Abstract
Hypertrophic scars, resulting from alterations in the normal processes of cutaneous wound healing, are characterized by proliferation of dermal tissue with excessive deposition of fibroblast-derived extracellular matrix proteins, especially collagen, over long periods, and by persistent inflammation and fibrosis. Hypertrophic scars are among the most common and frustrating problems after injury. As current aesthetic surgical techniques become more standardized and results more predictable, a fine scar may be the demarcating line between acceptable and unacceptable aesthetic results. However, hypertrophic scars remain notoriously difficult to eradicate because of the high recurrence rates and the incidence of side effects associated with available treatment methods. This review explores the various treatment methods for hypertrophic scarring described in the literature including evidence-based therapies, standard practices, and emerging methods, attempting to distinguish those with clearly proven efficiency from anecdotal reports about therapies of doubtful benefits while trying to differentiate between prophylactic measures and actual treatment methods. Unfortunately, the distinction between hypertrophic scar treatments and keloid treatments is not obvious in most reports, making it difficult to assess the efficacy of hypertrophic scar treatment.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Cutaneous wounds inevitably heal with scars. For some individuals, the normal wound-healing process becomes derailed, resulting in an overabundance of scar tissue [17, 138]. Moreover, individuals with deep burn injuries are at a high risk for hypertrophic scarring, which is a serious cause of long-term impairment and disability [64]. Hypertrophic scars, often viewed as aesthetically displeasing [17], are among the most common and frustrating problems after injury, causing functional and cosmetic deformities, discomfort, itching, pain, psychological stress, and patient dissatisfaction, possibly affecting joint range of movement and reducing functional performance [17, 18, 154, 208]. The quality of life experienced by patients with keloid and hypertrophic scarring also can be impaired [29].
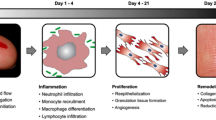
Hypertrophic scars, resulting from alterations in the normal processes of cutaneous wound healing, are characterized by proliferation of dermal tissue with excessive deposition of fibroblast-derived extracellular matrix (ECM) proteins, especially collagen, over long periods, and by persistent inflammation and fibrosis [122, 173, 188]. In the normal healing process after reepithelialization, the decrease in cellularity during the transition between granulation tissue and scarring is mediated by apoptosis and remodeling of ECM [59]. However, during hypertrophic scar formation, the granulation tissue does not regress, and the alpha smooth muscle actin-expressing myofibroblasts, the main cellular type observed in this tissue, are activated, producing excess ECM [173] and resulting in scar tissue that is red, raised, and rigid [5].
Although genetic makeup, regional variations, and age can influence the final result, the greater the insult, in general, the worse is the scarring [138]. It is estimated that hypertrophic scars affect 1.5% to 4.5% of the general population. However, the exact prevalence of hypertrophic scarring, particularly after burn injury, really is unknown [30], although some studies report a high prevalence, ranging from 32% to 67%. These studies also document a higher prevalence of hypertrophic scars in nonwhite individuals and higher rates of scarring in children [30, 64]. Currently, patients are expecting better outcomes from wound care [178], and wound management is increasingly important to the avoidance of excessive scar formation, especially in populations with Fitzpatrick 3 or higher skin types [178].
As current aesthetic surgical techniques become more standardized and results more predictable, a fine scar may be the demarcating line between acceptable and unacceptable aesthetic results [211]. However, hypertrophic scars remain notoriously difficult to eradicate because of the high recurrence rates and the incidence of side effects associated with available treatment methods [5].
Medical science and industrial development are devoting more effort to understanding and offering better therapy to control scars. However, advances in scar management have been hampered by the confusing or ambiguous terminology. There is no consensus on what amount of posttraumatic skin scar formation is “normal” and what should be considered “hypertrophic” [178]. Moreover, the persistent confusion with all forms of pathologic scars considered as one entity although hypertrophic scars and keloids clearly are separate entities [15] is making single uncontrolled studies about scar management almost impossible to evaluate [142].
In addition to the fact that the pathogeneses of the two conditions are different, a genetic predisposition plays a strong role in keloid formation [206, 211]. As early as 1970, Peacock et al. [159] defined hypertrophic scarring as a scar raised above the skin level that stays within the confines of the original lesion and a keloid as a scar raised above skin level that proliferates beyond the confines of the original lesion [42, 71, 148] (Figs. 1 and 2). Nevertheless, clinical differentiation between hypertrophic scars and keloids can be problematic [142]. This results in inappropriate management of pathologic scar formation, and occasionally contributes to inappropriate decision making related to elective or cosmetic surgery [178].
Numerous methods have been described for the treatment of keloids and hypertrophic scars, but to date, the optimal treatment method has not been established [181]. However, it must not be overlooked in discussions of scar management that it is important to differentiate between keloid and hypertrophic scars [206, 211]. Undoubtedly, an understanding of the cellular and molecular events implicated in the development of these fibroproliferative disorders will allow for optimization of wound healing [202] and subsequent scar management.
Considerable advances have been made in our understanding about the fundamental biology of scarring. As research methods become increasingly sophisticated, it will be even more crucial to characterize source material extensively, recognizing major differences not only between the keloid and the hypertrophic scar, but also among scars in varying stages of maturation and among histomorphologic, biochemical, and molecular variations within individual scars [39].
Because phases of scar evolution can be protracted, and because a tremendous range exists between the scar that becomes hypertrophic in the first few months, then completely resolves with little or no treatment, and the more severe hypertrophic scar that becomes permanently disfiguring, there also is tremendous confusion regarding what constitutes prophylaxis in contradistinction to actual treatment [142].
It has been stated that the transition from prophylaxis to a treatment regimen takes place when a true hypertrophic scar or keloid, and not an immature scar, is diagnosed, and that conceptually and practically, treatment and prevention regimens can be similar [142]. However, detailed pathophysiologic study of pathologic scarring invariably leads to the conclusion that prophylactic management is preventive and should be applied immediately after wound healing and full epithelialization. Prevention implies the use of a therapeutic method aimed at reducing the risk of a problem scar evolving [13, 14, 16, 142]. This is totally different from actual treatment of pathologic scarring irrespective of the scar stage at which the treatment is being applied because the actions of wound-healing cytokines and cellular mechanisms involved in the early phases of healing may be totally different from the actions and mechanisms in subsequent stages.
Definitely, it is much more efficient to prevent pathologic scars than to treat them [13, 14, 16, 142]. Needless to say, early diagnosis of a problem scar can have a considerable impact on the outcome [142]. Furthermore, the efficacy of any treatment method depends invariably on the stage of wound evolution for which the treatment is being initiated. As our knowledge about wound healing is expanding, it is clear now that prophylaxis and treatment should be, and in fact are, conceptually different.
Scars usually develop 6 to 8 weeks after reepithelialization, and a period of at least 6 to 18 months is required for their maturation [58]. It has been well proven that delayed wound healing results in unsightly hypertrophic scars [2]. Prophylactic treatment thus aims at accelerated wound healing [2, 14] under optimal conditions and at downregulating the persistent synthesis of proinflammatory/fibrogenic cytokines such as interleukin-1-beta, tumor necrosis factor-alpha (TNF-α), platelet-derived growth factor, and transforming growth factor-beta (TGF- β) from inflammatory cells [75, 173], leading to improved scarring.
Currently, new therapies designed to minimize scarring and accelerate wound healing after burn injury or any other type of injury rely on the optimization of systemic conditions, early wound coverage, and closure of lacerations and surgical incisions with minimal trauma to the surrounding skin [138]. These therapies augment three main treatment methods thought to have a beneficial influence on the aesthetic outcome of scars: wound support, hydration, and hastened maturity of scars [17, 211]. However, in most areas, but primarily in the area of burn scar management, the literature lacks strong evidence to support the usual standard of care interventions for rehabilitation [29, 64], particularly for the treatment of hypertrophic scarring [64]. Physicians need to identify different types of skin scars and treat them appropriately because misdiagnosis and mismanagement of scars can be costly for both the patient and the physician [21]. It is important also for practicing physicians and surgeons to know the full range of techniques available to control scar formation, and for any medical intervention to be planned such that potential problems are caught and minimized or even avoided [199].
A wide variety of treatments have been advocated for hypertrophic scars and keloids including surgical excision and/or grafting, occlusive dressings, topical and intralesional corticosteroids, interferon, cryosurgery, radiation, pressure therapy, laser therapy retinoic acid, and silicone gel sheeting [5, 27, 143] as well as a multitude of extracts, topical agents, and other promising, lesser known therapies directed at collagen synthesis [5, 13, 27, 35, 97, 128, 143, 182]. Unfortunately, the aspect of the usual interventions for the rehabilitation of burns and other major injuries that causes the most concern is treatment for hypertrophic scarring not supported in the available literature with strong evidence [64].
The current review explores the various treatment methods for hypertrophic scarring described in the literature including evidence-based therapies, standard practices, and emerging methods. An effort is made to distinguish between those with clearly proven efficiency and anecdotal reports about therapies of doubtful benefits as well as between prophylactic measures and actual treatment methods. Unfortunately, the distinction between hypertrophic scar treatments and keloid treatments is not obvious in most reports, making an accurate assessment of hypertrophic scar treatment efficacy difficult to ascertain.
Pressure Garments
Although pressure was described for the treatment of hypertrophic scars as early as the 16th century [123], and although pressure therapy often is prescribed, few controlled studies have examined its effectiveness in preventing or treating hypertrophic scarring [30]. Pressure therapy did not become popular until the reports from Larson et al. [112, 113] in the 1970s. Ever since that time, pressure garments have been the mainstay of hypertrophic scar treatment [127, 142, 197] and currently are the standard first-line therapy for hypertrophic burn scars in many centers [64, 142, 147, 166, 210].
Currently, elastocompression using elastic garments is the predominant means for both the prophylaxis and treatment of hypertrophic scars [46, 78, 79, 173] despite controversial evidence-based data about their value in reducing the prevalence or magnitude of scarring [30, 64, 142, 173] and despite little if any scientific evidence supporting their use [78, 79]. There is no level 1 or 2 literature to justify this form of therapy [30, 64]. In fact, studies investigating pressure garments have found no significant difference whether the treatment involves the use of high-pressure garments, lower-pressure garments, or no pressure at all [64]. Others, however, claim that pressure therapy achieves hypertrophic scar regression success rates of 60% to 85% [173], without any conclusive evidence.
