Abstract
Prolapse of the lacrimal gland is an acquired clinical condition caused mainly by relaxation of the local suspending ligaments. Before an aesthetic blepharoplasty, there should be a preoperative clinical suspicion of lacrimal gland pathology for patients with bulging lateral thirds of the upper eyelids. It should be borne in mind that inadvertent removal of the lacrimal gland can lead to important alterations in ocular lubrication. This report describes two clinical cases of patients with lacrimal gland prolapse associated with dermatochalasis and their treatment.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Among the various causes for bulging upper eyelids, lacrimal gland prolapse should be remembered because unintentional removal of the lacrimal gland due to a diagnostic error from thinking the gland is part of a local tumor or the orbital fat pad leads to the potential risk of corneal lubrication compromise.
The secretory lacrimal complex is composed of the lacrimal gland situated in the lacrimal fossa and two other accessory lacrimal glands (Krause and Wolfring, or Ciaccio) [1].
The lacrimal gland is derived from the thickening of five to eight basal conjunctival cells. It begins to develop in the interior eyelid and migrates first to the lateral corner, then later to the upper eyelid [20].
During development, part of the gland may become separated and develop as a separate tissue of the lacrimal gland [10]. Ectopia is the displacement of lacrimal gland tissue anywhere other than in its natural habitat. Congenital ectopia of the lacrimal gland has been observed in numerous regions of the eyes. The most common are the bulbar conjunctiva and the limbus. Orbital involvement is infrequent, and ectopic intraocular occurrence is rare [7]. Lacrimal ectopia of the eyelids, tarsal plate, and even the nasal mucosa also has been described [1, 11, 16].
Ectopia can occur in an isolated form or in combination with other mesodermal tissues [18, 19]. The ectopic secretions can accumulate and create an inflammatory reaction in the ectropic site. If this occurs in the orbit, it can produce proptosis at any age.
Acquired ectopia is termed prolapse (or herniation, migration, or even dislocation) [12] of the lacrimal gland. Either one or two lobes of the gland can prolapse. Usually, this results from aging or orbital trauma. With aging, the suspending lacrimal ligaments loosen and the gland prolapses. When the orbital lobe prolapses, a bulging in the lateral region of the upper eyelid is observed, and associated palpebral ptosis may coexist [17].
Two clinical cases are presented, illustrating patients with lacrimal gland prolapse associated with dermatochalasis, and management during blepharoplasty is discussed.
Anatomy
The lacrimal gland is a bilobed structure composed of the palpebral and orbital portions. The latter is twice the size of the former and is its main part. The lateral horn of the aponeurosis of the elevator muscle is the anatomic structure that divides it into lobes (Figs. 1 and 2). It occupies the lacrimal fossa formed by the frontal bones and the major and minor wings of the sphenoids [6, 14] (Fig. 3) in the lateral temporal quadrants of each orbit.
Relationship between the palpebral and orbital lobes of the lacrimal gland. A large portion of the elevator muscle (L) was removed, except for its lateral horn (B), exposing the subjacent Muller muscle (K). The palpebral lobe (J) is exposed, displaying its relationship with the floor (Muller muscle [K]) and exposing the bilobed shape of the lacrimal gland separating the orbital lobe (A) from the palpebral lobe (B). The other visible structures are I (medial cantal tendon), H (lacrimal sac), E (orbital fat pads), and D (Whitnall transverse ligament) [17]
Excretory ducts of the lacrimal gland’s orbital lobe: Five excretory ducts are visible (M) draining the orbital lobe (A) directly into the palpebral lobe (J) before draining into the superior conjunctival fornix [17]
Cranium demonstrating the fossa of the lacrimal gland (A) [6]
Two to six excretory ducts drain the orbital lobe up to the palpebral lobe, and from the latter, the lacrimal secretion attains the conjunctiva. Interruption of all these ducts between the two glandular lobes or removal of the palpebral lobe can alter occular lubrication unless aberrant ducts exist that connect the orbital lobe directly to the conjunctiva [17].
