests; however, the overall trend was toward higher diversity inside park boundaries. These findings suggested that RNP has been effective at maintaining taxonomic diversity relative to surrounding unprotected areas and restricting the spread of non-native plants. The results also suggested that low functional redundancy of forests outside of RNP might be of concern, because residents in surrounding villages may have few other substitutes for the services provided by species that are of critical importance to their livelihoods. This study highlights the challenges of trying to reconcile biodiversity conservation with human use of natural resources in economically poor, remote areas.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Protected Areas
One of the major strategies for promoting biodiversity conservation is the establishment of protected areas. The World Conservation Union (IUCN 1994) defines a protected area as “a clearly defined geographical space, recognized, dedicated and managed through legal or other effective means to achieve the long-term conservation of nature with associated ecosystem services and cultural values” (Dudley 2008). The IUCN World Commission on protected areas presently estimates that there are 100,000 protected areas that fall into one of six conservation categories, ranging from areas that strictly limit human activity to those that allow for sustainable human use (IUCN 2007). Many of these protected areas are directed at conserving tropical forests, as these habitats contain two-thirds of the world’s terrestrial plant and animal species (Raven 1980). More importantly, many protected areas are located in developing countries (Terborgh and van Schaik 2002; Wilshusen and others 2003), where people are often directly dependent on natural resources for subsistence and, therefore, are often surrounded by a matrix that may include a mosaic of development, agriculture, pastures, and unprotected forests of varying condition and successional state. Due to the many surrounding pressures on natural resources, protected areas can be an important strategy to conserve habitat and species in tropical regions. These surrounding pressures, however, may also challenge conservation of protected areas.
Despite their prevalence in both developed and developing countries, there have been surprisingly few assessments on the ecological effectiveness of protected areas (sensu Gaston and others 2006). Studies that have attempted to assess the success of protected areas for safeguarding against environmental degradation have included qualitative assessments based on interviews with park managers (Bruner and others 2001), assessments on the degree of forest cover change from one time period to another (DeFries and others 2005), change in the abundance of a target species (Tuya and others 2006), and abatement of threats (Salafsky and Margoluis 1999; Mugisha and Jacobson 2004), among others. Hockings (2003) provides an extensive review of strategies used by different organizations for assessing the effectiveness of protected area management. Considering the prevalence and importance of protected areas, assessments of their effectiveness at preventing ecological degradation, such as biodiversity loss or invasive plant spread, are also of importance for conservation.
Deforestation within protected area boundaries can be measured with high accuracy using satellite imagery or aerial surveys, but these methods are unable to capture and describe the great variety of processes that reduce or alter forest cover without eliminating it (Stone and Lefebvre 1998; Sgrenzaroli and others 2002). This more subtle process of ‘forest degradation’ refers to the temporary or permanent decrease in the density, biomass, or overall structure of vegetation cover or its species composition (Grainger 1993; Sgrenzaroli and others 2002) and, in many cases, is a more prevalent process than deforestation (Lambin 1999; Nepstad and others 1999). Degradation can be a result of chronic selective extraction of forest resources for purposes such as fuelwood, construction materials, and non-timber forest products (NTFP).
Due to the changes in diversity, structure, and environmental conditions in degraded forest sites compared to intact analogues, forest degradation is often coupled with invasive species establishment (Von Holle and Motzkin 2007). However, the degree to which non-native plants are able to establish viable populations in degraded tropical forests is a function of the propagule pressure from source locations (Aragón and Morales 2003), such as abandoned agricultural lands. Protected areas that border highly degraded habitats may be more susceptible to invasion, because dispersal mechanisms that facilitate range expansion of non-native vegetation might not constrained by park boundaries. At certain spatial and temporal scales, establishment and proliferation of non-native invasive plants may lead to a decline in biodiversity in tropical forests (Brown and Gurevitch 2004; Brown and others 2006). Still other studies suggest that the relationship between non-natives and biodiversity is strongly dependent on the spatiotemporal scale examined (Sax and Gaines 2003; Allen and Shea 2006; Brown and others 2008). Thus, the threat of invasive species or high-intensity, small-scale forest degradation may be more difficult to measure with rapid assessment tools such as remote sensing. However, these challenges should not preclude the monitoring of such threats, since they are important to quantify individually and because such pressures may have synergistic effects and feedbacks that could negatively affect the biodiversity that protected forests are intended to conserve.