The early development of compression treatment was based on observed improvements of scars (i.e., increased rate of maturation or lack of hypertrophic scar development) under some kind of pressure in individual patients [123, 127, 166]. Although the clinical effectiveness of pressure therapy has never been scientifically proven, as mentioned earlier, a large body of dermatologic/histologic, clinical, and anecdotal or case study evidence supports its use [79, 127, 163].
To date, the working mechanism of pressure and the way pressure positively influences the development or maturation of hypertrophic scars are not fully understood, and explanations remain hypothetical [127, 173, 209]. At this writing, the exact mechanism remains unknown [79]. However, many have researched possible mechanisms of action, exploring the theories of hypoxia, biochemical changes, and cellular and collagenous influences [79].
Some valuable evidence suggests that pressure controls collagen synthesis by limiting the supply of blood, oxygen, and nutrients to the scar tissue [86, 127, 167]; reduces collagen production to the levels found in normal scar tissue more rapidly than the natural maturation process [167]; encourages realignment of collagen bundles already present [127, 163, 210]; restores in part the ECM organization observed in normal scarring; and induces the disappearance of fibrogenic alpha smooth muscle actin-expressing myofibroblasts and vascular cells, probably by apoptosis [51]. However, available data also suggest a role for prostaglandin E2 in the process of hypertrophy remission induced by pressure therapy [172].
Studies have demonstrated that mechanical compression also acts directly to modulate the remodeling phase of wound healing, altering the release and activity of matrix metalloproteinase MMP-28 in hypertrophic scars and inducing a significant reduction of the protein presence in hypertrophic scar keratinocytes [168]. It also has been suggested that pressure acts by accelerating the remission phase of the postburn reparative process [51].
All these effects may hasten scar maturation, reducing the incidence of contractures and negating the need for surgical intervention [210]. It is accepted also that application of pressure commonly alleviates the itchiness and pain associated with active hypertrophic scars [121, 127].
Currently, pressure garments are used as treatment for established hypertrophic scars and as a prophylactic for wounds requiring more than 10 to 14 days to heal spontaneously or those requiring grafts [49, 127, 177]. Much of the difficulty in scientifically assessing the efficacy of pressure garments lies in the current difficulty of objective scar assessment and the current lack of scientific evidence for details such as what pressures are effective, when to apply pressure, and for how long [79]. The amount of effective pressure generated by a given pressure garment also still is unknown and remains controversial [79, 209]. Problems of pressure loss of the garments over time and problems with compliance of the patients using the garments are yet other factors complicating the entire issue [46, 100, 209].
Recommendations for the amount of pressure and the duration of the therapy are based merely on empirical observations [209]. Currently, many authors recommend pressures of 20 to 40 mmHg [79, 158]. In general, pressures that exceed 24 mmHg are required to overcome capillary pressure [209]. This is based theoretically on the arterial capillary closing pressure of 25 mmHg rather than on any scientific evidence [46]. However, good clinical results (e.g., better appearance of the scar, less itching) have been reported with pressure levels as low as 5 to 15 mmHg [209, 210]. Yet it also is claimed that 15 mmHg is necessary to accelerate the maturation process and that effects of pressure below 10 mmHg are minimal. With pressures above 40 mmHg, maceration and paresthesia may occur [46, 167, 209].
Pressure garments, in general, generate a 9- to 90-mmHg increase in subdermal pressures, depending on the anatomic site, with a mean pressure increase of 22 mmHg through the skin into the subdermis [78, 79]. Garments over soft sites generate pressures ranging from 9 to 33 mmHg. Over bony prominences, the pressures range from 47 to 90 mmHg [78]. Anatomic zones with a small radius of curvature generate more pressure than those with a large radius of curvature. Similarly, positive radii and convex surfaces generate more pressure than concave surfaces with negative radii. This is explained geometrically by the La Place law, which states that pressure (for a given tension) is inversely related to the radius of the curvature [79], and by Cheng’s theory that scar response and pressures are related to compliance of the underlying tissues and the local anatomic geometry [46, 79].
Clinically, it is known that scars in different anatomic areas respond differently to pressure garments [79, 120]. Although this effect has not been fully explored, findings have shown that the most important factor in determining the effectiveness of pressure therapy is the anatomic area [79, 177]. It is thought that this is attributable to the different pressures generated by the garments because of a change in the underlying body shape and consistency [79]. Unfortunately, the data concerning areas of good scar response are not sufficiently detailed for a close comparison between recorded pressures in specific anatomic zones and the areas of clinical response. However, it can be said that areas of good clinical response generally correlate positively with the higher pressures generated [46, 120, 177]. Poor response areas such as flexural creases of joints and the trunk show pressures generally lower than 10 mmHg (respective means, 6.2 and 7.9 mmHg). Medium response areas such as the thighs, calves, and arms show mean pressures of 20.5 mmHg [79].
Many problems are associated with the use of pressure garments [127]. The least of these is poor compliance with the treatment, not exceeding 40% in most instances, due to the lack of perceived benefits or improvement of scars or problems with comfort, movement, appearance, or culture [38, 96, 100, 127, 177, 198]. Much of what is traditionally understood as “patient nonadherence” appears, however, to result largely from rational choices made by patients in the face of several difficulties they usually experience with the current form and nature of pressure garment therapy [198]. Discomfort from heat and perspiration, particularly in warm weather, also is a serious handicap [38, 127, 177, 198]. Swelling of extremities, eczema, rashes, and pruritus caused by pressure garments have been reported as well. Excessive friction, blistering, ulceration, and scar breakdown also may occur because of too much pressure applied too soon or because of humid or hot weather, resulting in suspension of treatment [44, 46, 119, 121, 167, 198].
Skeletal and dental deformity also has been caused by excessive pressure applied to growing children and even adults [69, 96, 120, 192]. Furthermore, some recent experimental studies have indicated that the pressure exerted by tight-fitting clothing adversely affects certain aspects of the normal physiologic balance [90, 127]. Problems inherent to the garment material and manufacturing such as poor quality, bulky seams, decay in pressures applied by a garment over time with continuous wear, and variability in manufacturing so that the ‘‘same manufacturing techniques’’ may make pressure garments that exert different pressures for the same patient [46, 79, 127, 209] also are serious handicaps affecting the efficacy of pressure garments. Moreover, it is not surprising that some authors have questioned the cost–benefit of pressure therapy [44, 209].
Despite overwhelming earlier reports, little sound data exist to show that pressure garments reduce the prevalence or magnitude of scarring. Currently, a fair body of evidence may support their use, but it is not definitive scientific evidence stemming from serious research [163]. A recent study objectively shows for the first time that pressure garments delivering a pressure of at least 15 mmHg tend to accelerate scar maturation, with clear acceleration in scar flattening. A significant difference in the thickness of postburn scars has been demonstrated during the first month of preventive treatment with garments delivering mean pressures of 15 mmHg, as compared with scars subjected to garments with a lower mean pressure (10 mmHg) [209].
Recently, a new balloon-compression wear that fits complicated uneven surfaces has been described. This garment, using air as a compression source, has a compression force greater than that of sponges or supporters, is easy to wear, and does not require tape fixation. It is claimed that this wear is especially useful for keloids and hypertrophic scars in the chest region [109].
To optimize pressure therapy further, studies definitely should be undertaken to examine the changes in pressure under dynamic circumstances, the effects of pressure on the local vector forces in the skin, and the optimal timing and duration of pressure therapy [79]. Similar studies investigating the pressures produced by the use of custom inserts in areas of concavities also should be performed to document their effectiveness [79]. Such studies and objective measurements would avoid the useless, expensive, and frustrating wearing of ineffective garments. This is particularly true for areas known to be poorly responsive to pressure therapy [79]. In any case, the selection of any treatment must follow negotiation and agreement with the patient who will be required to continue treating his scars at home [163].
Silicone Materials
Silicones (e.g., creams, gel sheets, silastic sheets, orthosis garments) have become a very useful tool in the treatment and prevention of hypertrophic scarring, especially after burns [76, 208]. Silicone materials are synthetic polymers based generally on a dimethyl siloxane monomer and containing a silicon–oxygen backbone, with organic groups attached directly to the silicon atom by silicon carbon bonds. Depending on the length of the polymer chain and the degree of cross-linking, the silicone can be a fluid, gel, or rubber [42, 118, 208]. Depending also on the amount and type of the catalyst used in the fabrication process, the final product can differ in physical and chemical properties [118]. The most common example in surgical practice is polydimethylsiloxane (PDMS), with an index of approximately 130 [208]. Silicone is inert and does not inhibit microbial growth, but it can act as a bacterial barrier [42]. In wound care and rehabilitation, three types of silicones are used:
-
1.
Silicone fluids: short, unbound, straight PDMS chains
-
2.
Silicone gels: lightly cross-linked PDMS chains (e.g., H-bridges), usually formed in the presence of a catalyst
-
3.
Elastomers: long, strongly cross-linked PDMS chains also formed in the presence of a catalyst (usually silica) [208].
The history of the use of silicones in burn wound care dates from the early 1960s. Silicone fluids were used as an immersion treatment to promote complete eschar separation, early formation of a granulation tissue bed, and early joint motion of a spontaneously healed or grafted burn wound [74, 137, 208]. Unfortunately, their use was stopped after reports of numerous complications stemming from impure industrial grades of silicone injected for soft tissue augmentation [164].
Subsequently, in the early 1980s, an Australian research group developed the earliest silicone gel sheet (elastomer). It was intended for use on scars located at anatomic depressions and flexures under the pressure garments. The researchers used the gel sheet 6 to 8 weeks after burn injury when the scars started to develop [42, 77, 131, 160].
With the introduction of silicone gel, the emphasis in burn scar management shifted from pressure to the use of contact media [54, 94]. Quinn et al. [164] introduced the concept of nonpressure treatment for hypertrophic scars in 1985 [42]. Since then, a range of “contact media” has been developed, allowing for individualization of therapy to both the patient and the scar [54].