The orbital lobe is suspended by four units of ligaments. Posteriorly, two bands of fascia connect to the subperiosteal tissue. Superiorly, additional bands of connective tissue adhere to the subperiosteum. Anteriorly, fascial bands extend from the lacrimal gland to the lateral margin of the elevator aponeurosis, and medially, a wide ligament fixes the gland to the Whitnall transverse ligament [17].
Case Reports
Case 1
C.S.L., a 19-year-old black woman, reported edema in the lateral third of her upper eyelids since the age of 7 years, with progressive and slow exacerbation since the age of 10 years (Figs. 4 and 5). She denied local pain, draining secretions, ocular irritation, or “dry eyes” and epiphora. Her edema worsened at waking and also after crying spells, with slight improvement as the day progressed. She reported sporadic palpebral itching with mild hyperemia. There were no concomitant diseases or prior surgeries and no use of medications.
At physical examination, she displayed tumors in the lateral third of the upper eyelid. These tumors were 2 cm in diameter, movable, fibroelastic in consistency, painless, and almost symmetric. They could be reduced manually up to the lacrimal fossa. The tumors decreased on the ventral decubitus and the upper palpebral eversion. Lobulated and rosy subconjunctival tumors were seen in the lateral thirds (Figs. 6–8).
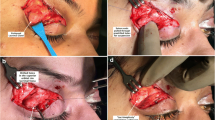
The patient underwent surgical treatment. Through a transverse skin incision along the upper palpebral sulcus, the palpebral portion of the orbicular muscles was exposed. Blunt dissection was performed until complete visualization of the orbital septum and its extensive dieresis was realized (Fig. 9). This exposed the medial and median orbital fat pads, where herniation of a whitish lobulated tissue was observed, with adherence to the median pad in the superior temporal region (Fig. 10).
After total and careful isolation of the gland in relation to the adjacent fat pad, we proceeded to redistribute the salient fat pads and to perform partial resection when they were very prominent. Using a retractor, the superior lateral orbital border was exposed, and the eyeball was dislocated caudally. We observed the region of the lacrimal fossa and its adjacent periosteum. Two or three sutures with nonabsorbable 5–0 monofilament nylon were placed between the orbital lobe of the lacrimal gland and the periosteum of the lacrimal fossa to reposition this structure, correcting the existing prolapse (Figs. 11–13). An incisional biopsy of the glandular lacrimal tissue was performed to rule out other possible anomalies.
After strict hemostasia, we proceeded with synthesis of the orbital septum and the orbicular muscle with simple interrupted sutures of 5–0 monofilament nylon (Fig. 14). The excess skin then was excised and synthesized with continuous sutures of 6–0 monofilament nylon, and a local dressing was applied.
Case 2
For 5 years, M.S.P., a 23-year-old black woman, reported edema in the lateral third of the upper eyelids, which was increasing slowly and progressively. She had no ocular complaints, palpebral itching, other systemic symptoms, or concomitant diseases. She was not using any medications.
At physical examination, she displayed bulging of the upper eyelids, with the same features described for case 1 (Figs. 15 and 16). The lacrimal gland prolapse was treated with a surgical procedure. Through a transverse skin incision along the upper palpebral sulcus, the orbital lobe of lacrimal gland was dissected from the adjacent fat pad and fixed on the periosteum of the lacrimal fossa. Glandular biopsies were performed.
In both reported cases, the excess skin was resected, and the orbital lobe of the lacrimal gland was repositioned bilaterally, as described. The glandular biopsies did not show pathologic changes, whereas chronic inflammatory infiltration in the skin was noted.
Literature Review
The medical literature shows that the difference between lacrimal ectopia and lacrimal prolapse is undefined. Because ectopia occurs in the same region as prolapse, it is difficult in certain cases to determine whether it is a congenital ectopia or a lacrimal prolapse. To resolve this controversy, we think that lacrimal prolapse should be considered a type of acquired lacrimal ectopia because for various reasons, the gland is no longer in its usual site. Furthermore, cases in which the lacrimal glandular tissue is detached from the main lacrimal gland, although in the upper lateral orbital quadrant, should be considered a congenital type of ectopia.