Protected Areas in Madagascar
As of 2003, it was estimated that 3.1% of Madagascar’s land area was located within a total of 53 protected areas (World_Resource_Institute 2006). The land area currently under administrative or governmental protection (e.g., national park, special reserves, or preserves) is set to triple to include approximately 10% of Madagascar’s total land area by 2008 (Norris 2006). However, the effectiveness of these protected areas at reducing threats to the island’s biodiversity remains unclear (McConnell 2002). An example of persistent threats to biodiversity is the degraded state of buffer or peripheral areas surrounding many protected habitats in Madagascar. DeFries and others (2005) report that forested habitats within periphery zones surrounding moist forests in Madagascar registered the greatest loss of 198 protected areas surveyed globally during a period ranging from 1982 to 2001. The decrease in forests surrounding protected areas is of concern for both biodiversity conservation (DeFries and others 2005) and the human wellbeing of rural communities living near these forests, many of whom depend directly on forest resources for their livelihoods.
We used Ranomafana National Park as a case study to address questions regarding the ecological role of protected areas in developing countries. Our study assessed the efficacy of protected areas at (1) promoting conservation of taxonomic and functional diversity, (2) protecting against overexploitation of natural resources, and (3) restricting the spread of non-native, invasive plants. We choose these variables because they can serve as effective proxies for the ecological effectiveness of protected areas and are not easily measured with frequently used monitoring tools such as satellite imagery. For instance, biodiversity plays a central role in regulating ecosystem processes and function and conveys much information about the extent and degree of environmental changes within a system (Tilman 1999; Chapin and others 2000; Naeem 2002; Hooper and others 2005). Taxonomic diversity has been the principal form of measurement of biodiversity. In recent years, functional diversity—which measures the range of roles that organisms play in a community—has gained increasing prominence for assessing species diversity (Petchey and Gaston 2002, 2006). We assessed diversity using both taxonomic and functional metrics. In regions where forest resources form a critical part of people’s daily livelihoods, the abundance and distribution of certain useful species may depend heavily upon the degree of their utility to surrounding communities, how frequently they are exploited and their regeneration potential. Thus, the range of utilitarian traits associated with a suite of species found within a landscape can provide important information about the state of resources critical for human well-being and the degree of unsustainable use of those resources. The abundance and distribution of non-native plant species can also be a valuable barometer of environmental degradation.
Methods
Ranomafana National Park
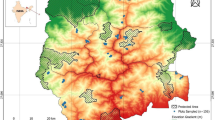
Ranomafana National Park was gazetted in 1991 (Randrianandianina and others 2003) and is located between 47°18′–47°37′ E and 21°02′–21°25′ S in Madagascar (Fig. 1). The designated park consists of 43,500 ha of continuous moist humid forest (midaltitude montane rainforest), with annual rainfall ranging from 1700 to 4300 mm. The high-rainfall season is from December to March, with the driest periods in September and October. Temperatures range from lows in June–September (4–12ºC) to highs in December–February (36–40ºC) (Wright and Andriamihaja 2003). Tropical cyclones are a dominant natural disturbance affecting Madagascar, with an estimated 155 cyclones hitting the island between 1848 and 1970 (Jury 2003) and 28 crossing over the island between 1985 and 2003 (U.S. Naval Pacific Meteorology and Oceanography Center 1985–2003). In 2001, a combination of assessments using aerial surveys and satellite imagery analyses revealed that, despite the extent of slash-and-burn agriculture outside of the park, 98% of the forest cover within the park was intact (Wright and Andriamihaja, 2003). However, Wright and Andriamihaja (2003) did not assess the condition of forest stand structure and composition in detail at the ground level.
Since the establishment of RNP, people have been relegated to the peripheral zones, which had been largely degraded throughout time and have continued to be degraded since the establishment of the park, but retain relict patches of relatively intact forest and mixed plantations (Peters 1998). Because of the pre-existent extreme degradation in the areas surrounding the desired park boundaries combined with the unregulated use of these areas, RNP’s periphery zones do not qualify as “buffer zones” as defined by the Integrated Conservation and Development Project (ICDP), which requires that there be relatively intact habitat directly bordering the protected area, with restricted land use (Margules and Pressey 2000). More specifically, “a buffer zone is the habitat surrounding a core protected area that is managed to help maintain values of a National Park, Preserve and/or Reserve” (Dudley and Phillips 2007). A buffer zone is intended to serve as a barrier against habitat degradation and other threats such as fire, disease or invasive species (Hansen and Rotella 2002). At RNP, the sharp juxtaposition between land uses outside and inside of the park suggests that threats may be higher at RNP than protected areas that have dense to moderate forest cover in the areas surrounding the park (Margules and Pressey 2000). Without a forested buffer area, the likelihood of threats such as invasive species spread or selective logging for purposes such as fuelwood and construction materials, which cannot be monitored as easily by park rangers, aerial surveys, or satellite imagery, could affect the forest within the park boundaries.
Currently available figures estimate that 160 villages surround the forest area of Ranomafana National Park, with a total population of 27,000 within a 500-km2 area (Peters 1998). A large portion of this population uses the forest area in the periphery zone for slash-and-burn agriculture and extract forest resources for fuelwood, construction materials, medicine, and a myriad of non-timber forest products (NTFP). The biggest threats to RNP when it was established were ongoing subsistence agriculture, extraction of plants and animals, and uncontrolled forestry activities within the park boundary (Peters 1998).
