Abstract
Background
Lacrimal gland prolapse (LGP) is considered to be one of the causes for upper eyelid contour abnormality that should be recognized and treated properly to yield satisfactory outcomes in blepharoplasty. To describe current findings about the prevalence, pre- and intraoperative diagnosis of LGP and its treatment options.
Methods
PubMed and Google Scholar were thoroughly searched for articles published describing the diagnosis and treatment of LGP.
Results
The reported prevalence of LGP by various authors varies between 10 and 60% based on their preoperative or intraoperative reports. Techniques such as dacryoadenopexy, modified dacryoadenopexy, and dacryoplasty have been described to secure the prolapsed lacrimal gland back into its original position. Additionally, creating a Whitnall’s barrier has also been suggested as a method to reposition the gland. While all these surgical procedures have shown promising immediate results, there is a lack of published data on their long-term outcomes.
Conclusion
Diagnosis and proper treatment of LGP could enhance the cosmetic results of upper eyelid blepharoplasty.
Level of Evidence IV
This journal requires that authors assign a level of evidence to each article. For a full description of these Evidence-Based Medicine ratings, please refer to the Table of Contents or the online Instructions to Authors www.springer.com/00266.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Lacrimal gland prolapse (LGP) should be recognized as one of the reasons for upper eyelid contour abnormalities. Leaving this problem unattended during blepharoplasty surgery leads to unacceptable post operation appearance due to prominent bulging of the lateral third of the upper eyelid [1]. Its prolapse might even become more obvious due to excision of the orbicular muscle during blepharoplasty and leads to unwelcome cosmetic results [2].
Around 10% of the patients who underwent upper blepharoplasty had clinically diagnosed LGP preoperatively [3]. This incidence is influenced by age and can increase to 15% in patients over 60 [4]. Intraoperative exploration for prolapsed lacrimal gland has discovered LGP in 60% of patients who underwent upper blepharoplasty [5]. This suggests that most mild cases of LGP are missed in preoperative examinations. It seems that a lesser degree of LGP is expected to be frequently found intraoperatively [6], and as the severity of prolapse increases by age, preoperative diagnosis of LGP increases as well [4]. Most reports have found LGP to be more frequent in females [3, 4, 7].
Lacrimal gland prolapse is also the second common manifestation, following eyelid ptosis, in patients with blepharochalasis. It affects approximately 44.09% of these patients and occurs due to atrophic changes of the orbital septum and the weakening of attachments between the upper eyelids and the lateral orbit [8].
It is also unfortunate that prolapsed lacrimal glands have been excised under various incorrect clinical diagnoses, including tumors and dermoid cysts. This suggests that the surgeons involved were unaware of this particular clinical condition [9]. Both inadvertent excision and overlooking the prolapsed lacrimal gland during eyelid surgery can pose potential problems [10]. The objective of this review is to provide an overview of current opinions on the diagnosis and treatment options for lacrimal gland prolapse. This includes discussions on its clinical presentation, risk factors, underlying pathophysiology, diagnostic techniques such as physical examination and imaging modalities, differential diagnoses to consider, and available treatment options. Describing the causes and treatment of pathologic gland enlargement (including inflammation and neoplasm) and ectopic lacrimal gland is beyond the scope of this manuscript and is not going to be discussed here.
The Anatomy of Lacrimal Gland
The lacrimal gland is situated within the lacrimal fossa of the frontal bone in the upper temporal orbit. It has an average length of 20mm and is divided into two lobes by the lateral horn of the levator aponeurosis. The larger and thicker lobe, located just inside the lacrimal fossa, is called the orbital lobe, while the smaller part beneath the levator aponeurosis (in the subaponeurotic Jones’ space) is known as the palpebral lobe. Approximately 8–12 excretory ducts drain the secretions of the lacrimal gland into the superotemporal conjunctival fornix [11].
The gland is attached to the subperiosteal tissue posteriorly by two fascia bands and superiorly by connective tissue. It is confined to the orbital septum, preaponeurotic fat pad, and lateral horn of the levator aponeurosis anteriorly, and to the Whitnall’s ligament and intermuscular membrane between the superior and lateral recti medially [11, 12].
Etiology and Pathology of LGP
Soft tissue weakness and ligamentous relaxation caused by age is considered to be the main reason for prolapsing the lacrimal gland. Weakening of the orbital septum and Whitnall’s ligament, as well as the gland attachment to the lacrimal fossa, leads to anterior and inferior displacement of the lacrimal gland [2, 13, 14].
