Abstract
Breast implant-associated anaplastic large-cell lymphoma (BIA-ALCL) is a rare peripheral T cell lymphoma. BIA-ALCL is a disease of the fibrous capsule surrounding the implant and occurs in patients after both breast reconstruction and augmentation. More than 300 cases have been reported so far, including two in a transgender patient. Here we describe BIA-ALCL presented with a mass in a transgender patient and the first case of BIA-ALCL in the Czech Republic. In 2007, a 33-year-old transgender male to female underwent bilateral breast augmentation as a part of his transformation to female. In June 2014, the patient developed a 5-cm tumorous mass in her left breast. Magnetic resonance imaging of the chest revealed a ruptured implant and a tumorous mass penetrating into the capsule and infiltrating the pectoral muscle. An R0 surgery was indicated—the implant, silicone gel and capsule were removed, and the tumorous mass was resected together with a part of the pectoral muscle. Histology revealed anaplastic large-cell lymphoma. The patient underwent standard staging procedures for lymphoma including a bone marrow trephine biopsy, which confirmed stage IE. The patient was treated with the standard chemotherapy for systemic ALCL—6 cycles of CHOP-21. The patient was tumor-free at the 2-year follow-up. BIA-ALCL has been reported mostly in women who received implants for either reconstructive or aesthetic augmentation. This is the third report of BIA-ALCL in a transgender person, the first in the Czech Republic.
Level of Evidence V This journal requires that authors assign a level of evidence to each article. For a full description of these Evidence-Based Medicine ratings, please refer to the Table of Contents or the online Instructions to Authors www.springer.com/00266.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Anaplastic lymphoma kinase-negative (ALK−) anaplastic large-cell lymphoma (ALCL) is a rare peripheral T cell lymphoma [1]. In 1995, Duvic et al. suggested there might be an association between silicone implants and ALCL [2]. In 1997, Keech et al. [3] reported the first case of ALCL associated with a saline-filled breast implant.
So far, breast implant-associated ALCL (BIA-ALCL) has been reported exclusively in women who received implants for both reconstructive and aesthetic augmentation and in women with both silicone and saline-filled breast implants [4]. BIA-ALCL is a disease of the fibrous capsule surrounding the implant [5]. Morphologically, the neoplastic cells are large, epithelioid and pleomorphic with abundant cytoplasm, vesicular irregular nuclei, and frequent mitoses. The lesional cells typically show strong and diffuse immunoreactivity for CD30 and often express T cell markers. Almost all reported cases are negative for anaplastic lymphoma kinase [6]. There are two distinct clinical subtypes of BIA-ALCL. Most of the patients present with an accumulation of fluid around the implant, and a smaller group of patients present with a palpable tumor mass [7, 8]. The etiology, pathogenesis and predisposing risk factors have not been identified yet [9]. Possible risk factors are textured implants, chronic inflammation, subclinical biofilm, genetic predispositions or even autoimmune disease [9,10,11,12]. BIA-ALCL is clinically and pathologically distinct from systemic ALCL, ALK-negative (sALCL) and primary cutaneous ALCL (cALCL) [6]. In 2016, the World Health Organization classified breast implant-associated anaplastic large-cell lymphoma as a new entity and highlighted the importance of surgical management of the disease [13]. Clemens et al. [14] proposed TNM Staging for BIA-ALCL based on solid tumor TNM, due to its local progression.
According to National Comprehensive Cancer Network (NCCN) guidelines, symptomatic peri-prosthetic effusions greater than one year after implantation should be aspirated and screened for CD30 by immunohistochemistry and flow cytometry. BIA-ALCL localized in the capsule may be treated in the majority of cases with surgery alone. Extended BIA-ALCL with lymph node involvement should be treated with adjuvant chemotherapy. Local residual or unresectable disease may require radiation therapy treatment to the chest wall in the salvage setting. Distant organ involvement with lymphoma cells follows established NCCN guidelines for systemic ALCL treatment [15].