The spread of non-native, invasive plants has also been identified as a threat to forest biodiversity in RNP (Brown and Gurevitch 2004). There are a number of common invasive trees and shrubs established within the park. These include Clidemia hirta (Melastomacaceae), Psidium cattleianum Sabine (Myrtaceae), Eucalyptus robusta (Myrtaceae), Lantana camara (Verbenaceae), and Syzygium jambos (Myrtaceae).
Vegetation Sampling
Vegetation sampling was carried out from May to July 2006. The park was separated into four regions—north, south, west, and central—from which we preselected nine sites in which to sample. Two sites were selected from each of the outer regions, and three sites within the central region. Each site was associated with one of the main villages in a given region. We established paired 100 × 4-m (400-m2) vegetation plots inside the park and another outside. The plots established outside the RNP (hereafter referred to as “village plots”) were situated within the peripheral zone in remnant forested areas. Those plots began at the forest edge closest to the village and radiated away from the village toward RNP (Fig. 1). The plots established inside the park (hereafter referred to as “park plots”) began at the boundary of RNP closest to the village and extended 100 m into the park away from its boundary. A total of 18 plots, each 400 m2 in size, were sampled for this study—nine within the park boundary and the same number in remnant forests outside the park (Fig. 1).
In each 400-m2 plot, all woody stems >1.5 cm in diameter at breast height (DBH) were identified to species, measured, and mapped to nearest meter along the transect line. Diameters were measured with 5-m DBH tapes and recorded to nearest millimeter. Trees with multiple stems that were connected near the base were counted as single individuals and assigned the DBH of the largest stem. These plants were counted as one individual during our analyses. All species were subsequently identified as native or non-native by cross-referencing several Malagasy botanical sources (Schatz 1994; Turk 1997; Boiteau 1999; Schatz 2001). The herbaceous layer was sampled at every 1-m interval along the 100-m transect line and the dominant herbaceous species were identified and categorized as native or non-native. In addition, location along the transect was recorded for woody species, and diversity metrics (below) were calculated separately for the four segments, 0–25, 25–50, 50–75, and 75–100 m from the edge of the park.
Utilitarian Species
Utilitarian species recorded within all transects were sorted by use category. The number of individuals was calculated for trees that were identified by Malagasy researchers as being used for fuelwood, construction materials, and other non-timber forest products (NTFPs; Appendix). Construction in this context refers to the provisioning of shelter and other critical needs. Fuelwood in this context is predominantly for firewood. Construction materials, fuelwood, and NTFPs are considered the most prominent selective uses of forests in Madagascar (Consiglio and others 2006) and are extremely high in intensity (Kremen and others 1998). These use practices are the dominant drivers of forest degradation in contrast to deforestation, which is caused primarily by slash-and-burn agriculture. It is important to note that the villages surrounding Ranomofana may vary in their degree of dependence and use of forest products (Ferraro 2001). Ideally, this analysis would be accompanied by interviews with local people to ascertain which species within each use category are preferred, as well as the species that are commonly substituted when preferred species are not available (Kremen and others 1998). However, analysis of this detail was not possible for this study.
We divided the utilitarian species for all use categories into four size classes (e.g., 1 cm ≤ DBH < 5 cm, 5 cm ≤ DBH < 10 cm, 10 cm ≤ DBH < 15 cm, and DBH > 15 cm). These size class intervals effectively broke the distribution of utilitarian species into quartiles. We calculated the frequency of utilitarian species in each size class for park and village sites and assessed significance using a contingency table (see Statistical Methods). These size class distributions included medicinal species as well as other non-destructively harvested species whose abundance may be important for human health purposes (Kremen and others 1998).
Analytical Methods
Diversity Metrics
Four indexes were used to characterize plot-level species diversity: species richness, S, the number of species per 400 m2; Shannon’s index, H’ = –ΣP i log2 P i , which accords greater weight to contrasts in rare species; inverse of Simpson’s index, 1/λ, which emphasizes differences in common species, where λ = \( \Upsigma p_{i}^{2} , \) and P i is the proportion of the number of ith species to total number of individuals; and Fisher’s α, \( S = \alpha \ln (1 + N/\alpha ) \), where S is species richness, N is number of individuals, and α is estimated from the species-individual relationships in each plot (Magurran 2004). Fisher’s α is commonly used for highly diverse tropical forest datasets (Condit and others 1996) and is a scale-independent measure of biodiversity (Schulte and others 2005). Each diversity metric assesses a different aspect of community structure (Magurran 2004). Using only a taxonomic measure of diversity overlooks the variation in socially important traits between species; therefore, we calculated the utilitarian functional diversity in conjunction with taxonomic metrics. For our purposes, “functional diversity” refers to the diversity of utilitarian traits across species in a given ecological community. Utilitarian traits were defined by a species’ utility to local residents surrounding RNP (Appendix).