LGP can be observed in patients without any other eyelid or orbital adnexal disorders, or it may be seen in conjunction with other abnormalities associated with laxity of the orbital adnexa, such as blepharochalasis and floppy eyelid syndrome [3, 8, 15, 16]. LGP can also manifest in patients with elevated intraorbital pressure, including those with thyroid eye disease and congenital craniofacial malformations [17, 18].
It is usually recommended to obtain a biopsy specimen from the prolapsed lacrimal gland if there are suspicions of an underlying malignancy or inflammatory disorder. A biopsy segment of approximately 4 mm from the leading edge of the gland should be sufficient [12]. The most common pathologic findings in LGP is mild chronic inflammation in an otherwise normal looking lacrimal gland; this is likely a result of the mechanical and repetitive movements of the displaced lacrimal gland [3, 7].
Clinical Features of LGP
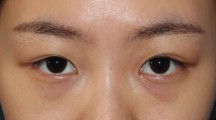
Recently, there has been a significant increase in awareness of LGP among patients, which can be attributed to the influence of social media. According to reports, as much as 72% of individuals considering upper lid blepharoplasty have expressed concerns about bulging specifically on the lateral side of the eyelid; 20% of them have suspected the prolapsed lacrimal gland. Therefore, it is crucial to thoroughly evaluate patients’ complaints regarding their eyelid abnormalities [2, 3]. Prolapse of the orbital lobe of the lacrimal gland demonstrates as a visible bulge beneath the skin on the outer part of the upper eyelid (Figs. 1a and c and 2a) [12]. It is the most commonly reported as a chronic and painless mass that could even give the impression of temporal ptosis in severe cases [19].
a and c show the preoperative photographs of the patient in front and side view, respectively, displaying lateral bulging of the upper eyebrow (indicated by the white arrow), which is caused by the prolapsed lacrimal gland. b and d depict the postoperative photographs of the same patient after undergoing combined upper eyelid blepharoplasty and dacryoadenopexy, demonstrating no evidence of LGP
The differentiation between prolapsed lacrimal gland and preaponeurotic fat pad could be a clinical challenge. Even though in the absence of lateral bulging the presence of LGP is highly unlikely (negative predictive value of 96%), its presence does not necessarily indicates LGP (positive predictive value of 30%); more than two third (70.6%) of the patients with lateral eyelid bulging did not have LGP which highlights the importance of distinguishing between these two conditions [4]. It is worth noting that unlike herniation of orbital fat, LGP commonly manifests bilaterally and symmetrically [3, 14].
In patients with LGP, a pink subconjunctival mass could be seen in the upper fornix especially when asking the patient to look down. It is important for the examiner to note that the orbital portion of the lacrimal gland cannot be directly observed from the conjunctival side of the upper eyelid. Nevertheless, the presence of this finding should raise suspicion regarding a prolapsed orbital portion of the lacrimal gland [20].
The “Supine test” can also be used for the clinical diagnosis of LGP. During this test, patients who are suspected to have LGP are asked to lie down and look down, while their eyebrow is gently lifted (Supplemented Video 1). If a bulge of the lacrimal gland is visible beneath the skin, it should be considered a positive test result that suggests LGP. It has been observed that a majority of the patients with positive supine test exhibit considerable intraoperative gland prolapse which suggests that the chance for suspension requiring lacrimal gland is high enough to justify surgical attempt to explore LGP in patients with positive supine test [4]. The necessity of exploring for LGP in patients with a negative supine test remains uncertain. However, some other authors recommend intraoperative LGP exploration and gland resuspension, if necessary, for all patients who undergo upper blepharoplasty. This approach is suggested to prevent future involutional changes related to LGP [6]; however, currently the latter approach lacks enough clinical evidence that can be suggested to the surgeons.
Orbital imaging, such as CT or MRI, in patients with LGP can reveal a slightly enlarged gland that is displaced forward forming a “J-shape” as the gland prolapses around the lateral orbital rim (Fig. 3). Lack of involvement of other orbital structures can also be supportive of the diagnosis of LGP [7].
LGP in Thyroid Associated Orbitopathy (TAO)
In TAO, the inflammatory edema affecting the retrobulbar structures results from the infiltration of lymphocytes and hyperplasia of adipose tissue. This condition stresses the fibrous periosteal connections between the lacrimal gland and the orbital septum, ultimately leading to the development of LGP [18].
