Abstract
The objective of this study was to investigate the effects on the vascular regeneration of adipose-derived stem cells (ASCs) by using red light-emitting diode (LED) irradiation in ischemic hind limbs. Low-level light therapy (LLLT) has been shown to enhance proliferation and cytokine secretion of a number of cells. ASCs are an attractive cell source for vascular tissue engineering. This approach is hindered because transplanted ASCs decline rapidly in the recipient tissue. Ischemic hind limbs were treated with LLLT from an LED array (660 nm) at an irradiance of 50 mW/cm2 and a radiant exposure of 30 J/cm2. LLLT, ASC transplantation, and ASC transplantation with LLLT (ASC + LLLT) were applied to ischemic limbs, and cell survival and differentiation, and secretion of vascular endothelial growth factor and basic fibroblast growth factor of the ASCs were evaluated by immunostaining and Western blot analyses. Vascular regeneration was assessed by immunostaining and hematoxylin and eosin staining. In the ASC + LLLT group, the survival of ASCs was increased due to the decreased apoptosis of ASCs. The secretion of growth factors was stimulated in this group compared with ASCs alone. The ASC + LLLT group displayed improved treatment efficacy including neovascularization and tissue regeneration compared with ASCs alone. In particular, quantitative analysis of laser Doppler blood perfusion image ratio showed that blood perfusion was enhanced significantly (p < 0.05) by ASC + LLLT treatment. These data suggest that LLLT is an effective biostimulator of ASCs in vascular regeneration, which enhances the survival of ASCs and stimulates the secretion of growth factors in ischemic limbs.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Ischemia is a common and fatal human disorder associated with decreased blood supply to certain organs or tissues. For example, peripheral arterial obstructive disease, which is mainly primed by atherosclerosis, causes lower limb ischemia and usually results in amputation [1]. The goal of therapeutic angiogenesis is to treat limb ischemia by stimulating new blood vessel growth from pre-existing vessels [2].
Adipose-derived mesenchymal stem cells (ASCs), which are found in many adult tissues, are an attractive cell therapy source for the regeneration of damaged tissues because they are able to self-renew and are capable of differentiating into various cells and tissues [3, 4]. In addition, human ASCs (hASCs) could be very useful for clinical application since they inhibit the immunological responses and do not need a major histocompatibility match for allogeneic transplantation [5]. Transplanting hASCs induces neovascularization and improves blood flow to ischemic limbs in animal models of hind limb ischemia [6, 7]. It has also been demonstrated that growth factors and cytokines released by hASCs promote in vitro and in vivo arteriogenesis in ischemic tissue through paracrine mechanisms [6, 7]. ASCs can differentiate into endothelial cells (ECs) in EC growth medium containing vascular endothelial growth factor (VEGF) and fibroblast growth factor (FGF) [8]. Thus, ASC could be a novel source for cell therapy of ischemic tissues.
However, several studies have reported minimal effects of stem cell therapy in the absence of scaffolds or stimulators. Most of the applied stem cells die within 1 week of transplantation. Therefore, to develop successful stem cell therapies, it is necessary to cultivate stem cells that can survive in ischemic tissue while being capable of differentiation into vascular cells [9, 10].
Low-level light therapy (LLLT) has long been used for various purposes, such as relief of pain and inflammation and improvement in the local circulation [11]. Use of LLLT to stimulate new blood vessel growth has recently received considerable attention. Wavelengths in the red and near-infrared spectrum (600–1300 nm) generate an optical window to produce effects on biological systems in vivo and in vitro [11–13]; 660-nm red light-emitting diode (LED) radiation also enhances tissue healing by stimulating angiogenesis in various animal models of ischemia [14]. In addition, mesenchymal stem cell proliferation, differentiation, and secretion of growth factors including VEGF and FGF are also enhanced by LLLT [15, 16]. However, little is known about the therapeutic effect of LLLT on transplanted ASCs in animal models.
This study was performed to determine the effect of LLLT on transplanted ASCs in a mouse model of hind limb ischemia. We compared the therapeutic angiogenesis effects between the ASC transplantation group and the ASC transplantation with the LLLT (ASC + LLLT) group.