Despite initial skepticism about silicone gel sheeting (SGS), good evidence now proves of its efficacy, and it has become standard care for plastic surgeons [36, 43, 142, 184, 200]. Topical silicone gel has shown promise for the treatment of hypertrophic and keloid scars [87], effectively reducing the bulk of these lesions [89].
The semiliquid, sticky gel is easy to apply and remains on the skin for many hours [42]. However, SGS has been more widely used as a clinical scar management option since the early 1980s. It has been advocated that treatments with SGS should begin as soon as an itchy red streak develops in a maturing wound [70]. It is applied directly to the scar without any intention to augment or establish pressure and needs to remain in contact with the skin surface as long as possible [208]. Various reports indicate that the duration of silicone sheet use usually ranges from 12 to 24 h daily, after which it needs to be washed and reapplied [42].
It is necessary, however, to distinguish between silastic (elastomer) and gel sheets [208], although the two have similar clinical results and indications for use [40, 117]. Silicone gel was used initially for scar treatment rather than prevention [42]. However, the introduction of “the adhesive technique” over the past few years has allowed for earlier therapy with the aim of preventing or minimizing scar hypertrophy with better short- and long-term cosmetic results [54] while causing limited damage to the stratum corneum at removal, as compared with nonadhesive silicone gel dressings [129].
The application of topical SGS in a variety of settings appears to result in flattening, softening, and increased pliability of the scar [40, 42, 81, 141, 160, 164]. Existing scars that are years old also may be effectively treated [77, 174], although it has been shown that silicone materials may not have any effects on mature hypertrophic scars [85]. Observed benefits appear to be independent of patient age, method of gel attachment, anatomic location, scar age, or scar etiology [141].
Although SGS is widely used and assumed to be beneficial in the treatment of pathologic scars, it is worth noting that the evidence supporting its effects is class 3 at best [141]. Nevertheless, topical SGS, with more than a 20-year history of satisfaction in the treatment of hypertrophic scars and keloids, now appears to be useful in the prevention of pathologic scarring [82], although this still is highly controversial [148]. There is some weak evidence of benefit in that silicone materials could be used to prevent abnormal scarring in both high-risk individuals [154] and patients undergoing scar revision [82]. More recent evidence suggests also that the semiliquid form of silicone gel is effective in preventing hypertrophic scar development in sternotomy wounds [42]. It seems that the effective regimen for preventing hypertrophic scars with silicone sheets begins about 2 weeks after wound healing [77, 174]. However, it must be stressed that when SGS is applied during the healing phase, widened, atrophic, and depressed scars usually develop [13].
The mechanism of action of topical silicone materials on hypertrophic scars is not well understood [104, 108, 141]. Various mechanisms of action have been proposed [90, 208]. It has been suggested that their therapeutic effect is not because of pressure, difference in oxygen tension or temperature, or silicone leakage into the dermis [16, 141, 148, 164]. Under the electron microscope, the surface of the silicone gel sheet is flat and has no pores [42]. Although the silicone acts as a barrier, sufficient oxygen does reach the skin for respiration [141]. There is evidence also that SGS affects the hydration status of the scar by decreasing the water vapor transmission rate to almost half that of normal skin [16, 141, 164, 208], causing a buildup of moisture on the skin surface under the silicone sheet [77]. This suggests that the stratum corneum acts as a water reservoir, with fluid accumulating below the gel, although when visualized directly, this is not evident [141, 208]. Hydration and occlusion seem therefore to be the principal modes of SGS action, and the presence of silicone apparently is not essential to obtain beneficial clinical effects [174, 185]. Increased skin hydration probably is responsible for a decrease in capillary activity, a reduced hyperemia, and a reduced collagen deposition [148].
Despite earlier reports, the significant and sustained elevation in scar’s surface temperature, which can be detected after gel application, is definitely not related to an acute alteration in microvascular flow within hypertrophic scars. This raises the possibility that temperature alteration may be involved in the mechanism of action as well [141]. Although evidence is conflicting, it is conceivable that SGS may well exert its effect through a temperature-mediated activation of collagen breakdown [141, 202].
Altered hydration is thought also to cause electrostatic changes that influence collagen deposition and remodeling within the scar [140]. Static electricity generated by friction also has been proposed as a plausible reason for SCS antiscarring effects [10, 25, 89, 92, 148]. However, when the efficacy of a silicone cushion filled with liquid silicone gel reported to induce a greater negative static electric charge was compared with SGS in the treatment of hypertrophic and keloid scars, no statistically significant differences were found between the two treatment methods [25] although a much faster response was demonstrated with silicone cushions in a more recent report [10]. Silicone gel sheeting probably also produces a favorable condition for the skin by protecting it from various environmental stimuli while keeping the skin in an adequately hydrated but not an overhydrated condition [200]. It also appears that silicone sheeting may act by downregulating fibroblasts and decreasing fibrogenic cytokines [110]. Modulation of growth factors that orchestrate the tissue repair process, such as the expression of basic fibroblast growth factor (bFGF), also has been suggested [87].
Combinations of methods or therapies may be applied. The most common combination is that of a silicone silastic sheet, gel sheet, or pad with a classical pressure garment [208]. Garments with varying degrees of stiffness or rigidity, depending on the therapeutic aims, can be made [55]. The latest development in this regard is that of inflatable silicone inserts for treating scars in which the pressure on the scar can be adjusted by means of a pump. The system is indicated for the treatment of scars or keloids in concave areas (presternal, axillary, subclavicalar) or in soft tissue parts of the face and neck [208]. When combined therapy is used, particularly in anatomic areas that do not respond well to silicone sheeting because of the difficulty maintaining gel contact with the skin (e.g., chin, breast, clavicle, neck, and face), it is evident that the presumed working mechanisms of the individual methods (pressure, hydration, occlusion, and static electricity) may combine and reinforce each other [208].
Because the silicone gel sheet has a water vapor transmission rate lower than skin, the water that accumulates below it can cause skin maceration [42, 53]. Other common problems associated with gel sheeting include persistent pruritis, skin breakdown, skin rash, foul smell from the gel, poor durability of the sheet, failure of the sheet to improve hydration of dry scars, poor response of the scar to treatment, and poor patient compliance [42, 53, 150, 208]. Similar to pressure therapy, detailed multimedia patient education improves compliance with SGS, resulting in a better scar outcome [195]. Obviously, the key to the success of this therapy is to ensure that hygienic precautions are taken, particularly when it is used in combination with pressure for children or in warm weather or climates [208]. It must be noted, however, that complications are reported to increase with the use of combined pressure and SGS therapy [208].
Materials other than silicones have shown the same ability [77, 174]. Silicone and nonsilicone gel dressings are equally effective in the treatment of hypertrophic scars [19, 58]. A hydrogel sheet wound dressing product is a well-characterized nonsilicone material with efficacy proven in a prospective, controlled trial. Treatment with a self-adhesive hydroactive polyurethane dressing (Cutinova thin; Beiersdorf AG, Hamburg, Germany) applied over a period of 8 weeks has been shown to have a beneficial effect on hypertrophic scars as well [186]. The positive results with other materials clearly establish that silicone is not needed for efficacy [77, 174, 185], and it seems that a program providing support, hydration, and hastened scar maturity is the most effective scar management to date [211].
Intralesional Corticosteroid Injections
Intralesional corticosteroid injections, used for the treatment of pathologic scars since the mid-1960s, continue to play a major role in the regression of hypertrophic scars and keloids [45]. Injections produce objective reductions in scar volume for significant numbers of patients, with improvement of scar pliability, height, and symptoms such as pruritus [102, 132, 142, 153]. Insoluble triamcinolone acetonide (10–40 mg/ml), the most common corticosteroid used for the treatment of scars, may be administered alone or in combination with lidocaine to reduce the pain associated with the injection. Several treatments at once or twice a month usually are required to achieve the desired results [132, 153].
Despite relatively few randomized, prospective studies, there is a broad consensus that injected triamcinolone is efficacious. It is first-line therapy for the treatment of keloids and second-line therapy for the treatment of hypertrophic scars if other easier treatments have not been efficacious [9, 142, 149, 175, 207].
Although the use of corticosteroids to suppress abnormal scar formation has been relatively effective for the most part, it also has been a troublesome therapy [68]. Intralesional corticosteroid injection is associated with significant injection pain, even using standard doses of triamcinolone (40 mg/ml), with up to 63% of patients experiencing some side effects [142, 196]. The most common side effects of this treatment therapy are hypopigmentation, skin and subcutaneous fat atrophy, telangiectasias, rebound effects, and ineffectiveness [68, 132, 153]. After intralesional injection, linear hypopigmentation also may develop secondary to lymphogenous uptake of the corticosteroid crystals] [72].
The mechanisms involved are complex and remain unclear [102, 142]. Corticosteroids suppress healing by three major different mechanisms. First, inflammation is suppressed by inhibition of leukocyte and monocyte migration and phagocytosis. Second, corticosteroids are potent vasoconstrictors that may reduce the delivery of oxygen and nutrients to the wound bed. Third, and perhaps most significantly, the antimitotic effect inhibits keratinocytes and fibroblasts, slowing reepithelialization and new collagen formation. The inhibition of fibroblast proliferation by corticosteroids may be dose dependent and may not be seen at lower dosages, as evidenced by tissue culture studies [68].
The rates of response to intralesional corticosteroid injections vary from 50% to 100%, with a recurrence rate of 9% to 50% [149]. Results may be improved when corticosteroids are combined with other therapies such as surgery, pulsed-dye laser (PDL) irradiation, 5-fluorouracil, and cryotherapy [5, 12, 34, 132, 142]. Surgical excision with intraoperative local injection of triamcinolone acetonide followed by repeat injection at weekly intervals for 2 to 5 weeks, depending on the symptomatic relief, and then monthly injections for 4 to 6 months may yield a good result. Complete symptomatic relief can be achieved with this combination for all patients within 5 weeks of surgery. An objective response in terms of no recurrence can be noted in 91.9% of patients with keloids and 95.24% of patients with hypertrophic scars. Local or systemic complications with this combination therapy may be insignificant. Because of promising results, further use and evaluation of this combination method of treatment are recommended [47].
Topical steroid creams have been used with varying success, but it must be noted that absorption through an intact epithelium into the deep dermis is limited. A prospective, randomized study showed that topical steroids do not reduce scar formation in postburn deformities [214]. It also has been demonstrated recently that limited use of corticosteroids topically fails to reduce scar formation [211].