The degree of LGP in TAO could be measured on axial T2 weight images as the vertical distance from the top of the lacrimal gland to the interzygomatic line [17]. The severity of LGP in TAO obtained from orbital magnetic resonance images were positively correlated with Clinical Activity Score, proptosis, and extra-ocular muscle volume. These findings suggest that LGP could serve as an indicator of disease activity in patients with TAO [18].
Surgical Procedures for Treating LGP
Currently, there is no general consensus regarding the grading of LGP severity. Grading is primarily determined by the extent of forward transposition of the lacrimal gland’s tip (Fig. 4b) and is categorized as follows: mild (less than 3–4 mm), moderate, and severe (greater than 6–7 mm).[4, 5] This grading system is utilized to determine the appropriate procedure for relocating the lacrimal gland. While cautery of the tip of the lacrimal gland has been utilized for mild cases of LGP (prolapse of less than 3–4 mm) [4, 5], surgical fixation of the prolapsed lacrimal gland should be considered in more severe gland protrusion. In general, the surgical methods consist of re-adhesion of lacrimal gland inside the lacrimal fossa and establishing a barrier to hinder its forward displacement.
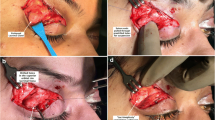
a displays an intraoperative photograph of the patient diagnosed with LGP, revealing the prolapsed lacrimal gland (white arrow) following the resection of the orbicularis oculi muscle and the opening of the orbital septum. The preaponeurotic fat pad is also visible (white asterisk), while the dotted line delineates the superolateral orbital rim positioned above the lacrimal gland. b exhibits the measurement of lacrimal gland prolapse severity, extending from the supralateral orbital rim to the tip of the gland
Dacryoadenopexy
Following the incision of the skin and removal of the temporal orbicularis oculi muscle (as indicated), the dissection is extended upward to the temporal two-thirds of the superior orbital rim. Subsequently, the orbital septum is incised, revealing the protruded lacrimal gland (Fig. 4a). Gently applying pressure on the globe can assist in further protrusion of the lacrimal gland through the incision.
The preaponeurotic fat may protrude from the incision (Fig. 4a), particularly when digital pressure is applied, and it is important to distinguish it from the glandular tissue. Unlike the yellowish, amorphous, and soft texture of orbital fat, the lacrimal gland exhibits a pinkish-gray color, distinct shape, and firm consistency. The excessive herniated orbital fat should typically be excised and cauterized using standard procedures [12,13,14, 19].
At this point, a pocket can be created above the lacrimal gland and beneath the orbital bone through gentle dissection, forming a space for the lacrimal gland to rest [1, 13]. To securely position the herniated lacrimal gland within the lacrimal fossa, a mattress suture with non-absorbable material (ideally a double-armed 5–0 or 6–0 prolene) passed through the lacrimal gland; each needle is then inserted from the back to the front through the periosteum of the orbital roof near the orbital rim. It is important to take a bite from inferior part of the visible large orbital lobe of the prolapsed gland capsule and fix it to a point just behind lateral margin of the superior orbital rim to ensure correct positioning of the gland. To prevent the recurrence of lacrimal gland prolapse, sufficient whip stitches should be placed within the lacrimal gland, ensuring that the full thickness of the periosteum in the orbital roof is engaged (Fig. 5a and b) [12, 20, 21].
Periosteal fixation of the lacrimal gland with horizontal mattress sutures have also been described. In this so called “modified” technique, the needle passes through the lacrimal gland once posteriorly and once through the anterior part of the orbital lobe in horizontal fashion; then, the needle passes horizontally through the periosteum of the lacrimal fossa. By placing the knots behind the orbital rim, this technique minimizes skin irritation. Additionally, it claims to have the advantage of suspending the gland both anteriorly and posteriorl [2].
Unlike previous emphases on closing the orbital septum [20], the general belief today is that the septum should not be sutured to prevent unwanted vertical eyelid shortening [12]. The skin and soft tissue are then sutured in a standard blepharoplasty manner.
Hemostasis is crucial during dacryoadenopexy due to the glandular tissue’s tendency to bleed, which can lead to hematoma formation and potential wound complications. It is also important to employ none absorbable suture to prevent LGP in the future [20]. Additionally, surgeons should pass the sutures parallel to the excretory ducts of the orbital lobe to avoid inadvertent ligation of the ducts.