Measures of functional diversity summarize the range and values of the traits of species present in a community using a single metric and, thus, provide a way to compare the diversity of species’ roles in a community between plots. In contrast, functional richness (e.g., the number of different traits present in a community) provides limited information about the total range in trait values and sheds little light on the functional trait composition of a community. We used Petchey and Gaston’s (2002) FD as a measure of utilitarian species functional diversity. While several other indexes have recently been developed for functional diversity measurement (e.g., Rao’s Q (Leps and others 2006)), Petchey and Gaston’s (2006) FD performs well in comparison, is relatively easy to calculate and interpret, and easily incorporates mixed data types. Using this dendrogram-based index of functional diversity requires several decisions; we used the Gower method to calculate multivariate distances between species because our trait data were of mixed types (continuous, ordinal, and binary) and unweighted pair-group method with arithmetic means (UPGMA) to create the functional dendrograms, because this clustering algorithm gave the highest cophenetic correlation with the original multivariate distances (Petchey and Gaston 2007). Utilitarian traits were as follows: construction (ordinal), firewood (ordinal), medicinal/tea (binary), fruit/food (binary), other wood (binary), and other leaves (binary). Species used for construction and firewood were separated and ranked into three categories based on their preference to local residents (e.g., poor, good, and very good).
Statistical Analyses
The effects of plot location on species richness, Shannon’s index, inverse of Simpson’s index, and Fisher’s α were tested using separate paired t-tests. Paired t-tests were used to analyze the effects of plot location on both woody and herbaceous non-native plant percentage. A single classification ANOVA was used to examine diversity along the transect distance classes (e.g., from forest edge to interior). The data for percentage of non-native vegetation were arcsine transformed.
To establish that all of the plots had a similar basic compositional structure and that village- and park-forest comparisons were appropriate (Aubad and others 2008), we used non-metric multidimensional scaling (NMDS [Minchin 1987]) to determine the compositional similarities between plots inside RNP and those in forest remnants associated with villages.
We used a 2 × 4 contingency table (habitat type × size class) to determine differences in the number of individuals in each size class compared across habitats (i.e., park and village). The importance of different size classes were tested using a post hoc cellwise standardized residual analysis (MacDonald and Gardner 2000). The post hoc test was based on standardized residuals defined as
where f ij is the observed frequency in the cell at the ith row and jth column of the table; and F ij is the expected frequency in the cell at the ith row and jth column. Residuals >2 were considered to be of most importance (Portney and Watkins 2000).
Since multiple diversity metrics were calculated from the same sample data, we applied the False Discovery Rate (FDR) correction for multiple comparisons (Benjamini and Hochberg 1995). The FDR is a smaller correction than a familywise error rate correction such as Bonferroni and considers the rank of each significance value in determining the appropriate α. This procedure has been recommended for ecological studies as a reasonable alternative to the sequential Bonferroni (Garcia 2004). All diversity calculations and statistical analyses were carried out using the statistical program R (R_Development_Core_Team 2007).
Results
Taxonomic and Functional Diversity and Non-Native Plants
Analysis of the diversity patterns within RNP and the surrounding villages revealed that there were significant differences in diversity between the two habitat types. The results showed that species richness (Fig. 2a; P = 0.007), Shannon’s diversity (Fig. 2b; P = 0.043), and Fisher’s α (Fig. 1d; P = 0.007) were all significantly higher inside the park than in remnant forests associated with villages. Simpson diversity was marginally non-significantly different between the two habitats (Fig. 2c; p = 0.056). These results were based on the corrected α values. In contrast to taxonomic diversity measures, utilitarian functional diversity did not differ across the park boundary (Fig. 3; P = 0.136). However, the park sites exhibited greater functional redundancy than the village sites, meaning that the FD values plateau at the species richness values in the park sites but change with species richness values for the village sites. In other words, high FD values are maintained even under low levels of species loss in the park sites. The exceptions to this trend were the park sites near the villages of Sahateza and Anjamba (Fig. 1). The FD of the Sahateza park plot was similar to that of the other village plots and both park and village plots for Anjamba exhibited both low species richness and low FD (Fig. 3). The result from the NMDS showed that there were few compositional differences between park and village plots, which supported the assumption that basic compositional structure was common to all plots (data not shown).