Materials and methods
ASC culture
hASCs supplied from CEFO (Seoul, Korea) were cultured in low-glucose Dulbecco’s modified Eagle’s medium F-12 (DMEM/F-12; Welgene, Daegu, Korea) supplemented with 10 % fetal bovine serum (FBS, Welgene), 100 units/ml penicillin, and 100 μg/ml streptomycin at 37.0 °C in a 5 % CO2 incubator. hASCs between passage 5 and 8 were used for all experiments.
Fluorescence-activated cell sorting
Cells were washed with phosphate-buffered saline (PBS) containing 0.5 % bovine serum albumin (BSA; Sigma-Aldrich, St. Louis, MO, USA). The cells were stained in PBS containing 1 % BSA with either isotype controls or antigen-specific antibodies for 60 min. The used antibodies were human CD34 (BD Biosciences, San Jose, CA, USA), KDR (Beckman Coulter, Brea, CA, USA), CD31 (Beckman Coulter), CD45 (Abcam, Cambridge, MA, USA), CD90 (BD Biosciences), CD105 (Caltac Laboratories, Burlingham, CA, USA), and CD29 (Millipore, Waltham, MA, USA). The cells were washed three times with PBS containing 0.5 % BSA and resuspended in PBS for flow cytometry using an Accuri device (BD Biosciences). Isotype control IgG was used as a negative control.
Histological staining
Ischemic limb muscles were harvested 21 days after treatment. Specimens were fixed in 10 % (v/v) buffered formaldehyde, dehydrated in a graded ethanol series, and embedded in paraffin. Specimens were sliced into 4-μm-thick sections and stained with hematoxylin and eosin (H&E) to examine muscle degeneration and tissue inflammation. Masson’s trichrome collagen staining was performed to assess tissue fibrosis in ischemic regions.
Immunofluorescence staining
Indirect immunofluorescence staining was performed using a standard procedure. In brief, tissues cryosectioned at 4-μm thickness were fixed with 4 % paraformaldehyde, blocked with 5 % BSA/PBS (1 h, 24 °C), washed twice with PBS, treated with 0.1 % Triton X-100/PBS for 1 min, and washed extensively in PBS. The sections were stained with specific primary antibodies and fluorescent-conjugated secondary antibodies (Supplementary Table 1) using a M.O.M. kit according to the manufacturer’s instructions (Vector Laboratories, Burlingame, CA, USA). The cells were counterstained with DAPI (4,6-diamino-2-phenylindole dihydrochloride; Vector Laboratories). Negative control-mouse IgG (Dako, Carpinteria, CA, USA) and -rabbit IgG (Dako) antibody were used as negative controls. To detect transplanted human cells, sections were immunofluorescently stained with anti-human nuclear antigen (HNA, Millipore). Stained sections were viewed with a model DXM1200F fluorescence microscope (Nikon, Tokyo, Japan). Processed images were analyzed for fluorescence intensity using ImageJ software (NIH).
Western blot analysis
Samples were solubilized in lysis buffer (20 mM Tris-HCl, pH 7.4; 150 mM NaCl; 1 mM EDTA; 1 % Triton X-100; 0.1 % sodium dodecyl sulfate (SDS); 1 mM phenylmethylsulfonyl fluoride; 1 μg/ml leupeptin; 2 μg/ml aprotinin) for 1 h at 4 °C. Lysates were then clarified by centrifugation at 15,000g for 30 min at 4 °C, diluted in Laemmli sample buffer containing 2 % SDS and 5 % (v/v) 2-mercaptoethanol, and heated for 5 min at 90 °C. Proteins were separated by SDS polyacrylamide gel electrophoresis (PAGE) using 10 or 15 % resolving gels followed by transfer to nitrocellulose membranes (Bio-Rad, Hercules, CA, USA). The membranes were incubated with primary antibody for 1 h at room temperature. For detection, peroxidase-conjugated anti-mouse IgG or anti-rabbit IgG and enhanced chemiluminescence (Amersham Pharmacia Biotech, Piscataway, NJ, USA) were used as described by the manufacturer. Membranes were scanned to create chemiluminescent images and to quantify with an image analyzer (Kodak, Rochester, NY, USA).