Chaudhari et al. [22] described an alternative surgical approach involving the placement of two drilled holes made parallel to the orbital rim and about 2 mm immediately above the location of prolapsed lacrimal gland. Non-absorbable suture (4–0 prolene) is then passed through the lateral orbital rim hole to the inferior border of the prolapsed lacrimal gland, and then, the swaged end of the needle is passed through the medial hole from inside-out. By tightening the suture tie the orbital lobe of the lacrimal gland recedes back into lacrimal fossa. They named this technique “lacrimoplasty” and claim that it provides superior outcomes compared to periosteal fixation, especially for patients with severe tissue laxity, as it reduces the chances of recurrence.
To prevent superotemporal hollowness after repositioning the lacrimal gland in Asian upper eyelid blepharoplasty, a suggested technique involves preserving and laterally moving the prolapsed preaponeurotic fat tissue as a pedicled fat flap [23]. It is claimed that by securing the flap to the lateral margin of the orbital septum with non-absorbable sutures, a natural and smooth upper eyelid contour can be achieved. However, it is important to note that some authors have argued against the necessity of this fat flap technique in non-Asian blepharoplasty, as postoperative hollowness in the upper eyelid has not been reported as a concern among Caucasian patients who underwent upper eyelid blepharoplasty with lacrimal gland prolapse fixation [24]; as a result, the use of a fat flap over the prolapsed lacrimal gland site remains a topic of debate.
Creating the Whitnall’s Barrier
Creating a barrier to prevent herniation of lacrimal gland has been a part of LGP treatment since the very beginning. Authors have recommended suturing the orbital septum over the repositioned lacrimal gland to prevent its future displacement [14, 20]. However, to reduce the risk of vertical shortening, repairing the orbital septum has been discarded. Instead, Whitnall’s ligament has emerged as an intriguing alternative for creating a barrier. After exposing the lacrimal gland (as previously described), it should be gently repositioned without further manipulation to maintain the integrity of the supporting structures. Then, the lateral third of Whitnall’s ligament is carefully moved and bent in a rectangular shape over the repositioned gland. Non-absorbable suture is used to affix the ligament to the periosteum of the orbital rim which is rather thick in contrast to the thin periosteum of the orbital fossa. In this procedure, dissection of Whitnall’s ligament is not necessary; however, care should be taken to not incorporating orbital septum and/or levator ligament into the suture inadvertently [19, 25].
LGP Repair in Blepharochalasis
There are reports about repositioning the herniated lacrimal gland in patients with blepharochalasis. Currently, the preferred procedure involves suspending the lacrimal gland to the lacrimal fossa using non-absorbable sutures [15, 16]. This approach is typically combined with upper blepharoplasty to address both skin laxity and lacrimal gland ptosis (LGP). However, the duration for which this surgery effectively maintains the lacrimal gland in its corrected position has not been specified.
Surgical Complications
The authors have not reported any major complications associated with lacrimal gland repositioning. It is important to note that the lacrimal gland contains a rich vascular tissue; thus, meticulous hemostasis is crucial to prevent intraoperative and postoperative hemorrhage and hematoma. Patients may experience postoperative pain that is usually mild but could be bothersome for some others. Nevertheless, the most significant surgical complication, which can be quite problematic, is the potential for persistent dry eye. This may occur due to disruption of the lacrimal gland ductules when engaged by sutures passing through the gland tissue [26, 27].
As previously mentioned, advancements in surgical techniques aim to reduce the likelihood of recurrent lacrimal gland prolapse in the future. However, there is a lack of definitive data regarding the long-term recurrence rates for any specific surgical procedure. There is a limited amount of data available regarding the chances of LGP recurrence, and its correlation with the severity of LGP. Additionally, it remains unclear whether specific or more advanced suturing techniques are necessary to secure the lacrimal gland in patients with thicker glands, greater gland mobility during surgery, and more severe prolapse. Further research is needed to shed light on these aspects. All of the procedures discussed above have favorable early postoperative outcomes, and it is our opinion that properly securing the gland to the lacrimal fossa’s periosteum using non-absorbable sutures is a sufficient method to maintain the gland in its proper position for a considerable duration of time.
Skin discomfort and suture erosion can also be potential issues for patients who undergo dacryoadenopexy, particularly in cases where non-absorbable sutures are utilized in patients with thin skin and prominent lateral orbital rim. Proper suture burial techniques can help prevent postoperative discomfort in these patients.