Some findings help to elucidate the diagnosis of lacrimal prolapse. When we evaginate the upper eyelid asking the patient to look down, the palpebral lobe prolapses, and a rosy lobulated mass below the palpebral conjunctiva is visible in the superior lateral fornix next to the tarsal plate [14, 17]. Comparison with the opposite side shows that the prolapse of the orbital fat generally is asymmetric. Prolapse of the lacrimal gland usually is bilateral and symmetric [12].
Compression of the eyeball increases orbital pressure and prolapses both the orbital fat and the already prolapsed lacrimal gland. With the pressure, the orbital fat brings the lacrimal portion of the gland anteriorly. However, the lacrimal gland is a deep structure and posterior to the preaponeurotic fat, thereby giving the site a more diffuse appearance [12]. In cases involving prolapse of the orbital portion, palpation between the thumb and index finger shows a slightly movable mass with well-defined margins that can be repositioned within the lacrimal fossa [17].
Most studies on lacrimal ectopia are based on case reports. The major study located in the literature to date is a retrospective study over the past 50 years that attempted to identify the lacrimal gland by histologic confirmation via products of tumor excision of the orbit except in the region of the lacrimal fossa [1]. The 120 cases were divided into two groups: 61 ectopic lacrimal gland cases (51%) and 59 prolapsed lacrimal gland cases (49%) [1]. Nevertheless, as in most case reports, no explanation was given about the difference between lacrimal ectopia and prolapse. Of those cases considered prolapsed, 54 (92%) occurred in the superior temporal bulbar conjunctiva (prolapse of the palpebral lobe), and the remaining 5 occurred in the orbital region (prolapse of the orbital lobe). Of the ectopic cases, 43 (70%) also were in the superior temporal conjunctiva.
These findings show that the so-called lacrimal ectopia predominates in the same region as the lacrimal glandular prolapse [1]. Furthermore, of the 59 prolapse cases, only 2 were correctly diagnosed preoperatively. The remainder were removed surgically and repositioned in the lacrimal fossa [1]. This demonstrates the large number of erroneous surgeries that could produce changes in ocular lubrication.
If the latter third continues to be prominent after superior blepharoplasty with the usual resection of palpebral fat pads, lacrimal prolapse should be presumed [8]. Between 10% and 15% of patients submitted to blepharoplasty can exhibit lacrimal gland prolapse [12]. Therefore, all patients between the ages of 20 and 50 years with bulging of the lateral third of the upper lid should be suspected of lacrimal gland prolapse [12]. Prolapse of the orbital region is not uncommon, but recognizing it as a prolapse is much rarer [1, 12, 17].
If the lacrimal gland is prolapsed but of normal size, many plastic surgeons, supported by reports in the literature, believe that partial excision of one-half to one-third of the orbital portion is harmless [8]. However, the dry eye syndrome occasionally reported in the literature after blepharoplasties (even in patients without glandular resection) is so distressful that it is frightening [8].
In a study by Schens and Dohlman [8] various dry eye syndrome findings appeared after partial resections of the lacrimal gland, leading to controversies in the literature. Because of this, a more conservative approach with reattachment of the gland to the lacrimal fossa is the option of choice for the majority of surgeons. These surgeons keep in mind that a wider resection around the lacrimal gland can shred it when it is being refixed.
Horton et al. [8] treat bulging of the lateral third of the upper eyelid by fixing the prolapse to the orbital periosteum above and behind the gland. Beer and Kompatscher [2] fix the prolapse between the Whitnall ligament and the periostium of the lacrimal fossa, indirectly reducing the glandular prolapse and avoiding direct sutures on the gland.
The primary goal in surgical treatment of prolapse is not to treat the palpebral lobe but to refix the orbital portion, taking with it the palpebral portion. Therefore, a prolapse of the palpebral portion leads to the assumption that prolapse also is present in the orbital portion. The orbital lobe is never visible through the superior conjunctiva of the eyelid, which occurs only with the prolapsed palpebral portion [12].
