Abstract
Introduction
Breast implant-associated anaplastic large cell lymphoma (BIA-ALCL) is a rare and recently described type of peripheral T-cell lymphoma. Fewer than 550 cases have been reported worldwide. Although BIA-ALCL is usually indolent, early diagnosis and treatment have been shown to improve outcome.
Case Description
This case report describes the management of a 50-year-old healthy Caucasian woman presenting with rapid painful enlargement of the left breast. Imaging revealed findings consistent with BIA-ALCL. This diagnosis was confirmed by fine needle aspiration cytology and subsequent pathological analysis. Bilateral removal of implants, complete left capsulectomy and immediate bilateral implant exchange were performed.
Conclusion
No consensus currently exists regarding optimal time of implant exchange and management of the contralateral capsule. The immediate replacement with smooth implants was thoroughly discussed with the patient and endorsed by expert opinion, given complete removal of the disease. There was no sign of recurrence at 6 months. Close clinical and radiological visits are planned for the next years.
Level of Evidence V
This journal requires that authors assign a level of evidence to each article. For a full description of these Evidence-Based Medicine ratings, please refer to the Table of Contents or the online Instructions to Authors www.springer.com/00266.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Anaplastic large cell lymphoma (ALCL) is one of the most common forms of peripheral T-cell lymphomas, a heterogeneous and usually aggressive sub-type of non-Hodgkin lymphoma. To date, four types of ALCL have been identified by the World Health Organization (WHO) based on differences in histological, cytological and clinical presentations and prognosis. Common to all four types of ALCL, however, is an expression of the CD30 T-cell marker [1].
Types 1 and 2 are primary systemic ALCL, which usually affect peripheral and/or retroperitoneal lymph nodes and may involve the lungs, liver, skin and bones. The main distinction between the two types is whether or not the anaplastic lymphoma kinase (ALK) gene is rearranged. ALK-positive (i.e., with a rearrangement of the ALK gene) is the most common type of primary systemic ALCL and usually has a better prognosis than its ALK-negative counterpart [2].
Type 3, known as primary cutaneous ALCL, is exclusively ALK-negative and has a very favorable prognosis. It is classified by the WHO as a lymphoproliferative disease rather than a lymphoma, underscoring its indolent nature. It is further distinguished from other types of ALCL by its characteristic skin tumors and lack of extracutaneous manifestations [3].
Type 4, the most recently recognized type, is breast implant-associated ALCL (BIA-ALCL). First described in 1997 by Creech and Keech [4], BIA-ALCL is a very rare form of the disease, usually indolent and poorly understood. The Food and Drug Administration (FDA) only officially recognized it in 2011. Fewer than 550 cases have been reported worldwide, but this figure is thought to underestimate the real number of cases because of unreliable implant sales data and insufficient recognition of the disease [5, 11].
Most cases present with a spontaneous unilateral capsule-confined seroma, which develops on average 8–10 years after implant placement and may be associated with pain or capsular contracture [3, 8, 9, 11]. Lymphadenopathy or extracapsular masses may be found initially in a minority of cases. The average age at diagnosis is 50 years [6,7,8,9,10,11]. To date, approximately half the diagnoses were made in cases of breast reconstruction following oncologic resection [8]. Although the specific pathogenesis of BIA-ALCL remains unclear, many believe the higher surface area of textured implants favors greater bacterial biofilm growth, which in turn induces chronic inflammation and subsequent T-cell dysplasia in genetically susceptible patients [8, 9].
Clinicians must keep a high degree of suspicion when faced with a delayed peri-prosthetic seroma. Poor knowledge of the clinical presentation, pathophysiology and treatment protocols have most likely lead to missed diagnoses, inadequate workup and ineffective treatment plans, which have all been shown to reduce 5-year survival rates [10].
Two distinct systems have been established for the staging of BIA-ALCL. The Lugano revision of the Ann Arbor algorithm, a common lymphoma staging system, was initially used. However, its stages failed to adequately differentiate subtypes of the disease, regrouping most cases in low-stage disease (IE or IIE). Clemens et al. therefore developed an adaptation of the TNM staging system at the MD Anderson Cancer Center (MDA). The MDA TNM staging is used in this report and is detailed in Table 1 [10].
Current treatment protocols have been defined by the National Comprehensive Cancer Network (NCCN) and depend on initial presentation and physical examination [12]. All patients diagnosed with BIA-ALCL should undergo surgical removal of the affected implant and surrounding capsule, as well as any associated masses and suspicious lymph nodes. If a contralateral implant is present, it too may be removed, as 4.6% of cases have incidental contralateral breast lymphoma [10,11,12,13]. There is no established surgical recommendation regarding the contralateral capsule [11,12,13]. Patients presenting with advanced disease (MDA Stage IIB–IV) may benefit from adjuvant systemic chemotherapy [11]. Currently prescribed chemotherapy is based on established protocols for ALK-negative systemic ALCL and includes cyclophosphamide, doxorubicin, vincristine and prednisolone (CHOP) as well as Brentuximab Vedotin regimens. Although radiation therapy has been attempted as an adjunctive treatment in many cases, its effect on the disease remains unclear and no reliable study has demonstrated a measurable benefit [3, 8, 10].