ELISA assay for angiogenic growth factor production
Angiogenic growth factor production in the L-spheroid was assayed with a commercially available ELISA kit (R&D Systems) according to the manufacturer’s protocols. Concentrations are expressed as the amount of angiogenic growth factor per 104 cells at a given time.
Preparation of the experimental animal model
Experiments involving mice were performed in accordance with the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health (NIH Publication No. 85-23, revised 1996). All aspects of the animal care and experimental protocols were approved by the Dankook University Committee on Animal Care. Hind limb ischemia was induced in 5-week-old BALB/c nude mice (20 g body weight; Narabio, Seoul, Korea) as previously described [9, 10]. The femoral artery and its branches were ligated through a skin incision using 5-0 silk suture. The femoral artery was excised from its proximal origin as a branch of the external iliac artery to the distal point where it bifurcates into the saphenous and popliteal arteries.
Treatment of limb ischemia
The animals were randomly distributed into four groups: PBS group, LLLT-treated group, ASC-treated group, combined treatment group (ASC + LLLT). hASCs (1.5 × 106 cells; hASC group, n = 9) cultured on TCP were mixed with PBS (300 μl) and injected intramuscularly into three sites of the gracilis muscle at the medial thigh of the ischemic limb. The PBS group received an injection of PBS (n = 9). Additionally, the LLLT group (n = 9) received light-emitting diode treatment. The physiological status of ischemic limbs was followed up to 3 weeks after treatment. The outcome was rated in three levels: limb salvage (similar limb integrity and morphology as normal limb control of the same animal), foot necrosis (death of the foot tissue), or hind limb loss. An equivalent number of cells were injected in both conditions. After 3 weeks, cutaneous blood flow was measured by a laser Doppler blood flowmeter (Laser Doppler Perfusion Imager System PeriScan PIM 3 System; Perimed AB, Stockholm, Sweden). Before scanning was initiated, mice were placed on a heating plate at 37 °C. After laser Doppler color image had been recorded twice, the average perfusion at ischemic and non-ischemic limbs was calculated on the basis of the colored histogram pixels. To minimize variables including ambient light and temperature, perfusion is expressed as the ratio of the ischemic to non-ischemic hind limb. Euthanasia was conducted by intravenous injection of thiopental sodium (40 mg/kg).
Low-level light therapy
Light-emitting diode (WON Technology, Daejeon, Korea) was applied for 10 min daily from day 1 to 20. The distance from the LED to the ischemic hind limb was 8 cm. This LED model exhibited an irradiated wavelength of 660 nm and power density of 50 mW/cm2. The fluence of each skin site was 30 J/cm2 (1 mW s = 0.001 J).
Statistical analyses
All the quantitative results were obtained from triplicate samples. Data were expressed as mean ± SD. Statistical analysis was carried out using two-sample t test for comparing two groups of samples and one-way analysis of variance (ANOVA) for three groups. A p value <0.05 was considered to be statistically significant.
Results
Characterization and phenotype of hASCs
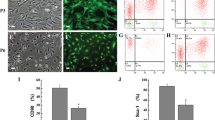
Adherent cells obtained from human adipose tissue were expanded in vitro. The cells were positive for human MSC markers CD29 (β1 integrin 96.9 %), CD90 (Thy-1 96.9 %), and CD105 (endoglin 70.9 %). However, the cells were negative for human endothelial cell markers CD34, CD31, KDR (VEGF receptor), and hematopoietic cell marker CD45 in immunofluorescence staining and flow cytometry analyses (Supplementary Fig. 1A, B). These results indicated that the expanded cells included a large population of hASCs and were not contaminated with endothelial cells.