Laser Therapy
Advances in laser technology over recent years have led to progress in the treatment of many dermatologic conditions [153]. The advent and development of laser technology may represent the most promising treatment method for the cosmetic and functional improvement of cutaneous scars [9]. It has been claimed that the appropriate choice and use of lasers can significantly improve most scars [126]. A variety of lasers can be used. It is, however, of paramount importance that the type of scar be properly classified at initial examination so that the most appropriate method of treatment can be chosen [6, 126].
Laser treatment of hypertrophic and keloidal scars, which began with the carbon dioxide (CO2), argon, and neodymium:yttrium-aluminum-garnet (Nd:YAG) lasers [153], has been used for nonspecific destruction of tissue to produce less scarring [142]. Argon lasers were first used in the 1970s for the management of keloids, but subsequent studies have failed to show long-term improvements [142, 153]. They produce more nonspecific thermal damage than CO2 lasers and are associated with high levels of keloid recurrence [4, 95, 142, 153].
Despite early promising results, CO2 laser scar treatment also does not seem to be effective [4, 151]. Hypertrophic scars and keloids excised or vaporized with a continuous-wave CO2 laser demonstrate similar high recurrence rates [153]. Carbon dioxide laser resurfacing with thin skin grafting as a “camouflage” operation has been used successfully to convert self-inflicted scars to a socially acceptable appearance similar to a burn scar [1]. However, the final result has not been an improvement in scar quality, but rather a trade-off between an unacceptable scar with bad social connotations and another more acceptable scar.
Carbon dioxide laser for excision of a hypertrophic scar and application of a skin substitute (Apligraf, Novartis, East Hanover, NJ) also have been reported for the treatment of a large painful hypertrophic scar on the plantar aspect of the foot to gain coverage and resolution of the painful condition] [115]. Two newer types of CO2 laser (high-energy short-pulsed CO2 lasers and scanned continuous-wave CO2 lasers) also were used for the treatment of keloids without any convincing results [142]. Currently, the CO2 laser is not widely accepted for the treatment of keloids [142]. Proliferative keloids and hypertrophic scars should not be vaporized because of the high risk for scar recurrence or progression [126] in addition to the disadvantage of aerosolizing hepatitis B and C, human immunodeficiency virus (HIV), and other viruses, putting the surgical personnel at risk [36]. Use of the continuous-wave 1064-nm Nd:YAG laser, which selectively inhibits collagen production according to in vivo and in vitro studies, initially demonstrated softening and flattening of keloidal scars [153]. The results, however, were transient, and scar recurrences were common [153]. After mixed and even conflicting results in larger long-term trials, the use of CO2, argon, and Nd:YAG lasers to reduce severe scars fell out of favor and has been largely discredited [142, 153].
Pulsed erbium:YAG lasers, with wavelengths of 2,940 nm, are 10 times more selective for water than their CO2 counterparts at 10,600 nm, reducing thermal damage [153]. Since 2001, the erbium:YAG laser has become an integral part of the treatment for postburn scars at some burn centers, proving to be a valuable supplementary tool for the improvement of cosmetically disturbing mild postburn scars. It seems to be particularly handy in areas difficult to treat, such as around the eyes, nose, lips, and fingers [62], but the long-term benefit of this method has not been established to date by well-controlled comparative studies. Combined ablative CO2 and erbium:YAG laser treatments also have been proposed as a valid treatment method for atrophic scars [126].
More recent wavelength-specific lasers (YAG and PDL), used for selective ablation of blood vessels [142], have been successful in the treatment of hypertrophic scars. The benefits of the 585-nm PDL in that regard have been well established over the past decade, and PDL has been recognized generally as an excellent first-line treatment option [7]. The conventional short-pulsed dye laser (585-nm PDL) is reported to be the most appropriate and effective system for the treatment of varied traumatic and surgical scars, with improvement in scar texture, color, and pliability, as well as minimal side effects [106, 126]. Findings have shown it to improve the appearance of hypertrophic scars, keloids, erythematous scars, and striae, [23, 41, 126] resulting in a normal number of dermal fibroblasts with decreased sclerosis at histologic examination [8]. It inhibits hypertrophic scar implant growth in nude mice. This effect likely is mediated by selective photothermolysis of the implant microvasculature [169].
Early PDL treatments also can fundamentally change the physiology of wound healing if applied in the early phases by reducing scar microcirculation and preventing excessive scar formation [134, 189]. The 585-nm flashlamp-pumped PDL also is an effective treatment for the intense pruritis often experienced during the healing process after a burn injury [3].
The PDL method carries a low risk of side effects and complications when used at appropriate treatment parameters and time intervals [126]. More recent studies have raised concerns, however, about its effectiveness [41]. These studies show that although significant symptomatic improvement occurs, there is a statistically insignificant degree of objective improvement in terms of scar redness, scar thickness, viscoelasticity, reduction in height, and textural quality, contradicting the results of several earlier reported studies [3, 41].
Currently, it seems that the suprapurpuric PDL should not be considered the standard of practice for the treatment and prevention of hypertrophic scars [41]. Prolonged purpura after treatment has led to the development of the newer long-pulsed dye laser (LPDL) [23]. The LPDL (595 nm) with a cryogen-spray cooling device also is described to be an effective treatment for hypertrophic scars. It can improve scar pliability and texture while decreasing scar erythema and associated symptoms [106]. Findings have shown another method, intense pulsed light, to be as effective as LPDL in improving the appearance of hypertrophic surgical scars and minimizing the risk of purpura [23].
Further improvements have been reported with intense pulsed light in combination with intralesional corticosteroids [142, 190]. However, the adjunctive use of intralesional corticosteroids with 585-nm PDL irradiation does not significantly enhance clinical outcome except in the case of the most symptomatic scars, for which the combined treatment approach provides a greater benefit in improving scar pruritus [5]. Improvement in nonerythematous, minimally hypertrophic scars also was found after combination treatment involving CO2 laser vaporization to achieve deepithelialization followed by PDL irradiation, resulting in significant and prolonged clinical and textural improvement [153].
For prophylaxis, findings have shown that early scar treatment with PDL irradiation effectively prevents scar formation or worsening of an existing scar, yielding a better and more prolonged clinical improvement. The concomitant use of corticosteroids, 5-fluorouracil, or other treatments is proving to be of particular importance in reducing scar bulk and symptoms of more proliferative scars [7]. Although optimal management for pathologic scars has yet to be determined, PDL irradiation will no doubt continue to play a role in their treatment [7]. However, it must always be remembered that laser therapy remains an emerging technology with limited follow-up study and lack of controlled studies. Further studies in the future are required to define its exact role [142].
Fractional photothermolysis (1550-nm Fraxel SR laser, Reliant Technologies, Inc., Mountain View, CA) is a newly proposed method with a claimed 75% clinical improvement of scarring achieved 2 weeks after a single treatment session at a pulse energy of 8 mJ (MTZ) and a final density of 2,000 MTZ/cm2. The observed improvement was persistent at a 1-month follow-up assessment, suggesting that fractional photothermolysis may offer a new, effective, and safe method for the treatment of surgical scars [22].
Adhesive Tape Support
Longitudinal stretching of wounds that cross the relaxed skin tension lines appears to be the stimulus that induces the body to form hypertrophic scars [170]. It is generally thought that tension plays a major pathophysiologic role [26]. After suture removal, surgical scars are susceptible to skin tension [17]. Apparently, the use of a nonstretch microporous contact media fulfills the criteria for effective scar support and management [17, 142]. Application of microporous hypoallergeninc paper tape with an appropriate adhesive to fresh surgical incisions, beginning at 2 weeks and used for several weeks after surgery, has been effective in controlling scar tension, eliminating stretching forces, and preventing hypertrophic scarring [17, 142, 170]. Tape with an elastic component may be useful for scars over mobile or complex surfaces, including joints [142, 170].
Long-term use of paper tape is proposed to prevent the formation of a hypertrophic scar because it is inflexible, provides good scar support, is microporous, and is thought to mimic the stratum corneum and accelerate healing without creating the bacterial growth seen with more occlusive media. It can be worn for 4 to 7 days continuously, even during bathing or swimming. Moreover, it places fewer demands on the patient than methods such as silicone and compression, which have daily hygiene requirements. It also is cost effective, with most scars requiring only a single roll of 2.5-cm tape for treatment, costing less than $1 [17].
Evidence for the effectiveness of paper tape in reducing scar volume and preventing hypertrophic scar formation has been well established [17]. Its use to support surgical scars after suture removal is standard practice. However, it usually is continued for only a few weeks [17, 142]. This appears to be insufficient, considering that the maximum strength of a scar is not achieved until approximately 12 weeks after wounding [17].
Although its real mechanism of benefit is unknown, it seems that micoporous tape support may be able to reduce multidirectional forces and eliminate scar tension. It has been suggested also that its action may in part be mechanical (analogous to pressure therapy) and occlusive (analogous to silicone gel therapy) [17, 142, 170]. It is hard, however, to see how simple application of a tape, similar to silicone gel application, may exert pressure on the underlying tissues. Moreover, in one study comparing Micropore and Blenderm (3M, St. Paul, MN, USA), better cosmetic results were achieved in the areas treated with Blenderm, a clear plastic tape capable of maintaining better hydration, than in the areas treated using Micropore paper tape or no treatment. The areas treated with Blenderm were less red and less hypertrophic than the areas treated with Micropore. However, the difference was not significant [183], suggesting that hydration is not the major mechanism of action with adhesive tape support of scars. In fact, paper tape may act by preventing an exacerbation of the inflammatory response during wound healing, allowing the more stable, closely woven type 1 fibers to form their covalent bonds and create a cross-linking pattern more comparable with normal skin [17].
In one study, the development of hypertrophic and stretched scars occurred only after the tape was removed. This implies that the cellular derailing of the wound-healing process results from a mechanical stimulus (scar tension) that occurs only after scar support has been removed [17].