Until February 1, 2017, the United States Food and Drug Administration (FDA) received a total of 359 reports of breast implant-associated ALCL, including nine deaths [16]. In this article, we describe a unique case of primary BIA-ALCL in a transgender patient.
Case Report
In 2001, a 27-year-old white transgender male to female started his transformation to a female with hormonal therapy. In 2007, the patient underwent bilateral breast augmentation as a part of his transformation to female. During the operation, silicone gel-filled permanent anatomical textured implants McGhan 360 ml (Allergan, Irvine, CA, USA) were used and placed under the pectoral muscle from the periareolar incision. The operation and postoperative course were without any complications. Afterward, the patient’s matricular sex was changed into a female.
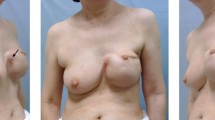
In January 2014, the patient developed a mass in the lower inner quadrant of her left breast. In June 2014, the patient sought a doctor. Besides the palpable mass, the patient had no other complaints. Physical examination revealed a poorly defined 5 cm tumorous mass and redness of the skin (Fig. 1). There was no evidence of capsular contracture or effusion. Because of the hormonal therapy due to transformation to female, a doctor first thought of breast carcinoma, so an ultrasound of the left breast was performed and showed the rupture of the implant and tumor mass. Subsequent magnetic resonance imaging (MRI) of the chest revealed the tumorous mass with multiple necrotic foci which indicated a very aggressive tumor. Imaging also showed infiltration of the pectoral muscle and penetration into the capsule (Fig. 2). Staging examinations were indicated—chest X-ray, ultrasound of the abdomen and skeletal scintigraphy were performed, which all turned out negative. The patient was then indicated for an ultrasound-guided core-cut biopsy, which revealed necrosis and deposits of purulent inflammation with surrounding production of fibrous tissue. A vacuum ultrasound-guided re-biopsy was indicated (11 samples were taken with a 10-G needle), but the results were not conclusive for a malignant disease revealing only abscess deposits. Routine blood tests were normal.
Breast MRI. a Native scan—T2 WI STIR (Short Time Inversion Recovery), spectral suppression of the silicon signal. The breast implant is shifted ventrally by the exudate and tumorous mass in the lower internal quadrant between the large pectoral muscle and the implant wall. Edema affects the surrounding subcutaneous tissue as well as part of the breast muscle. The size of the tumor corresponds to the subsequent contrast image and the hyperintense fluid seen inside the tumorous mass is central necrosis. On the right side, the normal state after augmentation can be observed. b Native scan—T1WI GRE (Gradient Echo). In the sagittal plane, there is a clear infiltration of the part of the large pectoral muscle by the tumor, the fascia of the muscle (the black line) is completely interrupted in the caudal course. Also, disruption of the wall of the breast implant is evident, especially cranial wall embossment (intracapsular rupture confirmed during the surgery). Thickening of the skin in the lower half of the breast clinically correlates with its redness
Extirpation of the tumorous mass was indicated. The incision was led in the submammary fold. A thin capsule was found, together with a ruptured silicone implant. Leaked silicone gel was found between the capsule and the implant. The implant, silicone gel and capsule were removed, and the tumorous mass was resected together with a part of the pectoral muscle (Fig. 3). During the operation, several smears were taken, coming back negative for both aerobic and anaerobic cultivations as well as negative for tuberculosis and actinomycosis.
Surprisingly, the histology revealed anaplastic large-cell lymphoma (ALCL), ALK 1 negative (Fig. 4). The excised capsule revealed infiltration with malignant lymphocytes highly positive for CD30 and CD4 and also diffuse high expression of cytotoxic markers perforin and granzyme B. The proliferation index of the malignant T cells according to Ki-67 marker was high (approx. 90%). The cells were negative for B-cell markers CD20 and PAX5 and also lacked expression of CD45R0, CD3, CD8, and ALK1. There was no microscopic residual tumor (R0 resection).