Production of angiogenic factors by hASCs
The LLLT group showed an increase in the expression of hypoxia-induced survival factors such as hypoxia-inducible factor (HIF)-1α when compared to cells in the no LLLT treatment culture (Fig. 1a). The LLLT group showed considerable expression of the angiogenic growth factors, i.e., hepatocyte growth factor (HGF), vascular endothelial growth factor (VEGF), and fibroblast growth factor 2 (FGF2) (Fig. 1b).
Enhanced expression of hypoxia-induced survival factors and angiogenic growth factors in hASC + LLLT groups. hASC + LLLT and hASC were cultured for 3 days. a Western blot analysis and quantification of HIF-1α in hASCs. b ELISA measurement of hASC cultured for 3 days. Concentrations of growth factors are presented as picograms corrected for 104 cells (*p < 0.05 compared with 6 J/cm2 group, t test, n = 3 in each group)
Survival of ASCs in the ischemic limbs
At 21 days, apoptotic factor caspase 3-positive cells and HNA-positive cells were identified using fluorescence microscopy throughout the ischemic tissue to determine whether locally transplanted ASCs were incorporated into the ischemic hind limb. In the ASC and ASC + LLLT groups, ASCs were observed in the regenerated ischemic tissue (Fig. 2a), but no ASCs were found in the LLLT group. The ASC + LLLT group exhibited significantly increased numbers of HNA-positive cells (ASC group 12 %; ASC + LLLT group 35 % per DAPI-positive cells) (Fig. 2b) and decreased proportions of caspase 3-positive ASCs (ASC group 48 %; ASC + LLLT group 12 % per HNA-positive cells) (Fig. 2c). The HNA+ cell per DAPI+ cell ratio of the ASC + LLLT group was three times higher than that of the hASC group.
Survival of transplanted hASCs in hind limbs. a In the hASC and hASC + LLLT groups, DAPI-positive (blue) and caspase 3-positive (apoptotic marker; red) cells were detected after immunostaining at 21 days. hASCs were stained with HNA (green). Apoptosis of transplanted hASCs (arrows) was reduced in the hASC + LLLT group. b Ratio of HNA-positive cells (transplanted hASCs) to DAPI-positive cells (total cells) in the ischemic region (*p < 0.01, n = 4). c Ratio of caspase 3-positive cells plus HNA-positive cells (apoptotic transplanted hASCs) to HNA-positive cells (transplanted hASCs) in the ischemic region (*p 0.01, n = 4)
Enhanced secretion of angiogenic growth factors from grafted hASCs
Transplantation of hASCs into ischemic tissue enhanced paracrine secretion of angiogenic growth factors. Double immunofluorescent staining of HNA and the human angiogenic growth factors VEGF and FGF2 indicated secretion from transplanted hASCs in the ASC or ASC + LLLT group (Fig. 3a). Secreted human growth factors were mainly distributed in the vicinity of transplanted hASCs (HNA-positive cells). Compared with the ASC group, more growth factor-positive ASCs were observed in the ASC + LLLT group (Fig. 3a). Western blot assay showed that significantly higher levels of VEGF and bFGF were secreted by the LLLT and ASC + LLLT groups than by the PBS group, and a greater amount of growth factors was observed in the ASC + LLLT group than in the ASC group (Fig. 3b, c). However, there was no significant difference between ASC-treated tissues and PBS-injected tissues, indicating that the ASC + LLLT group was more effective than the ASC group at increasing transplanted cell retention and VEGF expression.