References
Alyahya GA, Bangsgaard R, Prause JU, Heegaard S (2005) Occurrence of lacrimal gland tissue outside the lacrimal fossa: comparison of clinical and histopathological findings. Act Ophthal Scand 83:100–103
Beer GM, Kompatscher P (1994) A new technique for the treatment of lacrimal gland prolapse in blepharoplasty. Aesth Plast Surg 18:65–69
Boccato P, Blandamura S, Midena E, Carollo C (1992) Orbital ectopic lacrimal gland tissue simulating a neoplasm: report of a case with fine-needle aspiration biopsy diagnosis. Acta Cytol 36:737–743
Cockerham KP, Hidayat AA, Cockerham GC, Depper MH, Sorensen S, Cytryn AS, Gavaris PT (2000) Melkersson-Rosenthal syndrome: new clinicopathologic findings in 4 cases. Arch Ophthalmol 118:227–232
Collin JRO (1991) Blepharochalasis: a review of 30 cases. Ophthal Plast Reconst Surg 7:153–157
Goldberg RA, Kim AJ, Kerivan KM (1998) The lacrimal keyhole, orbital door jamb, and basin of the inferior orbital fissure: three areas of deep bone in the lateral orbit. Arch Ophthalmol 116:1618–1624
Green WR, Zimmerman LE (1967) Ectopic lacrimal gland tissue: report of eight cases with orbital involvement. Arch Ophthalmol 78:318–327
Horton CE, Carraway JH, Potenza AD (1978) Treatment of lacrimal bulge in blepharoplasty by repositioning the gland. Plast Reconstr Surg 61:701–702
Jordan DR (1990) The blepharochalasis syndrome. Arch Ophthalmol 108:1633
Kaushik NC (1985) Ectopic lacrimal gland in the eyelids. Ind J Ophthalmol 33:65–66
McCulley TJ, Yip CC, Kersten RC, Kulwin DR (2002) An ectopic site of lacrimal gland secretion mimicking epiphora. Arch Ophthalmol 120:1586–1587
Smith B, Lisman RD (1983) Dacryoadenopexy as a recognized factor in upper lid blepharoplasty. Plast Reconstr Surg 71:629–632
Smith B, Petrelli R (1977) Herniation of the lacrimal glands. Trans Am Acad Ophthalmol Otolaryngol 84:988–990
Smith B, Petrelli R (1978) Surgical repair of prolapsed lacrimal glands. Arch Ophthalmol 96:113–114
Owsley JQ Jr (1980) Resection of the prominent lateral fat pad during upper lid blepharoplasty. Plast Reconstr Surg 65:4–9
Pe’er J, Ilsar M (1982) Ectopic lacrimal gland under the nasal mucosa. Am J Ophthalmol 94:418–419
Petrelli RL (1988) Management of prolapsed lacrimal glands. In: Hornblass A (ed) Oculoplastic, orbital, and reconstructive surgery. vol. 1, Cap. 59. Williams & Wilkins, New York
Pfaffenbach DD, Green WR (1971) Ectopic lacrimal gland. Int Ophthalmol Clin 11:149–159
Pokorny KS, Hyman BM, Jakobiec FA, Perry HD, Caputo AR, Iwamoto T (1987) Epibulbar choristomas containing lacrimal tissue: clinical distinction from dermoids and histologic evidence of an origin from the palpebral lobe. Ophthalmology 94:1249–1257
Rush A, Leone CR Jr (1981) Ectopic lacrimal gland cyst of the orbit. Am J Ophthalmol 92:198–201
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Friedhofer, H., Orel, M., Saito, F.L. et al. Lacrimal Gland Prolapse: Management During Aesthetic Blepharoplasty: Review of the Literature and Case Reports. Aesth Plast Surg 33, 647–653 (2009). https://doi.org/10.1007/s00266-008-9222-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00266-008-9222-y