Boxplots of diversity metrics, summarizing medians, upper and lower quartiles, and minimum and maximum data values. a Species richness outside (village) and inside (Park) RNP; t-ratio = −4.496, P = 0.007. b Shannon-Weiner H’ diversity index outside (village) and inside (Park) RNP; t-ratio = −2.732, P = 0.043. c Inverse of the Simpson diversity index, 1/D, outside (village) and inside (Park) RNP; t-ratio = −2.371, P = 0.056. d Fisher’s α diversity outside (village) and inside (Park) RNP; t-ratio = −4.254, P = 0.007. For all figures, dark bars indicate average diversity metric at village sites and white bars indicate average diversity metric at park sites (N = 9)
The results showed that both woody and herbaceous non-native species were significantly less abundant within RNP (Figs. 4a and b). Distance from park edge was not a significant predictor for any of the diversity metrics or for percentage of non-native species (data not shown).
Comparison of non-native plant species percentage outside and inside RNP using boxplots. a Percentage of woody non-native plant species present within and outside Ranomafana National Park (RNP). Asterisk indicates significance at P = 0.043 based on paired t-test (N = 9). b Percentage of herbaceous non-native plant species present within RNP and village sites. Asterisk indicates significance at P = 0.011 based on paired t-test (N = 9). See Fig. 1 for interpretation of boxplots
Size Class Analysis of Utilitarian Species
The chi-square test indicated that there were significant differences in the size of utilitarian individuals inside RNP versus in the village (χ2 = 58.186, P > 0.001, df = 3) (Fig. 5a). The post hoc residual analysis showed that the smallest and largest size classes were most important in driving the differences between the size of individuals inside RNP and the villages, with residual scores of −2.72 and 4.09, respectively. There were fewer individuals in the size class ranging 1 cm ≤ DBH < 5 cm inside the park (Fig. 5a) and more individuals in size class DBH > 15 cm inside RNP. The abundance of utilitarian trees used for construction and firewood were significantly higher at the village sites for stems within the small size class (residual value = −2.11) (Fig. 5b). The trend was opposite for the larger size class, with a significantly greater number of large stems within RNP (residual value = 3.15) (Fig. 5b).
Discussion
Taxonomic Diversity and Non-Native Plants
Although there has been considerable environmental degradation in the peripheral zones, habitats within RNP’s boundaries have managed to maintain higher levels of taxonomic biodiversity and fewer established non-native plants relative to village sites. This result is quite robust, considering that the park plots were established on the margins of RNP, just 100 m from the park’s edge. The analyses consistently revealed higher biodiversity inside RNP than within forest remnants in surrounding villages. Despite the degraded state of the peripheral zone directly adjacent to RNP, park boundaries seemed to have been able to protect taxonomic diversity. It should be noted that a more conclusive assessment of RNP’s effectiveness would require information regarding the possible prevailing baseline states at the park’s inception, but those data were not available. Nonetheless, protected areas are not always established in regions with high biodiversity or less degraded habitats. Instead, they are chosen based on a variety of factors including remoteness, areas of least economic value, and habitats with high aesthetic value (Terborgh and van Schaik 2002).
The presence of non-native plants showed a similar pattern: although there is no ecological buffer to stem invasive species spread into the protected forest, non-natives have been largely restrained to the peripheral zone. There was no evidence that the abundance or density of non-natives increased from forest edge to forest interior for park plots. Habitat fragmentation and the consequential formation of edges often leads to increased exposure to non-native species in surrounding areas, so the abundance of invasive, non-native species is often higher along fragmented edges (Green and others 2004; Hansen and Clevenger 2005). Our result is a promising finding for native plant conservation in this area considering that the survey plots for the park sites were established in close proximity to the park’s edge. These observations of non-native plant distribution may be due to any number of factors, ranging from recruitment and dispersal limitations to biotic and abiotic factors that limit germination and establishment. More research is needed to understand the specific factors that drive non-native invasive species establishment and distribution in the area.
Functional Diversity and Size Class Analysis
There were no statistically significant differences in the functional diversity of species inside the park vs. village forests. However, the results showed a trend toward higher functional redundancy for plots inside RNP, where a relatively high diversity of utilitarian traits would remain even if some species were lost from park sites. This trend suggested that plant populations in the park have been less impacted by selective removal than those in the villages, and are more likely to maintain a high diversity of utilitarian functional traits. Several authors have addressed the impacts and costs born by local residents by RNP’s establishment (Peters 1998; Ferraro 2002). The issue of lack of access to a particular resource may be of special concern around the park because of the highly degraded state of natural resources in the peripheral zone. At RNP, efforts to restore the peripheral zone (e.g., into an IUCN-defined ‘buffer zone’) in concert with managing the park could be a way to help relieve threats to biodiversity while providing local people with alternative forest resources. Similar findings on the importance of alternative resource availability have been highlighted in other studies (Aubad and others 2008; Lamb and others 2005), and our results lend further support to the idea that access to alternative resources, particularly for firewood and construction, may decrease potential pressure from local communities on native protected forests.