Paper tape is a noninvasive, inexpensive means of preventing hypertrophic scarring that places minimal demands on patients [17]. It is obvious that compliance with the paper tape–wearing regimen for at least 12 weeks is essential for maximizing treatment outcome. Moreover, to ensure success using this treatment, it is recommended that individuals at greater risk of developing hypertrophic scars should support their scars using paper tape for a longer period until the scar matures [17]. However, this treatment seems to be less effective than more established treatments such as silicone gel, but it could be used as preventive treatment for low-risk patients, or before silicone gel is used in fresh incisions [142, 170]. It is not effective and definitely not practical for plaque-like scars such as those after deep burns. The adverse reactions experienced are few and involve mainly a localized red rash beneath the tape. These reactions are minor and transient, resolving without medical intervention [17].
Massage
Massage therapy, manual or mechanical, routinely used by therapists for the treatment of various conditions [157], is standard therapy in rehabilitation centers specializing in the treatment of scars and burns [176]. A significant method for almost all burn rehabilitation teams, it occupies a place, on the average, in 52% of the treatment protocols (range 25–60%) [176]. Increased scar pliability and decreased scar banding with the use of massage have been reported [157]. Although various techniques can be applied, none have been validated. Their use is thus based on the experience of various teams and does not have any scientific basis [176]. Massage indications depend on the burn zone and age as well as characteristics of the scar, and they may be adapted according to its development.
Scar hypertrophy is treated by cutaneous hydration, cutaneous mobilization, and pulpar massage, whereas for keloids, 50% of teams practice hydration and 40% use cutaneous mobilization [176]. Methods vary also according to topography. The rolled pleat is used more for the thorax, hands, and limbs. Lymphatic drainage is used more for the face, hands, and limbs, with hydration, cutaneous mobilizations, and pulpar massage used in all locations [176]. The majority of products used for massage are hydrating and thermal. Corticosteroids, itch-relieving products, and keratolytics are used less frequently. Some authors have proposed adjusting the intensity and depth of massage according to the skin inflammatory state being evaluated with the vitro-pressure test [52, 162].
Contraindications for massage are cutaneous fragility, open wounds, infection, pain, and inflammation. Complications include inflammation, cutaneous lesions, epithelial breakdown, and infection [176]. Although concrete beneficial effects on scars are hard to document, reported benefits of massage include improved relationships with the patient, improved skin quality, relieved sensitivity, increased cutaneous hydration, improved scar quality, and better acceptance of the lesion by the patient [176]. However, the few reported studies failed to demonstrate any appreciable effects of massage therapy on the vascularity, pliability, and height of the hypertrophic scar. Only reduction of pain and itching have been documented in studies investigating adult patients [66, 67].
Massage also reduces anxiety and improves the mood and mental status of patients [157, 176]. It has been demonstrated that massage therapy applied even to acutely burned patients before debridement sessions decreases the anxiety and cortisol levels while it improves behavior ratings of state, activity, vocalizations, and anxiety after the massage therapy sessions on the first and last days of treatment. Longer-term effects also were significantly better for the massage therapy group, including decreases in depression and anger as well as decreased pain according to the McGill Pain Questionnaire, present pain intensity scale, and visual analog scale [67].
In addition to manual massage, other techniques allow for mechanized massage such as compressed air, threadlike showers, and vacuotherapy, none of which have been validated. The use of these techniques is thus based on the experience of the rehabilitation teams, and the results are not yet proven [176]. Useful compressed air can reach a pressure of 10 kg/cm2. With the threadlike showers, the effect of pressure is associated with the water hydrating and thermal properties. Water pressure application can be 20 kg/cm2. The vacuotherapy technique uses a vacuum to aspirate the treated zone, and with application of specific heads, carries out cutaneous mobilization capable of going to the rolled pleat. A progress report on clinical experiments using the Louis Paul Guitay (LPG) (LPG World, Valence, France) on burn scars also has been published. Again, the technique has not been validated [176]. Further scientific studies are required to prove or invalidate the effectiveness of the various massage techniques in the treatment of scars and burns [176].
Cryotherapy
Contact or spray cryosurgery with liquid nitrogen can yield significant improvement or even complete regression of hypertrophic scars and keloids [45, 88, 142, 179]. It results in flattening of keloid scars in 51% to 74 % of patients after two or more sessions [142]. It has been proven to reduce the volume of keloid by induced ischemic destruction and consequent necrosis of the hypertrophic scar tissue [34]. Up to 20 treatment sessions may be required [88].
Compared with corticotherapy and laser-therapy, cryotherapy is a very effective method [150]. The usefulness of cryotherapy, however, is limited to the management of very small scars. A delay of several weeks between sessions usually is required for postoperative healing, and the commonly occurring side effect of permanent hypopigmentation is a major handicap. Other side effects also include hyperpigmentation, moderate skin atrophy, and pain [142, 179]. Better results have been reported, however, after treatment combining cryotherapy and intralesional triamcinolone than after triamcinolone or cryotherapy alone [27, 34, 98, 215]. Apparently, cryotherapy combined with intralesional triamcinolon injection is the most common traditional therapy for hypertrophic scars and keloids [145].
To avoid the many drawbacks associated with the classical cryotherapy technique, an intralesional needle cryoprobe method for the treatment of hypertrophic scars and keloids has been developed recently [88]. A specially designed cryoneedle can be inserted into the long axis of the hypertrophic scars and keloids to maximize the volume of tissue to be frozen. After one session of intralesional cryosurgery treatment, 50% of scar volume reduction, on the average, can be achieved with significant alleviation of objective and subjective clinical symptoms. Only mild local edema and epidermolysis occurs, followed by a short reepithelialization period. The mild pain or discomfort during and after the procedure can be managed easily. Rejuvenation of the treated scars (i.e., parallelization) and the more organized architecture of the collagen fibers than of the pretreated scars can be demonstrated by histomorphometric analysis [88]. The better efficacy of this method than that obtained with contact/spray probes results from increased freezing of the deep scar material. As a result, fewer treatment cycles are needed. Moreover, because the reepithelialization period is short, treatment intervals, if any, can be shortened to between 2 and 3 weeks. It seems that this intralesional cryoneedle method is simple to operate and safe to use. It necessitates less postoperative care of the wound and can easily be added to any preexisting cryosurgical unit [88].
Radiotherapy
Superficial x-ray, electron beam therapy, and interstitial radiotherapy have been used in the past for effective treatment of keloids [143]. Currently, there is a consensus that ionizing irradiation is an effective way to treat keloids [155]. It seems that electron beams are more capable than soft x-rays of selectively reaching the area related to keloid generation located at the border of the papillary layer and the reticular layer in the dermis [155]. The mechanism of its effect is the control of collagen synthesis in striking the abnormal activated fibroblast and the promotion of the existing normal fibroblast [155].
Radiotherapy has been used as a monotherapy or in combination with surgery [142]. Although primary radiotherapy has been reserved primarily for cases of unresectable keloids, low-dose fractionated radiotherapy as an adjuvant to surgical excision may be safe and efficacious. Various regimens have been described and appear to be well tolerated [45, 60, 130]. It is reported that best results can be achieved with 1,500 to 2,000 rads (15–20 Gy) over five or six sessions in the early postoperative period [142].
In many institutions, electron beam irradiation is started 24 to 48 h after keloid surgical excision, and the total dose is limited to 40 Gy over several administrations [155]. However, a total dose of 15 Gy could be the optimum sufficient dose (i.e., the dose at which side effects such as pigmentation and malignant tumor generation are minimal, but beneficial effects are recognized). This dose can be increased to 21 Gy for recurring keloids [155]. Pigmentation, however, increases when the radiation dose is increased to 21 Gy [155].
Radiotherapy, however, is difficult to evaluate because most studies are retrospective, do not define the term “recurrence,” and use a variety of radiation techniques with varying follow-up periods ranging from 6 to 24 months [142]. Nevertheless, although the risk of malignant change has always been exaggerated, and although reports of carcinogenesis several years after the procedure are mainly anecdotal, this treatment method remains controversial despite the fact that there are no current reports of malignant tumors being induced by electron beam irradiation up to 128 months after irradiation [155]. The use of potentially harmful radiation therapy to treat benign lesions may be ill advised and probably cannot be justified [142, 143, 152].
Vitamin E
Vitamin E is tremendously popular among the public for skin care [20, 91, 142]. Although many physicians and patients believe vitamin E speeds wound healing and improves the cosmetic outcome of healed wounds, there is little scientific support for this idea in the literature [20]. Vitamin E is a generic term for four pairs of racemic stereoisomers that are derivatives of tocol and tocotrienol [20]. It comprises a class of related compounds, the tocopherols, of which alpha-tocopherol is the most important component [21, 144].
Vitamin E, particularly in the form of topical alpha-tocopherol in an oil base, is a popular agent in the treatment of acute and chronic dermal wounds. Since its discovery, vitamin E has been used to treat almost every type of skin lesion. It has been used frequently by the general population to treat burns, surgical scars, and other wounds [20, 45, 156]. Quantitative studies have shown that vitamin E applied topically penetrates deep into the dermis and subcutaneous tissue. Probably for this reason, it is believed that vitamin E may improve wound healing when applied topically [20].
As a response to injury, the free oxygen radicals released by neutrophils in the inflammatory phase decrease healing by damaging DNA, cellular membranes, proteins, and lipids, leading ultimately to cell death. As a result, this damage is believed to be reduced by antioxidants enhancing wound healing [20, 133, 213]. Vitamin E is the major lipid soluble antioxidant that protects cells from oxidative stress [20, 144]. It functions primarily by inhibiting and preventing the spread of peroxidation of lipids in cellular membranes, thereby acting as a membrane-stabilizing agent [20, 45, 156]. Although the in vitro antioxidant effects of vitamin E have been well investigated, and although its role in cardiac protection is a topic of great scrutiny, research on the effects of vitamin E in vivo on skin healing is surprisingly scant [20, 45, 156]. Animal studies examining its effects on wound healing have shown contradictory results. These studies have been generally unhelpful because tocopherols, unlike other vitamins, have species-specific mechanisms of action [20].
The effect of vitamin E on wound healing is complex [91]. Systemic vitamin E inhibits the inflammatory response, inhibits collagen synthesis, and thereby yields decreased wound tensile strength. This action is similar to that of the glucocorticoids and can be mitigated by vitamin A, a lysomal destabilizing compound that reverses some of the deleterious effects of the glucocorticoids [90].