Enhanced secretion of angiogenic growth factors from hASCs in ischemic limb muscles. a Immunostaining was performed with anti-VEGF or anti-bFGF antibody (red) at 21 days. VEGF- or bFGF-positive ASCs are indicated by arrows. The scale bar indicates 100 μm. Injection of ASC + LLLT increased VEGF production 21 days after transplantation. b Western blotting showed the expression of VEGF and bFGF at 21 days. c The results of Western blotting were analyzed as relative density (*p < 0.05 compared to the hASC + LLLT group, n = 3)
Angiogenic efficacy
To verify the angiogenic effect of transplanted cells, smooth muscle actin (SMA)+ cell density and CD31+ vessel number were analyzed (n = 10 in each group; Fig. 4a–c). Many of the CD31+ cells in the ASC + LLLT group were double-stained for SMA. ECs and perivascular cells differentiated from injected human cells were detected by human αSMA and hCD31 antibodies, respectively (Fig. 4a–c). The area and number per unit area (1 mm2) of CD31+ vessel-like structures were compared among groups, as shown in Fig. 3b, c. The area of the ASC + LLLT group increased to four times that of the ASC group. Since we observed increased vessel formation in ischemic tissue and it is well known that the angiogenic process is stimulated by hypoxia, we further tested the hypothesis that the beneficial effect of LLLT could be due to activation of the transcription factor hypoxia-inducible factor-alpha (HIF-1α). Western blot assay demonstrated significantly higher levels of HIF-1α secreted by the LLLT and ASC + LLLT groups than by the PBS group (Fig. 4d, e) and greater amount of growth factors in the ASC + LLLT group than in the ASC group (Fig. 3b, c). However, there was no significant difference between ASC-treated tissues and PBS-injected tissues. HIF-1α could be responsible, at least in part, for some of the beneficial effects of LLLT therapy on ischemic tissues. These findings suggested the greater effectiveness of ASC + LLLT treatment in angiogenesis in ischemic limbs.
Endothelial cell and smooth muscle cell differentiation of transplanted cells. a The implants were removed on day 21 after transplantation and stained with anti-human CD31 and αSMA antibody. Scale bar 100 μm. All photographs were taken at the same magnification. Quantification of b capillary density and c arteriole density in the ischemic region (*p < 0.05 compared to the hASC + LLLT group, n = 4). d Western blotting shows the expression of HIF-1α at 21 days. e Western blot analysis quantification (*p < 0.01 compared to the hASC + LLLT group, n = 3)
Improvement of ischemic limb salvage by transplantation of hASCs
The therapeutic potential of hASCs was evaluated by physiological observation of ischemic limbs 21 days after treatment. In the absence of treatment, extensive muscle degeneration and inflammation were evident in the ischemic region (Fig. 5e, f), resulting in rapid limb necrosis at day 3 and complete limb loss due to autoamputation by day 21 (Fig. 5a). All mice in this group underwent limb loss (89 %) or severe limb necrosis (11 %) without limb salvage (0 %) (Fig. 5b). Transplantation of hASCs or LLLT treatment decreased limb necrosis after treatment (Fig. 5a), but limbs still showed muscle degeneration and inflammation in the ischemic region (Fig. 5e, f); 67 % of mice ultimately underwent limb loss at 21 days (Fig. 5b). In contrast, hASC+ LLLT therapy protected the limb muscle against necrotic damage induced by ischemia (Fig. 5e–g) and significantly reduced the rate of limb loss compared to the non-treatment and hASC treatment groups (Fig. 5a, b). Most mice receiving the hASC + LLLT treatment exhibited limb salvage (56 %) or only mild limb necrosis (22 %), although one of the ten mice did lose limbs (22 %) (Fig. 5b).
Improvement of ischemic limb salvage by ASC + LLLT treatment transplantation. a Representative photographs of PBS, hASC, and ASC + LLLT treatment-treated ischemic hind limbs on days 1, 7, 14, and 21 after treatment. b Physiological status of ischemic hind limbs 21 days after transplantation, n = 9 for each group. c Hind limb blood flow monitored in vivo by laser Doppler blood perfusion image ratio 21 days after femoral artery ligation and representative photos of the ischemic (right) and non-ischemic (left) hind limbs are shown. In color-coded images, normal perfusion is depicted in red, and marked reduction in blood flow of ischemic hind limb is depicted in blue. d Angiographic score (ischemic/non-ischemic leg) (*p < 0.05 compared to the LLLT group, n = 5 for each group). e, f Histological analysis of the ischemic hind limb muscles. e H&E staining and f Masson’s trichrome staining of histological ischemic limb sections 21 days after no treatment, LLLT treatment, hASC transplantation, and ASC + LLLT treatment transplantation. The scale bar indicates 200 μm. g Histological score
To determine the angiogenic potential of ASCs + LLLT treatment, we injected the cultured cells into the ischemic hind limb of the mouse 3 h after induction of ischemia by the ligation of the femoral artery. Figure 5c shows the relative blood flow images of the ischemic/normal hind limb and the quantitative analysis (Fig. 5d) of laser Doppler blood perfusion image ratio. Mice receiving PBS were considerably impaired with severe ischemic damage resulting in limb contracture, and other groups represented the increased blood flow index. In particular, quantitative analysis (Fig. 5d) showed that blood perfusion was enhanced significantly (p < 0.05) by ASC + LLLT treatment.