The findings on functional diversity were supported by the size class analysis of stems inventoried for park and village sites. Species used for fuelwood and construction materials are typically the most heavily exploited trees in a forest, because the utilization of trees for both of these purposes, particularly the former, is inherently chronic and often more destructive than other forest uses such as medicinal plant use or food. At RNP, many species of importance to local people may not be regenerating outside of the protected area, as evidenced by significantly lower abundances of trees in the largest size class. From our analyses, it appeared that it was not the species composition that differed between forest and village plots; instead, it was the abundance and size class of the species present that seemed to be driving the observed contrasts. Our study suggests that the ecological integrity of the park plots is being maintained through limited or restricted use of large-sized utilitarian species. Overall, the low rate of regeneration coupled with the low functional redundancy (e.g., little overlap in utilitarian function between species present) of forest remnants exemplifies the highly degraded nature of the peripheral zone. These conditions, combined with the extreme poverty of many of the residents living in remote villages, may indicate that the pressures facing RNP are likely to continue and increase.
Implications for Protected Areas Management
An analysis of both taxonomic and functional diversity of utilitarian species has provided insights into the ecological and social impacts of human pressures on forests within and surrounding RNP. A paucity of utilitarian species in the peripheral zone may result in increased pressure on the protected area, particularly because communities have few resource substitutes. The park’s boundary seemed to have adequately impeded the local community from harvesting utilitarian species from within RNP, but these activities might have shifted to areas adjacent to the park. Furthermore, increased usage and pressure on unprotected, less functionally diverse communities of utilitarian species could negatively affect vulnerable human populations (Igoe 2004), who could face shortages in fuelwood, construction materials, medicine, and/or food due to ongoing environmental degradation. Similarly, these communities would be more vulnerable to shocks, such as disease, fire, or flooding, that could further deplete or destroy remaining forest resources.
Most importantly, though, these results have profound implications for protected areas in developing countries that must conserve biodiversity amid poor human communities who often depend on forest resources for their livelihoods. Degradation of natural resources in developing countries represents a threat to both livelihoods and biodiversity: if restricting access to natural resources within protected areas increases pressures in surrounding unprotected areas and results in severe decreases in the redundancy of accessible unprotected utilitarian species, pressures may eventually increase on protected resources. Thus, as our results suggest, to stem ecological and human threats to intact forests, it is important to manage natural resources sustainably both within and outside protected areas for buffering pressures such as invasive species and for providing critical ecosystem services to human populations.
References
Allen MR, Shea K (2006) Spatial segregation of congeneric invaders in central Pennsylvania, USA. Biological Invasions 8:509–521
Aragón RM, Morales JM (2003) Species composition and invasion in NW Argentinian secondary forests: effects of land use history, environment and landscape. Journal of Vegetation Science 14:195–204
Aubad J, Aragón P, Olalla-Tárraga MÁ, Rodríguez MÁ (2008) Illegal logging, landscape structure and the variation of tree species richness across North Andean forest remnants. Forest Ecology and Management 255:1892–1899
Benjamini Y, Hochberg Y (1995) Controlling the false discovery rate: a practical and powerful approach to multiple testing. Journal of Royal Statistical Society Series B Methodological 57:289–300
Boiteau P (1999) Dictionnaire des Noms Malgaches des Vegetaux, vol II. Grenoble, France
Brown KA, Gurevitch J (2004) Long-term impacts of logging on forest diversity in Madagascar. Proceedings of the National Academy of Sciences of the United States Of America 101:6045–6049
Brown KA, Scatena FN, Gurevitch J (2006) The effects of an invasive tree on community structure and diversity in a tropical forest in Puerto Rico. Forest Ecology and Management 226:145–152
Brown KA, Spector S, Wu W (2008) Multi-scale analysis of species introductions: combining landscape and demographic models to improve management decisions about non-native species. Journal of Applied Ecology 45:1639–1648
Bruner AG, Gullison RE, Rice RE, da Fonseca GB (2001) Effectiveness of Parks in protecting tropical biodiversity. Science 291:125–128