Although vitamin E may help to minimize the damage, possibly potentiating healing after radiation injury, its impact on surgical wounds is less clear [90]. As a matter of fact, its effect on surgical wound healing, particularly scar formation, has never been adequately demonstrated [91]. Findings have shown, on the other hand, that topically applied vitamin E provides no more effect than other emollient-type ointments, and hydration appears to be its only beneficial effect. Despite its widespread use in cosmetics, topical vitamin E actually may cause more harm than good [20, 91, 211], possibly worsening scars and causing contact urticaria, eczematous dermatitis, erythema multiform-like reactions, and contact dermatitis in an unacceptably large percentage of patients [20, 35, 45, 57, 99, 156].
Recent publications [90] have highlighted the skin irritation and reduced breaking strength caused by vitamin E, finally putting to rest the long-standing myth that vitamin E has a part to play in early scar control [211]. Use of vitamin E later on in the scar’s maturity (4 to 6 weeks and later) may well flatten the scar because of its hydrative capabilities. However, because of its decreased breaking strength effect on the scar, the use of vitamin E may result in a stretched weakened scar at best. At worst, if used too early, it can result in wound separation [211].
The most recent report about vitamin E states that current evidence from the literature does not support the conclusion that topical use of vitamin E cream can reduce scar formation. In fact, studies report some adverse effects with its use. Further research is needed before the application of vitamin E cream becomes the standard of care [105]. Because no conclusive data exist to support its use, topical vitamin E application on surgical wounds should be discouraged [20].
Extracts and Topical Applications
A number of skin care products produced annually claim capability to diminish the skin flaws of aging, solar damage, or scarring from physical injury [182]. Over the years, multiple extracts and products have been used for scar management, with anecdotal reports claiming promising results. Although these are widely marketed, most need further investigation for conclusive evidence of their effectiveness [45]. Popular treatments include allantoin-sulfomucopolysaccharide gel [128], glycosaminoglycan gel [35], onion extract cream [97], moist exposed burn ointment (MEBO) [6–8], and other creams or ointments containing extracts from plants such as Bulbine frutescens, Centella asiatica [142], Anogeissus latifolia, and Butea monosperma [45]. Many other topical agents have been described such as mitomycin C that have made no difference in the prevention of keloid or hypertrophic scar recurrence after excision when applied topically [180].
Mederma Skin Care
Mederma Skin Care Gel (Merz Pharmaceuticals, Greensboro, NC, USA) is a topical gel [182] that has been marketed as a product to improve scar appearance and texture. However, although few data are available to substantiate these claims [48, 207], it is one of the most popular recent nonprescription topical agents used for wound management. Its active ingredient is Allium cepa, derived from a specific type of onion: Allium cepa Linn.
The principal constituent of Allium cepa is quercetin, a bioflavonoid with a demonstrated antiproliferative effect in both normal and malignant cells of various types as well as antiinflammatory and antihistamine release effects by stabilization of mast cell membranes [45, 182]. Quercetin also is found in apples, red wine, and gingko biloba [45, 182].
The significance of quercetin’s cellular effects may be pertinent to the treatment of hypertrophic scars [182]. It seems, however, that the antiproliferative effects of quercetin probably play less of a role in that regard [182]. Most of the observed results are primarily secondary to its true antihistamine effects. A product that blocks histamine release could perhaps normalize or at least decrease collagen production by fibroblasts, subsequently resulting in reduced dermal scar volume and relative normalization of the scar maturation process. In addition, a decrease in scar inflammation and erythema also would be expected [146, 182].
The antihistamine effects of quercetin present in Mederma also may play a role in downregulating the overproduction of collagen by fibroblasts that, in addition to decreased inflammation, leads to less scar hypertrophy [182]. Quercetin also produces a significant reduction in transforming growth factor beta (TGF-β) expression and in its downstream-signaling molecules, Smad2, Smad3, and Smad4, in fibroblasts [45, 161].
In histologic examination of Mederma-treated wounds, investigators have noted significantly better improvement of collagen organization [45]. A recent experimental study with the rabbit ear hypertrophic scar model demonstrated more mature, organized collagen in Mederma-treated scars, indicating a transformation from the immature collagen characteristics of hypertrophic scars to more mature scars [182]. There was, however, no simultaneous decrease in scar height, probably because the accelerated conversion of immature to mature collagen is not associated with a net decrease in overall collagen production by fibroblasts, or because the short treatment period of 4 weeks in this experimental study was too brief to detect a significant decrease in scar hypertrophy. The study also failed to demonstrate any improvement in scar erythema after topical scar treatment with Mederma Skin Care Gel [182].
This finding is commensurate with the finding of a pilot study that could not demonstrate statistically significant improvement in scar erythema or pruritis among patients using Mederma, although improvement in wounds covered with simple petrolatum-based ointment could be seen [97]. However, in a more recent study comparing the efficacy of Mederma with that of a petrolatum-based emollient (Aquaphor; Beiersdorf, Inc.), the onion extract gel did not improve scar cosmesis or symptomatology [48]. Considering the widespread popularity of this agent, it is surprising that more well-designed studies have not been performed to test its efficacy [45].
Contractubex
Contractubex gel (Merz Pharma, Frankfurt, Germany) (10% aqueous onion extract, 50 U heparin per gram of gel, 1% allantoin) is a well-known ointment in routine out-patient practice claimed to be effective in the treatment and prevention of hypertrophic scars and keloids [28, 93]. Its exact mechanism of action still is unknown. In vitro studies have shown, however, that the onion extract contained in Contractubex gel possesses fibroblast-inhibiting properties, which reduce both fibroproliferative activity and the production of ECM. It is believed that the flavonoids (quercetin and kaempferol) in onion extract account for its fibroblast inhibition and other antiproliferative effects.
Heparin, another active ingredient in Contractubex gel, also may play an important role. In vitro experiments have shown that heparin was able to interact strongly with collagen molecules. It induces the formation of thicker fibrils typical of a mature tissue and promotes intermolecular bonding in collagen [93]. Both heparin and onion extracts, through their inhibitory effects on inflammatory processes, fibroblast proliferation, and the synthesizing capacity of fibroblasts, influence scar development [93].
A study comparing Contractubex gel with intralesional corticosteroid therapy showed that Contractubex was significantly more effective than corticosteroid treatment with regard to erythema, pruritus, consistency of hypertrophic scars, and time to scar normalization and maturation [28]. Contractubex treatment also was associated with significantly fewer adverse events (e.g., teleangiectasias, cutaneous atrophy of scars and surrounding skin tissue) than intralesional corticosteroid injection [28]. It is believed that light itchiness after the application of Contractubex gel, encountered in approximately 7% of patients, probably is related to the pharmacologic action of active ingredients rather than to allergy [93]. Other studies suggested that Contractubex gel had a statistically significant lower rate of scarring in dark skin patients receiving laser treatments of tattoos [93], and that the increase in scar width after thoracic surgery was less in the Contractubex gel–treated fresh scars than in untreated scars after 1 year of treatment [93].
It was recently postulated that heparin-containing compounds (e.g., Contractubex gel) observed to improve blood rheology are effective in preventing the development of pathologic scars in freshly healing scars (6 to 12 days old). On the other hand, well-formed hypertrophic scars respond best to products containing enzymes with fibrinolytic activity [73].
Moist Exposed Burn Ointment
Moist Exposed Burn Ointment (MEBO; Julphar, Gulf Pharmaceutical Industries, Dubai, UAE) is a USA-patented formulation since 1995 composed of six herbal extracts. Its active ingredient is beta-sitosterol in a base of beeswax and sesame oil. The basis of moist exposed burn therapy (MEBT) popularized two decades ago by Xu Rongxiang from the Beijing Chinese Burn Center, it offers the advantages of a moist environment for wound healing by simple ointment application without the need for an overlying secondary, cumbersome, bulky, and expensive dressing [13, 14, 16, 101]. In a prospective study of linear traumatic or surgical facial wounds in humans, healing wounds treated prophylactically with MEBO were noted to have a significantly better cosmetic appearance, with less hyperemia and less postinflammatory hyperpigmentation [13, 45]. The ointment induced earlier restoration of the cutaneous physiologic barrier function commensurate with the observed better cosmetic results after epithelialization of split-thickness donor-site wounds [13, 14].
The observed beneficial effect of the moisture-retentive ointment MEBO on wound healing and scar quality cannot be explained only by moisture retention [101]. In a recent study analyzing experimentally induced burn injuries in rabbits, MEBO was found to have profound effects on mast cells, bFGF, TGF-β, interleukin-1, and nerve growth factor, explaining the previously reported beneficial effect of the ointment on wound healing as an effective prophylactic agent to improve scar quality [101]. This study demonstrated that various inflammatory cells, growth factors, and cytokines present in the wound bed may be modulated by application of local agents with drastic effects on their expression dynamics involving characteristic temporal and spatial regulation and changes in the expression pattern [101].
Madecassol and Alpha Centella Cream
Madecassol is an old “healing drug” [33]. It is the brand name given to a group of chemicals related to asiatic acid, which is extracted from the Centella asiatica plant [211]. Asiatic acid, madecassic acid, and asiaticoside, the principal terpenoids found in Centella asiatica, are shown to aid wound healing in a large number of scientific reports [211]. The oral form of Madecassol is as effective as the injectable intramuscular form [211]. Findings have shown Madecassol to be of clinical value in stopping the inflammatory phase of hypertrophic scars and keloids as well as other forms of connective tissue anomalies with an inflammatory phase, gradually bringing scars to the maturation phase [33]. In addition to the decreased inflammatory reaction and myofibroblast production, its most beneficial effect appears to be the stimulation of type 1 collagen production [31, 211], thus increasing the 1 to 3 ratio. Centella asiatica extracts may well be inducing more rapid maturity of the scar [211].
Madecassol also has been shown to have a preventive effect on burn and postoperative hypertrophic scars. It has been claimed that its effect compares favorably in effectiveness with compression bandaging, and gives more lasting results than intralesional corticosteroid or radiation therapy. It has, however, a placebo effect of 29%, well within acceptable limits [33]. To date, this drug has no known side effects other than occasional mild gastric intolerance and allergic reaction [33].