Shortly after the operation, on the patient’s demand due to the symmetry of the chest, the implant from the right breast was removed, and the patient was referred to a hemato-oncology center at the Charles University General Hospital in Prague. The patient underwent standard staging procedures for lymphoma including a positron emission tomography-computed tomography (PET-CT) scan and a bone marrow trephine biopsy, which confirmed stage IE disease according to the Ann Arbor classification (i.e., no systemic dissemination). According to BIA-ALCL tumor, lymph node and metastasis (TNM) staging and stages, adapted from Clemens et al. [14], our patient’s stage was IIA: T4N0M0. The patient had low-risk disease both according to the International Prognostic Index (IPI) for aggressive lymphoma (IPI = 0, age < 60 years, plasma level of LDH in normal range, disease stage = IE, number of involved extranodal organs = 1 and performance status according to ECOG score = 0) and according to the Prognostic Index for peripheral T cell lymphomas (PIT score = 0, no detectable bone marrow involvement). Despite the positive prognostic indexes, the patient was offered the standard chemotherapy treatment for systemic ALCL, namely because there is no standardized approach for BIA-ALCL, and the histological picture corresponded to a rather aggressive phenotype (high proliferation index according to Ki-67, expression of cytotoxic markers). Between September 2014 and January 2015, the patient received six cycles of CHOP-21 regimen in 3-week intervals (CHOP: cyclophosphamide 750 mg/m2 on day 1, hydroxydaunorubicin/doxorubicin 50 mg/m2 on day 1, oncovin/vincristine 1.4 mg/m2 on day 1, and prednisone 100 mg/day on days 1–5). The therapy was well tolerated, and the final PET-CT restaging in the February 2015 revealed complete remission of the disease. The last contact with the patient was in November 2016 with no clinical signs of the lymphoma relapse.
Discussion
We report the first case of BIA-ALCL in the Czech Republic and to our best knowledge, the third case of BIA-ALCL in a transgender person [17, 18]. Unlike the previous two cases of BIA-ALCL in a transgender patient, our patient presented with a 5-cm palpable mass, which infiltrated beyond the capsule to the major pectoral muscle. Surgical treatment was indicated, and an R0 resection was performed, which included removal of the implant, total capsulectomy and complete removal of the mass with negative margins. After the surgery, the patient received six cycles of the CHOP-21 regimen. Final PET-CT restaging revealed complete remission of the disease. The patient was tumor-free at the 2-year follow-up.
BIA-ALCL is an extremely rare subtype of T cell lymphoma first described in 1997 by Keech and Creech and included in the WHO Classification of Tumors of Haematopoietic and Lymphoid Tissue only in 2016 [13]. Most of the patients present with a seroma around the implant, and a smaller group of patients present with a palpable tumor mass [7, 8]. The etiology, pathogenesis and predisposing risk factors have not been identified yet [9]. Several risk factors like textured implants, chronic inflammation, subclinical biofilm, genetic predispositions or even autoimmune disease are discussed [9,10,11,12]. The most mentioned risk factor is a textured-surface implant. Brody et al. [10] who analyzed 173 cases of BIA-ALCL found two patients with smooth implants. Srinivasa et al. [19] reviewed 40 government authority databases and found 11 patients with smooth implants. Conversely, Doren et al. [20] conducted a US epidemiological study of BIA-ALCL and described no patient with smooth implants. Guillermo et al. [21] in their meta-analysis also did not find a patient with smooth implants. There are still many reported cases with an unknown surface of the implants, and therefore more research of this risk factor is necessary.