Discussion
As multipotent stem cells residing in the stromal fraction of adipose tissue, hASCs are an abundant source of the cells required for transplantation. In addition, their numbers can be expanded rapidly in vitro using standard cell culture techniques [17]. Significant levels of cell surface markers, including CD29, CD44, CD54, thyroxine-1 (CD90), and endoglin (CD105), are expressed in hASCs [18]. hASCs can also secrete angiogenic and anti-apoptotic factors, including VEGF and bFGF [19]. In addition, VEGF is one of the most important proangiogenic factors involved in therapeutic angiogenesis during and after ischemia [20]. bFGF is an important growth factor in the repair and regeneration of tissues because of the effects on migration and proliferation of fibroblasts, angiogenesis, and matrix deposition [21]. Endothelial progenitor cells and stem cells were previously examined for therapeutic angiogenesis in the treatment of cardiac infarction and ischemia [22]. Mostly, cells were injected into the target site. Some transplanted ASCs expressed endothelial or smooth muscle markers and were incorporated into vascular networks in ischemic sites, but the frequency was low because most cells die within the hypoxic and low-mass transfer microenvironment of the ischemic region [23, 24].
Although the exact mechanism is unknown, the final pathway of the photobiostimulation process seems to be the modification of the gene response. Measurement of this response can be determined through production of proteins that mediate inflammatory and healing responses. Thus, we decided to analyze the LLLT effect on the main transcription factor activated during hypoxia, HIF-1α. This protein is stabilized at low oxygen tensions, while at higher oxygen tensions, it is rapidly degraded by prolyl hydroxylase enzymes that are oxygen dependent. HIF-1α regulates the cellular response to physiological and pathological hypoxia by activating genes that are important to cellular adaptation and survival pathways under hypoxic conditions [25]. Of interest, increased HIF-1α expression and consequent induction of protein for VEGF, FGF, and HGF occurred at the fluence of 660 nm (Fig. 1). HIF-1α expression is primarily induced by hypoxia, but its induction can also be mediated by growth factors and cytokines. In this case, the induction does not depend on the oxygen tension and involves the activation of a different regulatory mechanism possibly mediated by mitogen-activated protein kinase and phosphatidylinositol 3-kinase/Akt signaling pathway [12]. In the current study, hASC transplantation with LLLT significantly improved angiogenesis (Fig. 4) as well as the subsequent survival of ischemic limbs compared to transplantation of hASCs (Fig. 2). This was likely due to the enhanced survival of hASCs (Fig. 2) along with increased paracrine secretion (Fig. 3). Specifically, enhanced paracrine secretion of angiogenic factors in the ASC + LLLT group at day 21 (Fig. 3) could have been due to enhanced cell survival (Fig. 2). These data suggest that LLLT enhances the survival of ASCs by the inhibition of apoptosis. Furthermore, in the ASC + LLLT group, more VEGF- or bFGF-positive ASCs were observed in the regenerated tissue, and greater amounts of growth factors were found in hind limbs than in the ASC group. Of interest, increased HIF-1α expression and consequent induction of protein for VEGF occurred at the fluence of 660 nm. LLLT increased the number of vessels in the tissue concomitantly with the increases in HIF-1α and VEGF expression [15, 16, 26]. It can be concluded that HIF-1α induction after LLLT does not occur due to a negative effect on the tissue, for instance, reducing even more the local oxygen tension, because LLLT enhances the survival and functionality of the transplanted ASCs in the hind limb [11–13]. Oxidative stress can also increase the expression of this transcription factor in the tissues.