Alpha Centella cream used for topical scar treatment has two main components. The first is an extract from the plant Bulbine frutescens that increases hydration under the covering tape by leaving a layer of fatty vesicles of glycoprotein on the skin surface that has antibacterial properties. The second component is the principal terpenoid extracted from the Centella asiatica plant [211]. It has been documented that a topical application of Alpha Centella cream to a sutured wound significantly increases the breaking strength of the wound (as opposed to vitamin E) [211]. Patients, however, must be instructed that the critical period for the use of Alpha Centella cream is the first 6 to 8 weeks [211].
Miscellaneous Extracts and Agents
Extract of Anogeissus latifolia, a deciduous tree native to India whose bark, containing leucocyanin and ellagic acids, is used in tanning has traditionally been used for a variety of skin diseases [45, 84]. Its antioxidant and antibacterial activity is well documented. When applied to fresh wounds, a significant reduction in epithelialization time, improved wound contraction, and significant improvement in tensile strength can be documented [45].
A topical extract of Butea monosperma bark, a tropical evergreen, has substantial antioxidant effects and is documented to improve the rate of epithelialization and wound contraction significantly, with increased wound tensile strength [45, 201].
The antioxidant activity of the polyphenol curcumin also is well documented. When it is incorporated into bovine collagen films, slow topical release of curcumin into the wound bed can be achieved. This particular delivery method provides optimal delivery of curcumin’s antioxidant activity on collagen scaffolding for optimal wound healing. Treated wounds have been found to heal faster, leave smaller scars, and contain histologically larger numbers of inflammatory cells as well as larger amounts of collagen [45, 83]. Although these extracts and several others appear to be promising in accelerating healing, further studies investigating their effects on scarring should be performed [45].
Topical tocoretinate, another agent used for the treatment of ulcers, also is a potent treatment for sclerotic skin diseases [139]. Its application to hypertrophic scars improves skin stiffness, the glossy appearance of scars, and telangiectasia. Histopathologically, the proliferated collagen fibers decrease in thickness, and the interfiber spaces increase. It markedly decreases immunoreactive tenascin-C, usually expressed in the proliferated deep dermal fibers of skin sclerosis as systemic sclerosis and hypertrophic scar lesions [139]. Vitamin A (0.05% retinoic acid) had some reported beneficial effects in scar management, but serious reported side effects of hypervitaminosis, skin irritation, and the like are limiting factors for its usefulness [211]. Further studies are required before any of these agents is routinely adopted for better healing and scar management.
Intralesional Injections
5-Fluorouracil Intralesional Injection
As one of the oldest chemotherapy drugs, 5-fluorouracil (5-FU), a pyrimidine analog with antimetabolite activity, has been used against many malignancies [11, 68, 143]. In the early 1980s, it was investigated as an adjunct to glaucoma-filtering surgery, a procedure in which inhibition of wound healing is desirable for the achievement of surgical success [68]. It also has been injected intralesionally for the treatment of nodular basal cell carcinoma and keratoacanthoma [11] as well as treatment of scars to induce regression of keloids and hypertrophic scars [45, 153]. The medication targets rapidly proliferating cells [45].
Intracellularly, 5-FU is enzymatically converted into its active substrate, which is ultimately incorporated into DNA, thus inhibiting DNA synthesis [11]. Rapidly proliferating and metabolizing cells, such as fibroblasts in dermal wounds responsible for excessive collagen production, are preferentially targeted by 5-FU [11, 45]. Although intralesional, 5-FU may be comparable with other therapies [132]. Softening of the scar is reported to occur faster with intralesional 5-FU than with PDL alone [153]. The use of 5-FU to inhibit fibroblast proliferation avoids the potential complications of intralesional corticosteroid injections and adds a new therapeutic agent for patients who did not respond well to corticosteroids [68].
Several studies have confirmed that 5-FU is effective in the treatment of keloid scars [11, 114]. Wound ulceration and hyperpigmentation are reported complications, but both may resolve spontaneously [11]. It also has been used for the treatment of inflamed hypertrophic scars, either alone or in conjunction with intralesional steroid injection of PDL treatments [11, 45, 68]. The use of 5-FU intralesionally for the treatment of hypertrophic scars, either alone or in combination with corticosteroids and PDL therapy, appears to be both effective and safe [68, 149, 153, 186].
Intralesional injection of 5-FU 50 mg/ml in combination with triamcinolone 10 mg/ml or with a very low concentration of betamethasone (5.7 mg/ml) may promote better regression without recurrence of keloid scars smaller than 2 cm in diameter [11, 114]. However, although the addition of corticosteroid to the injection mixture does have a beneficial antiinflammatory effect [11], the use of adjunctive corticosteroid probably is unnecessary because findings have shown 5-FU used alone to be efficacious and well tolerated, with perhaps fewer undesirable side effects such as atrophy, hypopigmentation, and telangiectasia than with the use of intralesional potent corticosteroids alone [11].
It also has been suggested that the addition of local anesthetic does not decrease the injection pain [11]. On the other hand, it seems that the triple combination of 5-FU to suppress fibroblast activity, corticosteroids to suppress inflammation as well as fibroblast activity, and PDL to suppress angiogenesis and endothelial cell growth factors is a successful multifaceted approach to the treatment of hypertrophic scars and keloids [68]. This combination seems to be more acceptable to patients and produces better results. Its effect on lightening of lesions is promising and seems to be the best approach for the treatment of keloid and hypertrophic scars [12].
Bleomycin Multipuncture Injection
Bleomycin, originally isolated from the fungus Streptomyces verticillus, is frequently used as an antitumor agent for treating various kinds of malignancy. In addition, bleomycin is used as a treatment for recalcitrant warts, hypertrophic scars, and keloids [213]. Findings have shown bleomycin to block the cell cycle at G2, cleave single-stranded and double-stranded DNA, and degrade cellular RNAs. In addition, bleomycin is known to induce apoptosis in vitro [213].
Findings have shown that administration of bleomycin by intradermal injections or the multipuncture method is effective against keloid and hypertrophic scars [63, 213], and intralesional multiple jet injections of bleomycin 0.1 ml (1.5 IU/ml) at a maximum dose of 6 ml seems currently to be indicated as a therapy for keloids and hypertrophic scars unresponsive to intralesional steroid injection [63, 181, 213]. The number of sessions required for successful treatment of these lesions ranges from two to six [181], and complete to excellent flattening of scars may be expected for most patients [213].
In a recent study comparing the efficacy of bleomycin tattoo and that of cryotherapy combined with intralesional triamcinolon injection for the treatment of keloids and hypertrophic scars, the therapeutic response in lesions smaller than 100 mm2 exceeded 88% in both groups, but with larger lesions (>100 mm2), the therapeutic response to bleomycin was significantly better [145].
Because bleomycin induces various adverse effects due to its toxicity, clinicians are encouraged to be aware of these potential problems. Bleomycin’s main dose-limiting toxicity is exerted on the lung [213]. Cutaneous side effects including “flagellate” erythema (scratch dermatitis), hyperpigmentation, Raynaud’s phenomenon, gangrene, fibrosis, neutrophilic eccrine hidradenitis, alopecia, edema, nail changes, and other miscellaneous reactions have been documented. It is reported that cutaneous toxicity usually occurs at total doses of 200 to 300 U, and that pulmonary fibrosis occurs at doses exceeding 400 U. However, there is much variation in the data reported [171, 213]. Because the toxic effects of bleomycin are thought to be attributable to its induction of free radicals, combined radiotherapy, which also induces production of free radicals, should be monitored carefully [213]. Discontinuation of bleomycin at the first signs of toxicity is crucial to avoiding a serious outcome [213]. Although the clinical findings show that administration of bleomycin for keloids and hypertrophic scars shows promise and results may be excellent, further investigation is needed and further studies using well-designed, double-blind, placebo-controlled, multicenter randomized clinical trials will be necessary [63, 213].
Interferon Subdermal Injection
Local and systemic TGF- β has been implicated as a fibrogenic cytokine in the pathogenesis of many fibrotic disorders [156]. Large regions of hypertrophic scar after injury, mainly after burns, have an elevated level of systemic TGF-β, contributing to the development fibrosis [203]. Moreover, high TGF-β levels are present not only in locally injured tissues, but also in the serum of thermally injured patients, which may contribute to the development of fibroproliferative hypertrophic scars in these patients. Furthermore, inhibition of TGF-β activity is associated with enhanced resolution of hypertrophic scarring in these patients similar to that found with other fibroproliferative disorders [203].
Elevated TGF-β could be antagonized by systemic interferon therapy [203]. Interferon alpha INF-α, interferon beta INF-β, and interferon gamma INF-γ have been shown to increase collagen breakdown [142]. Interferon alpha-2b (IFN- α2b) also has antiproliferative properties and may improve the pathologic features of dermal fibrosis directly or by antagonizing the effects of TGF-β and histamine [24, 143, 203]. In vitro, hypertrophic scar fibroblasts differ from dermal fibroblasts of uninjured normal skin by increased amounts of messenger RNA (mRNA) and protein for types 1 and 3 procollagen and fibronectin, reduced mRNA and active collagenase (MMP1), and more tissue inhibitor of metalloproteinase I (TIMP I), the net effect of which likely contributes to excessive production of extracellular matrix. Both IFN- γ and INF-α (types 1 and 2 interferon) reduce these and other features of fibrosis and contraction in both in vitro and in vivo animal models [68]. Importantly, only INF-α, and not IFN- γ, seems to activate dermal fibroblast synthesis of collagenase and to reduce its natural inhibitor TIMP I [68].
It is reported that INF-α2b is effective in reducing the size of hemangiomas and dermal lesions characteristic of systemic mastocytosis. These disorders contain activated and highly proliferating endothelial cells and mast cells, which also are prominent cellular components of dermal and other fibrotic conditions [203]. In vitro, INF-α2b has been effective in reducing collagen and fibronectin synthesis and TGF-β production by activated hypertrophic scar fibroblasts, in increasing collagenase activity, and in redirecting the phenotypic features of the hypertrophic fibroblast toward the normal dermal counterpart [203]. In vivo, systemic INF-α2b has been effective in antagonizing the fibrotic effects of TGF-β associated with bleomycin-induced pulmonary fibrosis [203, 205]. In vivo, intralesional IFN-γ is reported anecdotally to improve small region keloids and hypertrophic scarring [111, 203]. It is reported to be effective in improving the appearance of keloids and hypertrophic scars, and in reducing keloid recurrence after excision. Decreased collagen deposition, reduced production of TGF-β, and increased levels of collagenase activity have been proposed as possible mechanisms of action [26, 153]. However, limited efficacy of intralesional therapy may be related to circulating increases in fibrogenic factors such as TGF- β or N[tau]-methylhistamine [203].