The diagnosis of the disease can be difficult due to insignificant clinical manifestation or due to low awareness about the illness. For these reasons, there is a risk, and BIA-ALCL could be mistaken for a breast cancer. According to NCCN guidelines from 2017, BIA-ALCL localized in the capsule may be treated in the majority of cases with surgery alone [15]. Surgical treatment, however, requires removal of the implant, total capsulectomy, and removal of any mass with negative margins [14]. In case of extended BIA-ALCL, adjuvant chemotherapy should be used [15]. Even though our patient was treated in 2014, when there was no standardized approach for BIA-ALCL, her treatment was led correctly and according to the newest guidelines published just this year.
In the past years, the number of reported BIA-ALCL cases increased and several epidemiologic studies were conducted. However, they are limited by small study populations, inaccurate and unconfirmed reporting [20]. Guillermo et al. conducted a meta-analysis of 80 reported cases of BIA-ALCL. The majority of the reported cases were from the USA, followed by Australia and New Zealand. An interesting fact is there are no reported cases from South America, China or Africa as confirmed by Srinivasa et al. in their review of 40 government databases [19, 21]. According to Aladily et al. [22] the incidence of BIA-ALCL in women who received breast implants is approximately 1 in 500,000. However, not every case was reported and if so, there is a lack of detailed information; therefore, it is possible, the incidence will be higher. Increasing numbers of reported cases of BIA-ALCL question the safety of breast implants. For determination of more exact incidence, prevalence and risk factors, a global registry is necessary.
Conclusions
Most clinicians have never dealt with a patient with breast implant-associated anaplastic large-cell lymphoma, and it is necessary to raise awareness about this clinical entity. Every patient, not only women but also transgender male to female patients, who undergo reconstruction or augmentation of the breast with implants should be informed about the risk of developing BIA-ALCL. Even though BIA-ALCL is usually nonaggressive, early diagnosis is critical. If the patient is diagnosed early and treated appropriately, the prognosis is good. Treatment of a BIA-ALCL patient should be led according to the NCCN guidelines.
References
Li S, Lee AK (2010) Case Report Silicone implant and primary breast ALK1-negative anaplastic large cell lymphoma, fact or fiction ? Int J 3:117–127
Duvic M, Moore D, Menter A, Vonderheid EC (1995) Cutaneous T-cell lymphoma in association with silicone breast implants. J Am Acad Dermatol 32:939–942
Keech JAJ, Creech BJ (1997) Anaplastic T-cell lymphoma in proximity to a saline-filled breast implant. Plast Reconstr Surg 100:554–555
Health C. for D. and R. Breast Implants. Anaplastic Large Cell Lymphoma (ALCL) in women with breast implants: preliminary FDA findings and analyses. Center for Devices and Radiological Health. https://www.fda.gov/medicaldevices/productsandmedicalprocedures/implantsandprosthetics/breastimplants/ucm239996.htm. Accessed: 15 Oct 2017)
Thompson PA, Lade S, Webster H et al (2010) Effusion-associated anaplastic large cell lymphoma of the breast: time for it to be defined as a distinct clinico-pathological entity. Haematologica 95:1977–1979. https://doi.org/10.3324/haematol.2010.026237
Xu J, Wei S (2014) Breast implant-associated anaplastic large cell lymphoma: review of a distinct clinicopathologic entity. Arch Pathol Lab Med 138:842–846. https://doi.org/10.5858/arpa.2013-0068-RS
de Jong D, Vasmel WLE, de Boer JP et al (2008) Anaplastic large-cell lymphoma in women with breast implants. JAMA 300:2030–2035. https://doi.org/10.1001/jama.2008.585