In the current study, hASCs did undergo endothelial differentiation, supporting host angiogenesis by a paracrine effect in the ASC + LLLT group. The use of LLLT to stimulate new blood vessel growth has recently received considerable attention [27]. However, another study suggested that LLLT could avert the premature differentiation of these cells into other tissue types, such that stem cells could maintain their characteristics for longer periods [15]. Therefore, the effect of LLLT in the differentiation of stem cells is not fully understood, and further study is necessary.
Conclusions
Our study suggests that ASC transplantation with LLLT accelerates angiogenesis through differentiation and growth factor secretion. Furthermore, our results demonstrate that LLLT enhances the angiogenesis effect of the ASCs by enhancing survival of the ASCs and stimulating secretion of growth factors in hind limbs. In particular, the ASC + LLLT treatment enhances the functional recovery of the hind limb area with respect to the regeneration of tissues.
References
Mamidi MK, Dey S, Bin Abdullah BJ, Zakaria Z, Rao MS, Das AK (2012) Cell therapy in critical limb ischemia: current developments and future progress. Cytotherapy 14:902–916
Kyriakides TR (2009) The role of thrombospondins in wound healing, ischemia, and the foreign body reaction. J Cell Commun Signal 3:215–225
Huang JI, Jones NF, Zhu M, Lorenz HP (2004) Chondrogenic potential of multipotential cells from human adipose tissue. Plast Reconstr Surg 113:585–594
Gimble J (2003) Adipose-derived adult stem cells: isolation, characterization, and differentiation potential. Cytotherapy 5:362–369
Gonzalez-Rey E, González MA, Rico L, Büscher D, Delgado M (2009) Human adult stem cells derived from adipose tissue protect against experimental colitis and sepsis. Gut 58:929–939
Sumi M, Toya N, Yanaga K, Ohki T, Nagai R (2007) Transplantation of adipose stromal cells, but not mature adipocytes, augments ischemia-induced angiogenesis. Life Sci 80:559–565
Harada Y, Tsujimoto S, Matsugami H, Yoshida A, Hisatome I (2013) Transplantation of freshly isolated adipose tissue-derived regenerative cells enhances angiogenesis in a murine model of hind limb ischemia. Biomed Res 34:23–29
Heydarkhan-Hagvall S, Yang JQ, Heydarkhan S, Xu Y, Zuk PA (2008) Human adipose stem cells: a potential cell source for cardiovascular tissue engineering. Cells Tissues Organs 187:263–274
Bhang SH, La WG, Lee TJ, Yang HS, Sun AY, Baek SH, Rhie JW, Kim BS (2011) Angiogenesis in ischemic tissue produced by spheroid grafting of human adipose-derived stromal cells. Biomaterials 32:2734–2747
Park In S, Sang-Heon K (2013) A novel three-dimensional adipose-derived stem cell cluster for vascular regeneration in ischemic tissue. Cytotherapy 13:00681–00686
Hawkins D (2006) Effect of multiple exposures of low-level laser therapy on the cellular responses of wounded human skin fibroblasts. Photomed Laser Surg 24:705–714
Hu WP, Yu CL, Lan CC, Chen GS, Yu HS (2007) Helium-neon laser irradiation stimulates cell proliferation through photostimulatory effects in mitochondria. J Invest Dermatol 127:2048–2057
Alghamdi KM, Moussa NA (2012) Low-level laser therapy: a useful technique for enhancing the proliferation of various cultured cells. Lasers Med Sci 27:237–249
Whelan HT, Buchman EV, Whelan NT, Turner SG, Margolis DA, Cevenini V, Stinson H, Ignatius R, Martin T, Cwiklinski J, Philippi AF, Graf WR, Hodgson B, Gould L, Kane M, Chen G, Caviness J (2001) Effect of NASA light-emitting diode irradiation on wound healing. J Clin Laser Med Surg 19(6):305–314
Mvula B, Moore T, Abrahamse H (2008) The effect of low level laser irradiation on adult human adipose derived stem cells. Lasers Med Sci 23:277–282