Most patients with localized disease without masses achieve complete remission and have excellent progression-free survival with a 100% survival rate at 5 years, whereas patients with extracapsular disease have an overall survival of 12 years [14].
No consensus on appropriate timing for breast reconstruction following oncologic treatment of BIA-ALCL currently exists [8]. At least one report recommended delayed reconstruction after 1 year of disease-free follow-up with appropriate imaging [15]. However, the benefits of a delayed reconstruction have yet to be elucidated. Expert opinions currently do not discard immediate replacement with smooth implants given localized disease, adequate preoperative oncological staging, as well as complete surgical resection of the disease [3, 10, 13]. No reports of disease recurrence in patients with immediate reconstruction have yet been published.
Case Description
In June 2007, a 40-year-old non-smoking Caucasian woman with no relevant medical or familial history and a healthy BMI presented with right breast capsular contracture following prior breast augmentation. The original retropectoral saline implants were replaced by anatomic textured Allergan silicone implants using an inframammary fold approach. The patient had no postoperative complications.
In May 2017, the patient noticed a rapid augmentation of volume of her left breast associated with pain. She did not report any recent weight loss, night sweats, fever or fatigue.
On examination, a swollen left breast was noted with no associated palpable mass or axillary nodes. Taut skin and a laterally deviated nipple were also apparent (Figs. 1, 2). Ultrasonography revealed the presence of a seroma, which underwent fine needle aspiration. The clear serous fluid was sent for bacterial culture, cytology, flow cytometry and cellblock analysis, which showed a diffuse proliferation of CD30-positive cells, confirming the suspected diagnosis of BIA-ALCL. A computed tomography scan of the thorax, abdomen and pelvic region revealed no significant anomalies. Magnetic resonance imaging of the breasts confirmed a unilateral seroma and revealed moderate enhancement of the capsule and surrounding soft tissues (Fig. 3). No axillary or internal mammary adenopathy and no extracapsular mass were observed. The contralateral breast was normal. The preliminary staging was thus assessed as localized stage I disease according to the MDA TNM system.
The current lack of literature regarding long-term risks of immediate replacement with smooth implants was discussed with the patient, as was the low estimated risk of disease in the contralateral implant and capsule. The patient understood and expressed a clear desire for immediate replacement. The authors consequently consulted international experts on BIA-ALCL, who endorsed immediate replacement with smooth implants given appropriate surgical excision (Dr Mark W Clemens, MD Anderson Cancer Center, Houston, TX, written and oral communication, October 2017).
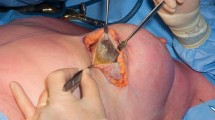
In October 2017, the patient underwent bilateral implant removal, total capsulectomy of the left breast and immediate bilateral replacement with smooth silicone implants. The capsule housed an evident seroma, estimated at 100 cc, 30 cc of which were sent for bacteriology, cytology and flow cytometry (Figs. 4, 5). The sample was not subjected to any special preparation prior to analysis. The left implant and capsule were sent “en bloc” to pathology, as was the right implant (Figs. 6, 7). Immediate bilateral breast implant replacement was performed using round, smooth, 560 cc silicone implants (Figs. 8, 9). No perioperative complications were encountered, and the patient was discharged the same day. No adjuvant chemotherapy was indicated given the localized nature of the disease, confirmed by the pathological analysis.
Clinical evaluation at 1- and 6-month postoperative visits showed no disease recurrence and proper implant placement (Figs. 10, 11, 12). An ultrasound completed at 4 months after surgery showed no evidence of seroma.
Histopathological Analysis
On macroscopic examination, the left implant was found to be almost entirely covered by a capsule with a thickness ranging from sub-millimetric dimensions to 3 mm. Microscopic examination of the capsule showed a thickened fibrous outer lining with numerous lympho-histiocitary inflammatory cells (Figs. 13, 14). The inner lining showed a continuous fibrin layer with intermittent agglomerations of large atypical pleomorphic cells with either large irregular and multi-lobed nuclei or multiple nuclei and large nucleoli. Mitoses were frequent. Tumor infiltration of the capsule and peri-prosthetic mammary tissues was not observed.
Immunohistochemistry of the atypical lymphoid proliferations revealed clear expression of CD3, CD5, CD30, EMA, Granzyne-B, bcl2, bcl6, c-myc, MUM1 and Ki-67. On the contrary, ALK1, CD20, CD79A, PAX5 and EBER-probe in situ hybridization study were all negative (Fig. 15).
The pathological findings thus confirm the diagnosis of CD30 +, ALK1 − malignant T-cell anaplastic large cell lymphoma of in situ sub-type associated to a breast implant. These findings correspond with Type IA disease according to the MDA TNM staging system.
Conclusion
At present, there is a paucity of conclusive literature regarding optimal time of implant replacement and contralateral capsulectomy. The rate of contralateral breast involvement of 4.6% should be discussed with the patient prior to surgical management. Furthermore, immediate implant replacement with smooth implants remains controversial. It may be considered, given localized disease and an appropriate discussion with the patient regarding the uncertainties of such a procedure. To date, the most commonly accepted etiology is the chronic inflammation induced by the biofilm and implant texture. Experts seem to agree that replacement with smooth implants is safe given complete surgical removal of localized disease. However, a close long-term follow-up is warranted.