Story SK, Schowalter MK, Geskin LJ (2013) Breast implant-associated ALCL: a unique entity in the spectrum of CD30+ lymphoproliferative disorders. Oncologist 18:301–307. https://doi.org/10.1634/theoncologist.2012-0238
Hu H, Johani K, Almatroudi A et al (2016) Bacterial biofilm infection detected in breast implant-associated anaplastic large-cell lymphoma. Plast Reconstr Surg 137:1659–1669. https://doi.org/10.1097/PRS.0000000000002010
Brody GS, Deapen D, Taylor CR et al (2015) Anaplastic large cell lymphoma occurring in women with breast implants: analysis of 173 cases. Plast Reconstr Surg 135:695–705. https://doi.org/10.1097/PRS.0000000000001033
Yoshida SH, Swan S, Teuber SS, Gershwin ME (1995) Silicone breast implants: immunotoxic and epidemiologic issues. Life Sci 56:1299–1310. https://doi.org/10.1016/0024-3205(95)00081-X
George EV, Pharm J, Houston C et al (2013) Breast implant-associated ALK-negative anaplastic large cell lymphoma: a case report and discussion of possible pathogenesis. Int J Clin Exp Pathol 6:1631–1642
Swerdlow SH, Campo E, Pileri SA et al (2016) The 2016 revision of the World Health Organization classification of lymphoid neoplasms. Blood 127:2375–2390. https://doi.org/10.1182/blood-2016-01-643569
Clemens MW, Medeiros LJ, Butler CE et al (2016) Complete surgical excision is essential for the management of patients with breast implant-associated anaplastic large-cell lymphoma. J Clin Oncol 34:160–168. https://doi.org/10.1200/JCO.2015.63.3412
Clemens MW, Horwitz SM (2017) NCCN Consensus guidelines for the diagnosis and management of breast implant-associated anaplastic large cell lymphoma. Aesthet Surg J 37:285–289. https://doi.org/10.1093/asj/sjw259
Health, C. for D. and R. Breast Implants. Breast implant-associated anaplastic large cell lymphoma (BIA-ALCL). Center for Devices and Radiological Health. https://www.fda.gov/MedicalDevices/ProductsandMedicalProcedures/ImplantsandProsthetics/BreastImplants/ucm239995.htm. Accessed 15 Oct 2017
de Boer M, van der Sluis WB, de Boer JP et al (2017) Breast implant-associated anaplastic large-cell lymphoma in a transgender woman. Aesthet Surg J. https://doi.org/10.1093/asj/sjx098
Orofino N, Guidotti F, Cattaneo D et al (2016) Marked eosinophilia as initial presentation of breast implant-associated anaplastic large cell lymphoma. Leuk Lymphoma 57:2712–2715. https://doi.org/10.3109/10428194.2016.1160079
Srinivasa DR, Miranda RN, Kaura A et al (2017) Global adverse event reports of breast implant-associated ALCL. Plast Reconstr Surg. https://doi.org/10.1097/PRS.0000000000003233
Doren EL, Miranda RN, Selber JC et al (2017) U.S. epidemiology of breast implant-associated anaplastic large cell lymphoma. Plast Reconstr Surg 139:1042–1050. https://doi.org/10.1097/PRS.0000000000003282
Ramos-Gallardo G, Cuenca-Pardo J, Rodríguez-Olivares E et al (2017) Breast implant and anaplastic large cell lymphoma meta-analysis. J Investig Surg 30:56–65. https://doi.org/10.1080/08941939.2016.1215576
Aladily TN, Medeiros LJ, Amin MB et al (2012) Anaplastic large cell lymphoma associated with breast implants: a report of 13 cases. Am J Surg Pathol 36:1000–1008. https://doi.org/10.1097/PAS.0b013e31825749b1
Acknowledgments
This work was supported by the Charles University Center of Excellence grant UNCE 20402, by the Ministry of Education, Youth and Sports Institutional Support for Long-term Development of Research Organizations PROGRES Q26/LF1 and PROGRES Q28/LF1 and by Grant Agency of Charles University GAUK 130717.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors have no conflicts of interest.
Rights and permissions
About this article
Cite this article
Patzelt, M., Zarubova, L., Klener, P. et al. Anaplastic Large-Cell Lymphoma Associated with Breast Implants: A Case Report of a Transgender Female. Aesth Plast Surg 42, 451–455 (2018). https://doi.org/10.1007/s00266-017-1012-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00266-017-1012-y