Hou JF, Yuan X, Li J, Wei YJ, Hu SS (2008) In vitro effects of low-level laser irradiation for bone marrow mesenchymal stem cells: proliferation, growth factors secretion and myogenic differentiation. Lasers Surg Med 40:726–733
Zuk PA, Mizuno H, Huang J, Futrell JW, Katz AJ (2001) Multilineage cells from human adipose tissue: implications for cell-based therapies. Tissue Eng Part A 7:211–228
Tapp H, Patt JC, Gruber HE (2009) Adipose-derived stem cells: characterization and current application in orthopaedic tissue repair. Exp Biol Med 234:1–9
Cai L, Cook TG, Liang Z, Traktuev D, Cornetta K (2007) Suppression of hepatocyte growth factor production impairs the ability of adipose-derived stem cells to promote ischemic tissue revascularization. Stem Cells 25:3234–3243
Nie C, Morris SF (2009) Local delivery of adipose-derived stem cells via acellular dermal matrix as a scaffold: a new promising strategy to accelerate wound healing. Med Hypotheses 72:679–682
Karsan A, Poirier GG, Zhou P, Craig R, Harlan JM (1997) Fibroblast growth factor-2 inhibits endothelial cell apoptosis by Bcl-2-dependent and independent mechanisms. Am J Pathol 151(6):1775–1784
Badorff C, Popp R, Rupp S, Urbich C, Dimmeler S (2003) Transdifferentiation of blood-derived human adult endothelial progenitor cells into functionally active cardiomyocytes. Circulation 107:1024–1032
Miyahara Y, Kataoka M, Yanagawa B, Tanaka K, Hao H (2006) Monolayered mesenchymal stem cells repair scarred myocardium after myocardial infarction. Nat Med 12:459–465
Nakagami H, Morishita R, Iguchi S, Nishikawa T, Takami Y (2005) Novel autologous cell therapy in ischemic limb disease through growth factor secretion by cultured adipose tissue-derived stromal cells. Arterioscler Thromb Vasc Biol 25:2542–2547
Botusan IR, Savu O, Catrina AI, Grünler J, Poellinger L, Brismar K, Catrina SB (2008) Stabilization of HIF-1a is critical to improve wound healing in diabetic mice. Proc Natl Acad Sci U S A 105:19426–19431
Peplow PV, Ryan B, Baxter GD (2011) Laser photobiomodulation of gene expression and release of growth factors and cytokines from cells in culture: a review of human and animal studies. Photomed Laser Surg 29:285–304
Zaidi M, Jones DW, Pritchard KA Jr, Struve J, Nandedkar SD, Lohr NL, Pagel PS, Weihrauch D (2013) Transient repetitive exposure to low level light therapy enhances collateral blood vessel growth in the ischemic hindlimb of the tight skin mouse. Photochem Photobiol 89(3):709–713
Funding
This study was supported by a grant of the Ministry of Science, ICT and Future Planning funded by the Korea government (2012K1A4A3053142, NRF-2014R1A1A1038199).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Figure 1
Immunofluorescence staining and flow cytometry analyses of hASCs. (A) hASCs (passage 4) were stained with CD29, CD90 and CD105 for mesenchymal stem cell identification, with KDR, CD31 and CD34 for endothelial lineage cell identification, and SMA for smooth muscle cell identification. Scale bar: 200 μm (B) Flow cytometry analysis; hASCs cultured for 1 days were stained for CD29, CD90, CD105, CD45, CD31, CD34 and KDR expression and analyzed by flow cytometry. (TIFF 1962 kb)
Table 1
List of antibodies for immunofluorescence staining. (TIFF 2112 kb)
Table 2
Histological scoring system (TIFF 896 kb)
Rights and permissions
About this article
Cite this article
Park, IS., Mondal, A., Chung, PS. et al. Vascular regeneration effect of adipose-derived stem cells with light-emitting diode phototherapy in ischemic tissue. Lasers Med Sci 30, 533–541 (2015). https://doi.org/10.1007/s10103-014-1699-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10103-014-1699-9