Conclusion
This comprehensive review aims to gather a wealth of information on the diagnosis and management of prolapsed lacrimal gland. It traces the historical development of knowledge on this condition, starting from the groundbreaking clinical description by Smith et al. [14] and covering important milestones up to the present day. The primary objective of this review was to provide aesthetic surgeons with a comprehensive understanding of importance of diagnosing LGP as they perform upper eyelid blepharoplasty procedures. By incorporating this knowledge into their practice, surgeons can optimize cosmetic outcomes and enhance patient satisfaction. The reported prevalence of LGP varies and is influenced by age with a higher incidence in older individuals. LGP can also be associated with blepharochalasis and thyroid-associated orbitopathy. Diagnosis of LGP can be challenging, and clinical features such as visible bulging, subconjunctival mass, and positive supine test can aid in its preoperative diagnosis. Surgical procedures for treating LGP include dacryoadenopexy, modified dacryoadenopexy, dacryoplasty, and creating a Whitnall’s barrier to secure the gland in its original position and prevent further displacement. Hemostasis and careful suturing techniques are important to minimize complications. Future research should focus on long-term outcomes of these surgical procedures. Overall, recognition and management of LGP are essential for achieving optimal aesthetic outcomes and patient satisfaction in eyelid surgery.
References
Horton CE, Carraway JH, Potenza AD (1978) Treatment of a lacrimal bulge in blepharoplasty by repositioning the gland. Plast Reconstr Surg 61(5):701–702. https://doi.org/10.1097/00006534-197805000-00006
Vatansever M (2023) Modified lacrimal gland suspension technique in patients who underwent upper eyelid blepharoplasty. J Craniofac Surg. https://doi.org/10.1097/scs.0000000000009538
Eshraghi B, Ghadimi H (2020) Lacrimal gland prolapse in upper blepharoplasty. Orbit 39(3):165–170. https://doi.org/10.1080/01676830.2019.1649434
Kashkouli MB, Tabrizi A, Ghazizadeh M, Khademi B, Karimi N (2019) Supine test: a new test for detecting lacrimal gland prolapse before upper blepharoplasty. Ophthalmic Plast Reconstr Surg 35(6):581–585. https://doi.org/10.1097/iop.0000000000001397
Massry GG (2011) Prevalence of lacrimal gland prolapse in the functional blepharoplasty population. Ophthalmic Plast Reconstr Surg 27(6):410–413. https://doi.org/10.1097/IOP.0b013e31821d852e
Fakih-Gomez N, Zarate JM, Martins L, Lindo Delgadillo LM (2023) Fakih-gomez I contemporary upper blepharoplasty: volumetric contouring concept. Am J Cosmetic Surg. https://doi.org/10.1177/07488068231171313
Huang S, Juniat V, James C, McNab A, Selva D (2023) Histopathological characteristics of lacrimal gland prolapse. Ophthalmic Plast Reconstr Surg 39(4):389–393. https://doi.org/10.1097/iop.0000000000002335
Zhou J, Ding J, Li D (2021) Blepharochalasis: clinical and epidemiological characteristics, surgical strategy and prognosis– a retrospective cohort study with 93 cases. BMC Ophthalmol 21(1):313. https://doi.org/10.1186/s12886-021-02049-4
Alyahya GA, Bangsgaard R, Prause JU, Heegaard S (2005) Occurrence of lacrimal gland tissue outside the lacrimal fossa: comparison of clinical and histopathological findings. Acta Ophthalmol Scand 83(1):100–103. https://doi.org/10.1111/j.1600-0420.2005.00365.x
Kashkouli MB, Heirati A, Pakdel F (2011) Lacrimal gland fistula after upper eyelid blepharoplasty. Middle East Afr J Ophthalmol 18(4):326–327. https://doi.org/10.4103/0974-9233.90139
Conrady CD, Joos ZP, Patel BC (2016) Review: the lacrimal gland and its role in dry eye. J Ophthalmol 2016;2016:7542929. https://doi.org/10.1155/2016/7542929
Petrelli RL (1988) The treatment of lacrimal gland prolapse in blepharoplasty. Ophthalmic Plast Reconstr Surg 4(3):139–142. https://doi.org/10.1097/00002341-198804030-00003