Chapin FS, Zavaleta ES, Eviner VT, Naylor RL, Vitousek PM, Reynolds HL, Hooper DU, Lavorel S, Sala OE, Hobbie SE, Mack MC, Diaz S (2000) Consequences of changing biodiversity. Nature 405:234–242
Condit R, Hubbell SP, Lafrankie JV, Sukumar R, Manokaran N, Foster RB, Ashton PS (1996) Species-area and species-individuals relationships for tropical trees: a comparison of three 50-ha plots. Journal of Ecology 84:549–562
Consiglio T, Schatz GE, McPherson G, Lowry PP, Rabenantoandro J, Rogers ZS, Rabevohitra R, Rabehevitra D (2006) Deforestation and plant diversity of Madagascar’s littoral forests. Conservation Biology 20:1799–1803
DeFries R, Hansen A, Newton AC, Hansen MC (2005) Increasing isolation of protected areas in tropical forests over the past twenty years. Ecological Applications 15:19–26
Dudley N (ed) (2008) Guidelines for applying protected areas management categories. IUCN, Gland, Switzerland
Dudley N, Phillips A (2007) Forest protected areas. IUCN World Commission on Protected Areas, pp 1–5
Ferraro PJ (2001) The local costs of establishing protected areas in low-income nations: Ranomafana National Park, Madagascar. Georgia State University, Atlanta
Ferraro PJ (2002) The local costs of establishing protected areas in low-income nations: Ranomafana National Park, Madagascar. Ecological Economics 43:261–275
Garcia LV (2004) Escaping the Bonferroni iron claw in ecological studies. Oikos 105:657–663
Gaston KJ, Charman K, Jackson SF, Armsworth PR, Bonn A, Briers RA, Callaghan CSQ, Catchpole R, Hopkins J, Kunin WE, Latham J, Opdam P, Stoneman R, Stroud DA, Tratt R (2006) The ecological effectiveness of protected areas: the United Kingdom. Biological Conservation 132:76–87
Grainger A (1993) Rates of deforestation in the humid tropics—estimates and measurements. Geographical Journal 159:33–44
Green PT, Lake PS, O’Dowd DJ (2004) Resistance of island rainforest to invasion by alien plants: influence of microhabitat and herbivory on seedling performance. Biological Invasions 6:1–9
Hansen AJ, Rotella JJ (2002) Biophysical factors, land use, and species viability in and around nature reserves. Conservation Biology 16:1112–1122
Hansen MJ, Clevenger AP (2005) The influence of disturbance and habitat on the presence of non-native plant species along transport corridors. Biological Conservation 125:249–259
Hockings M (2003) Systems for assessing the effectiveness of management in protected areas. Bioscience 53:823–832
Hooper DU, Chapin FS, Ewel JJ, Hector A, Inchausti P, Lavorel S (2005) Effects of biodiversity on ecosystem functioning: a consensus of current knowledge. Ecological Monographs 3–35
Igoe J (2004) Conservation and globalization: a study of national parks and indigenous communities from East Africa to South Dakota. Wadsworth Thompson Learning, Belmont
IUCN (1994) Guidelines for protected area managment categories. IUCN-World Conservation Union, Cambridge
IUCN (2007) World commission on protected areas. Available at http://www.iucn.org/themes/wcpa/
Jury MR (2003) The climate of Madagascar. In: Goodman SM, Benstead JP (eds) The natural history of Madagascar. University of Chicago Press, Chicago, pp 75–87
Kremen C, Raymond I, Lance K (1998) An interdisciplinary tool for monitoring conservation impacts in Madagascar. Conservation Biology 12:549–563
Lamb D, Erskine PD, Parrotta JA (2005) Restoration of degraded tropical forest landscapes. Science 310:1628–1632
Lambin EF (1999) Monitoring forest degradation in tropical regions by remote sensing: some methodological issues. Global Ecology and Biogeography 8:191–198
Leps J, de Bello F, Lavorel S, Berman S (2006) Quantifying and interpreting functional diversity of natural communities: practical considerations matter. Preslia 78:481–501
MacDonald PL, Gardner RC (2000) Type I error rate comparisons of post hoc procedures for I × J chi-square tables. Educational and Psychological Measurement 60:735–754
Magurran AE (2004) Measuring biological diversity, 2nd edn. Blackwell, Oxford, UK
Margules CR, Pressey RL (2000) Systematic conservation planning. Nature 405:243–253
McConnell WJ (2002) Misconstrued land use in Vohibazaha: participatory planning in the periphery of Madagascar’s Mantadia National Park. Land Use Policy 19:217–230
Minchin PR (1987) An evaluation of the relative robustness of techniques for ecological ordination. Vegetatio 69:89–107
Mugisha AR, Jacobson SK (2004) Threat reduction assessment of conventional and community-based conservation approaches to managing protected areas in Uganda. Environmental Conservation 31:233–241
Naeem S (2002) Disentangling the impacts of diversity on ecosystem functioning in combinatorial experiments. Ecology 83:2925–2935
Nepstad DC, Verissimo A, Alencar A, Nobre C, Lima E, Lefebvre P, Schlesinger P, Potter C, Moutinho P, Mendoza E, Cochrane M, Brooks V (1999) Large-scale impoverishment of Amazonian forests by logging and fire. Nature 398:505–508
Norris S (2006) Madagascar defiant. Bioscience 56:960–965
Petchey OL, Gaston KJ (2002) Functional diversity (FD), species richness and community composition. Ecology Letters 5:402–411
Petchey OL, Gaston KJ (2006) Functional diversity: back to basics and looking forward. Ecology Letters 9:741–758