Delayed reconstruction has some disadvantages including the need for a second surgery, a potential contracted soft tissue envelope rendering secondary surgery more challenging, and dissatisfaction with the appearance of the breasts and body image. Instead, immediate implant replacement offers a more satisfying initial surgical outcome and a hastier recovery for the patient. However, oncological data with regard to cancer recurrence in such cases is currently unknown. The authors recognize that 6-month follow-up is insufficient to rule out cancer recurrence and have therefore planned close clinical and radiological control visits.
Further studies involving cases of immediate reconstruction with long-term follow-up are warranted in order to better assess its outcome.
References
Swerdlow SH, Campo E, Pileri SA et al (2016) The 2016 revision of the World Health Organization classification of lymphoid neoplasms. Blood 127(20):2375–2390
Jacobsen E (2006) Anaplastic large-cell lymphoma, T-/null-cell type. Oncologist 11(7):831–840
Clemens MW, Jacobsen E. Breast implant-associated anaplastic large cell lymphoma [Document on the internet]. UpToDate; 19 April 2017 [Cited 2017 October 13]. https://www.uptodate.com/contents/breast-implant-associated-anaplastic-large-cell-lymphoma?search=bia%20alcl&source=search_result&selectedTitle=1~7&usage_type=default&display_rank=1
Keech JA Jr, Creech BJ (1997) Anaplastic T-cell lymphoma in proximity to a saline-filled breast implant. Plast Reconstr Surg 100(2):554–555
United States. U.S. Food and Drug Administration (2017) Breast implant-associated anaplastic large cell lymphoma (BIA-ALCL). [Document on the internet]. U.S. FDA: BIA-ALCL; 2017 [cited 2017 October 13]. https://www.fda.gov/MedicalDevices/ProductsandMedicalProcedures/ImplantsandProsthetics/BreastImplants/ucm239995.htm. Accessed 15 Oct 2017
Olack B, Gupta R, Brooks GS (2007) Anaplastic large cell lymphoma arising in a saline breast implant capsule after tissue expander breast reconstruction. Ann Plast Surg 59(1):56–57
Lazzeri D, Agostini T, Bocci G et al (2011) ALK-1-negative anaplastic large cell lymphoma associated with breast implants: a new clinical entity. Clin Breast Cancer 11(5):283–296
Leberfinger AN, Behar BJ, Williams NC, Rakszawski KL, Potochny JD, Mackay DR et al (2017) Breast implant-associated anaplastic large cell lymphoma: a systematic review. JAMA Surg 152(12):1161–1168
Loch-Wilkinson A, Beath KJ, Knight RJW, Wessels WLF, Magnusson M, Papadopoulos T et al (2017) Breast implant-associated anaplastic large cell lymphoma in Australia and New Zealand: high-surface-area textured implants are associated with increased risk. Plast Reconstr Surg 140(4):645–654
Clemens MW, Medeiros LJ, Butler CE, Hunt KK, Fanale MA, Horwitz S et al (2016) Complete surgical excision is essential for the management of patients with breast implant-associated anaplastic large-cell lymphoma. J Clin Oncol 34(2):160–168
Clemens MW, Brody GS, Mahabir RC, Miranda RN (2018) How to diagnose and treat breast implant-associated anaplastic large cell lymphoma. Plast Reconstr Surg 141(4):586e–599e
National Comprehensive Cancer Network. About NCCN. www.nccn.org/about. Accessed 10 Nov 2017
Clemens MW, Horwitz SM (2017) NCCN consensus guidelines for the diagnosis and management of breast implant-associated anaplastic large cell lymphoma. Aesthet Surg J 37(3):285–289
Miranda RN, Aladily TN, Prince HM, Kanagal-Shamanna R, de Jong D, Fayad LE et al (2014) Breast implant-associated anaplastic large-cell lymphoma: long-term follow-up of 60 patients. J Clin Oncol 32(2):114–120
Alhamad S, Guerid S, El Fakir EH, Biron P, Tourasse C, Delay E (2016) Breast implant-associated anaplastic large cell lymphoma. Case report of an undiagnosed form, management and reconstruction (ALCL). Ann Chir Plast Esthet 61(3):223–230
Acknowledgements
The authors would like to thank Dr. Mark W. Clemens of the MD Anderson Cancer Center for his valued advice regarding BIA-ALCL and contribution to this report. The authors would also like to acknowledge Dr. Tony Petrella for his generous contribution in the histopathological analysis of this case.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Human and Animal Rights
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards.
Informed Consent
Informed consent was obtained from all individual participants included in the study.
Rights and permissions
About this article
Cite this article
Shine, J.J., Boghossian, E., Beauchemin, G. et al. Breast Implant-Associated Anaplastic Large Cell Lymphoma: Immediate or Delayed Implant Replacement?. Aesth Plast Surg 42, 1492–1498 (2018). https://doi.org/10.1007/s00266-018-1204-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00266-018-1204-0