Castañares S (1979) Prolapse of the lacrimal gland: findings and management during blepharoplasty. Aesthetic Plast Surg 3(1):111–118. https://doi.org/10.1007/BF01577843
Smith B, Petrelli R (1978) Surgical repair of prolapsed lacrimal glands. Arch Ophthalmol 96(1):113–114. https://doi.org/10.1001/archopht.1978.03910050069017
Çakmak S, Göncü T (2014) Lacrimal gland prolapse in two cases of blepharochalasis syndrome and its treatment. Int Ophthalmol 34(2):293–295. https://doi.org/10.1007/s10792-013-9766-y
Hundal KS, Mearza AA, Joshi N (2004) Lacrimal gland prolapse in blepharochalasis. Eye (Lond) 18(4):429–430. https://doi.org/10.1038/sj.eye.6700668
Gagliardo C, Radellini S, Morreale Bubella R, Falanga G, Richiusa P, Vadalà M, Ciresi A, Midiri M, Giordano C (2020) Lacrimal gland herniation in Graves ophthalmopathy: a simple and useful MRI biomarker of disease activity. Eur Radiol 30(4):2138–2141. https://doi.org/10.1007/s00330-019-06570-5
Gao Y, Chang Q, Li Y, Zhang H, Hou Z, Zhang Z, Li Z, Li D (2022) Correlation between extent of lacrimal gland prolapse and clinical features of thyroid-associated ophthalmopathy: a retrospective observational study. BMC Ophthalmol 22(1):66. https://doi.org/10.1186/s12886-022-02270-9
Beer GM, Kompatscher P (1994) A new technique for the treatment of lacrimal gland prolapse in blepharoplasty. Aesthetic Plast Surg 18(1):65–69. https://doi.org/10.1007/bf00444250
Smith B, Lisman RD (1983) Dacryoadenopexy as a recognized factor in upper lid blepharoplasty. Plast Reconstr Surg 71(5):629–632. https://doi.org/10.1097/00006534-198305000-00008
Petrelli RL (1986) The prolapsed lacrimal gland in blepharoplasty. Orbit 5(4):267–271. https://doi.org/10.3109/01676838609036059
Choudhary S, Khanna S, Mantri R, Arora P, Jain R, Dutta S (2018) Lacrimoplasty: a new bone fixation technique for recurrent lacrimal gland prolapse. Eur J Plastic Surg. https://doi.org/10.1007/s00238-017-1388-6
Yang C, Guo X, Du L, Song G, Zong X, Zhang D, Du H, Dong X, Zhao J, Jin X (2021) A modified procedure for single-eyelid asian females with lacrimal gland prolapse: lacrimal gland reposition combined with fat transposition in double-eyelid operation. Aesthetic Plast Surg 45(4):1611–1619. https://doi.org/10.1007/s00266-021-02213-7
Jafarpour S, Kashkouli MB (2022) Re: a modified procedure for single-eyelid asian females with lacrimal gland prolapse: lacrimal gland reposition combined with fat transposition in double-eyelid operation. Aesthetic Plast Surg 46(1):214–215. https://doi.org/10.1007/s00266-021-02476-0
Mathewson PA, Athwal S, Hyer JN, Ezra DG (2023) Whitnall’s barrier: a technique for the management of lacrimal gland prolapse. Plast Reconstr Surg. https://doi.org/10.1097/prs.0000000000010563
Guyuron B, DeLuca L (1996) Aesthetic and functional outcomes of dacryoadenopexy. Aesthetic Surg J 16(2):138–141. https://doi.org/10.1016/S1090-820X(96)70037-7
Henares Chavarino ÁA, Estiragués Cerdá M, Ros Magallón A, Vicente Ruiz M, Arroyo Pérez Í, Bazán Álvarez A (2023) Correction of lacrimal gland ptosis in blepharoplasty: a systematic review. Ophthalmic Plast Reconstr Surg 39(5):427–432. https://doi.org/10.1097/iop.0000000000002388
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest to disclose.
Human and Animal Rights
This article does not contain any studies with human participants or animals performed by any of the authors.
Informed Consent
For this type of study, informed consent is not required.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Supplementary file 1 (MP4 2592 kb)
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Eshraghi, B., Najafi, M., Babaei, L. et al. Diagnosis and Treatment of Lacrimal Gland Prolapse: A Narrative Review. Aesth Plast Surg 48, 2786–2792 (2024). https://doi.org/10.1007/s00266-024-04017-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00266-024-04017-x