Petchey OL, Gaston KJ (2007) Dendrograms and measuring functional diversity. Oikos 116:1422–1426
Peters J (1998) Transforming the integrated conservation and development project (ICDP) approach: observations from the Ranomafana National Park Project, Madagascar. Journal of Agricultural & Environmental Ethics 11:17–47
Portney LG, Watkins MP (2000) Foundations of clinical research: applications to practice. Prentice Hall, Upper Saddle River, NJ
R_Development_Core_Team (2007) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria
Randrianandianina BN, Andriamahaly LR, Harisoa FM (2003) The role of the protected areas in the management of the island’s biodiversity. In: Goodman SM, Benstead JP (eds) The natural history of Madagascar. University of Chicago Press, Chicago
Raven P (1980) Research priorities in tropical biology. National Academy of Sciences Press, Washington, DC
Salafsky N, Margoluis R (1999) Threat reduction assessment: a practical and cost-effective approach to evaluating conservation and development projects. Conservation Biology 13:830–841
Sax DF, Gaines SD (2003) Species diversity: from global decreases to local increases. Trends in Ecology & Evolution 561–566
Schatz GE (1994) Botanical inventory of Ranomafana National Park. Missouri Botanical Garden, St. Louis, MO
Schatz GE (2001) Generic tree flora of Madagascar. Missouri Botanical Garden and Royal Botanic Gardens, Kew
Schulte RO, Lantinga EA, Hawkins MJ (2005) A new family of Fisher-curves estimates Fisher’s alpha more accurately. Journal of Theoretical Biology 232:305–313
Sgrenzaroli M, Baraldi A, Eva H, DeGrandi G, Achard F (2002) Contextual clustering for image labeling: an application to degraded forest assessment in Landsat TM images of the Brazilian Amazon. IEEE Transactions on Geoscience and Remote Sensing 40:1833–1848
Stone TA, Lefebvre P (1998) Using multi-temporal satellite data to evaluate selective logging in Para, Brazil. International Journal of Remote Sensing 19:2517–2526
Terborgh J, van Schaik C (2002) Why the world needs parks. In: Terborgh J, Schaik CV, Davenport L, Rao M (eds) Making parks work: strategies for preserving tropical nature. Island Press, Washington, DC, pp 3–14
The_World_Conservation_Union_(IUCN) (2007) World Commission on protected areas. Available at http://www.iucn.org/themes/wcpa/
Tilman D (1999) The ecological consequences of changes in biodiversity: a search for general principles. Ecology 80:1455–1474
Turk RD (1997) A guide to the trees of Ranomafana National Park and Central Eastern Madagascar. North Carolina State University, Raleigh
Tuya F, Garcia-Diez C, Espino F, Haroun RJ (2006) Assessment of the effectiveness of two marine reserves in the Canary Islands (eastern Atlantic). Ciencias Marinas 32:505–522
U.S., N (1985–2003) Annual tropical cyclone report. U.S. Naval Pacific Meteorology and Oceanography Center, Pearl Harbor, HI
Von Holle B, Motzkin G (2007) Historical land use and environmental determinants of non-native plant distribution in coastal southern New England. Biological Conservation 136:33–43
Wilshusen PR, Brechin SR, Fortwangler CL, West PC (2003) Contested nature: conservation and development at the turn of the twenty-first century. In: Brechin SR, Wilshusen PR, Fortwangler CL, West PC (eds) Contested nature: promoting international biodiversity with social justice in the twenty-first century. State University of New York Press, Albany, pp 1–22
World_Resource_Institute (2006) Earth trends: the Environmental Information Portal, biodiversity and protected areas. Available at http://earthtrends.wri.org/text/biodiversity-protected/country-profile-112html
Wright PC, Andriamihaja B (2003) The conservation value of long-term research: a case study from the Parc National de Ranomafana. In: Goodman SM, Benstead JP (eds) The natural history of Madagascar. University of Chicago Press, Chicago
Acknowledgments
We thank Dr. P. Wright for guidance and support with this project. We thank the Association Nationale pour la Gestion des Aires Protegees (ANGAP), Madagascar Institute pour la Conservation des Environnements Tropicaux (MICET), and Institute for the Conservation of Tropical Environments (ICTE) for valuable logical support. Special thanks go to our Malagasy research assistants, without whom this work would not have been completed: Paul Rasabo, Francois Ratalata, and Auguste Pela. This project was funded by the National Geographic Conservation Trust, Grant C81-05, and the Columbia University Science Fellowship. We thank three reviewers for their constructive comments.
Author information
Authors and Affiliations
Corresponding author
Appendix
Appendix
Rights and permissions
About this article
Cite this article
Brown, K.A., Carter Ingram, J., Flynn, D.F.B. et al. Protected Area Safeguard Tree and Shrub Communities from Degradation and Invasion: A Case Study in Eastern Madagascar. Environmental Management 44, 136–148 (2009). https://doi.org/10.1007/s00267-008-9257-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00267-008-9257-5