When INF-α2b was tested by intralesional injection of keloids, 1.5 million IU twice daily over 4 days, it was found to be much more efficient than intralesional corticosteroid steroid, resulting in a 50% reduction in keloid size by 9 days [24, 143]. Hypertrophic scars injected three times weekly with INF-α2b showed significant mean rates of improvement and sustained reduced serum TGF-β levels even after treatment [142, 203]. Interferon injections are reported to be significantly better than triamcinolone acetonide injections in preventing postsurgical recurrence of keloids (18.7% vs 58.5% recurrence). However, these painful injections may require regional anesthesia [142, 204]. Because the fibroproliferative disorder hypertrophic scarring often undergoes spontaneous resolution to a variable degree over a variable period of time, a phase 3 double-blinded trial will be necessary to establish the potential therapeutic role of interferon INF-α2b for this fibrotic disorder. Further research is warranted to assess the usefulness of subcutaneous INF-α2b treatment for hypertrophic scarring [203].
On the other hand, a newly developed dermal cream containing liposome-encapsulated INF-α2b (LIPO+INF) has been used to improve hypertrophic scarring in open and reepithelialized dermal wounds of a rabbit fibrotic ear model. The findings of this study suggest that LIPO+IFN is effective in reducing the scar formation. Further investigation is required to confirm these results [116].
Miscellaneous Products and Therapies
Collagenase
The unique feature of collagenase is its ability to cleave the native triple helix of intact collagen molecules [191]. It was demonstrated experimentally that collagenase degrades the collagen of the hypertrophic scar, lessening and softening the scar, suggesting that local injection of collagenase probably is a good therapy for hypertrophic scar [212]. Intralesional injection of pure collagenase (600–4,500 units) into keloids and hypertrophic scars has been proposed as an effective treatment strategy for reducing the volume of the pathologic scars [102]. The treatment resulted in a temporarily reduced scar volume. However, the scar volumes had returned to the same (or greater) levels 6 months later. Moreover, side effects are numerous and severe, including pain, swelling, blistering, ulceration, and ecchymosis at the site of injection. It seems that treatment of keloid and hypertrophic scars with intralesional injections of collagenase is ineffective [102]. It recently was established that collagenase D from the larvae Dermestes frischii, with observed effects superior to those of Clostridium histolyticum collagenase and collagenase PC from the hepatopancreas Paralithodes camtshatica, may be used for the treatment of keloids and hypertrophic scars [191].
Hyaluronic Acid
Hyaluronic acid is a major component of the extracellular matrix. Experimental and clinical evidence suggests that it enhances wound healing and reduces the extent of scarring [32]. Preliminary data after intralesional hyaluronic acid injections indicate that softening of the scar is the first observed effect 4 to 6 weeks from the beginning of the treatment. A progressive reduction of the scar thickness follows until the 12th week. It seems that high-molecular-weight hyaluronic acid (500,000–750,000 amu) in high-concentration solutions could be useful in the treatment of pathologic scars, and the clinical results show good correlation with the histologic and immunohistologic results [32].
Verampil
Verapamil is a widely used calcium-channel antagonist antiarrhythmic agent [50, 80, 143]. It also inhibits the synthesis/secretion of extracellular matrix molecules, including collagen, glycosaminoglycans, and fibronectin, and increases collagenase [50]. A few sporadic trials have reported verapamil to be effective in the treatment of keloids when administered intralesionally [80, 143]. It reduces the sustained basal IL-6 and vascular endothelial growth factor production in cultures of central keloid fibroblasts and inhibits cell growth and proliferation by 29%. It also increases apoptosis to an absolute value of 8% [80]. The beneficial effect of calcium antagonists on pathologic scars in vivo is yet to be determined, although it has been suggested that surgical excision with W-plasty or skin grafting and intralesional verapamil injection may be a good alternative for the treatment of keloid [50].
Epicatechin Gallate
Catechins are naturally occurring polyphenolic compounds believed to have antiinflammatory, antioxidant, and free radical scavenging properties [103]. Epicatechin gallate intradermal injections in a rat incisional wound model resulted in significantly improved quality of scar formation in terms of both maturity and orientation of the collagen fibers. An increase in inducible nitric oxide synthase and cyclooxygenase-2 and a decrease in arginase-I activity and protein levels also were observed. Better healing and scar quality may be partly attributable to an acceleration of the angiogenic response and an upregulation of the enzymes nitric oxide synthase and cyclooxygenase [45, 103].
ThermaCool TC Radiofrequency
Collagen fibril morphology and production changes are claimed to occur after the use of the thermage radiofrequency device on hypertrophic scars. ThermaCool (ThermaCool™ System, Thermage, Inc., Hayward, CA) alone did not, however, achieve clinical hypertrophic scar or keloid improvement. The collagen effects of this device should be evaluated further to optimize its therapeutic potential for all indications [136].
Dermatography
Dyspigmentation and textural abnormalities of large scars can be reduced with the multidisciplinary practice of dermatography, which includes microsurgical needle tattooing [45]. Cosmetic tattoos, simulating makeup, have become very popular in recent decades. The technique of micropigmentation consists of implanting pigment into the skin using a tattoo pen [56]. The procedure provides camouflage pigmentation, with the added benefit of inducing atrophy of scars via the cutting action of the needles [45, 153]. It also can be used as an adjunct to reconstructive surgery [56]. The usefulness of this method could be anticipated in flat or slightly elevated scars, but definitely cannot be extended to prominent hypertrophic scars or keloids.
Cosmetic camouflage using techniques from the theater and cinema to conceal a variety of conditions as well as cosmetic products has been applied to mask scars. It has been demonstrated to promote social and psychological well-being [95]. Even when the cosmesis is excellent, the techniques used are not effective at covering all skin lesions, particularly when the contour of the skin surface has been affected by scarring. Despite this limitation, individuals with scarring, who have a higher initial mean Dermatology Life Quality Index (DLQI) score, experience a greater reduction in mean DLQI than individuals with pigmentary or vascular disorders, suggesting that cosmetic camouflage clinics provide a valuable resource to individuals with a wide range of skin conditions, improving their quality of life [94].
Emerging Treatment Methods
Growth factors and cytokines have been involved in scar formation, and these factors are targeted for potential therapeutic use in scar management [135]. In a number of studies, TGF-β has been implicated in wound healing and hypertrophic scarring as well as several other scarring conditions [125, 142]. During the wound-healing process, TGF-β1, TGF-β2, and TGF-β3 have differential temporal effects and are important for optimal healing during the first week after wounding. Beyond 1 week, TGF-β1, TGF-β2, and TGF-β3 play a critical role in hypertrophic scar formation [125].
It also has been suggested that a reduction in Smad-3 proteins in parallel with an increase in Smad-7 contributes to the inhibitory effect and the reduction of ECM synthesis after blocking of TGF-β1 by treatment with neutralizing antibodies. This may indicate a molecular mechanism in the therapeutic intervention to reduce fibrosis, hypertrophic scar formation, and chronic wound-healing disorders [187]. In addition to blocking its effects with antibodies, researchers have proposed blocking TGF-β activation by means of the mannose 6-phosphate receptor and adding the TGF-β3 isoform. These approaches have shown efficacy in animal models.
Early human trials are in progress for some of these strategies [142]. Antagonism of TGF-β by hepatocyte growth factor also can be achieved by gene therapy, an emerging new treatment method with great potential. Hepatocyte growth factor administered via intradermal injection in an experimental animal model results in significantly flatter, smaller scars as well as histologically decreased inflammatory cell infiltrate and granulation tissue formation. Hepatocyte growth factor exerts antifibrotic effects via antagonism of TGF-β while promoting dermal regeneration by reducing inflammatory cell apoptosis, thus potentially providing a future therapy for “scarless wound healing” [45]. Administration of keratinocyte growth factor-expressing keratinocytes seeded onto a cell membrane carrier graft also may result in a much faster formation of a neoepithelium that is well formed and stratified [107, 142].
Scar prevention advancements include refinements in surgical technique, nutritional supplementation, and optimal wound care [45]. It currently is an accepted fact that accelerated wound healing may lead to improved scarring [2, 13, 14]. Among cytokines and growth factors, bFGF is clinically proven to accelerate acute and chronic wound healing. Clinical evaluation of pigmentation, pliability, height, and vascularity of bFGF-treated scars has demonstrated significant differences relative to conventional treatment [2]. Further investigations of this method are definitely warranted.
Other means to accelerate healing are being tested as well. Findings have shown that applying porcine acellular dermal matrix to deep partial-thickness burn wounds until the wound heals without dressing change maximally preserves residual dermal tissue and epithelium, helps to accelerate the regeneration of epithelial and stem cells thus shortening healing time, remodels the skin structure, and consequently has the effect of controlling hypertrophic scarring at its inception [65].
Excessive collagen deposition occurs relative to normal wounds. This extracellular matrix collagen accumulation makes a logical target for pharmacologic interventions, and researchers are attempting to modify collagen synthetic and degradative pathways [135, 142]. Penicillamine and other nonspecific inhibitors of collagen synthesis have been used as inhibitors, but these have shown unacceptable toxicity.
In recent years, several companies have looked for specific nontoxic inhibitors of collagen synthesis that could be applied locally. It seems likely that new therapies will be available within the next few years [142]. Bilidase (a hyaluronidase preparation) was studied in guinea pigs with experimental postburn scars and found to promote normalization of the structure and the histochemical picture of new scar tissue, suggesting that the drug can be used for the treatment of hypertrophic postburn scars [124]. The antifibrinolytic agent pentoxifylline also was shown to have a dose-dependent effect on proliferation and contraction of burn scar fibroblasts. It seems that pentoxifylline has a direct effect on inhibition of burn scar fibroblasts that could provide opportunities to reduce burn scar contractures in vivo [165]. Findings have shown that 50 mmol/l of putrescine inhibits tissue transglutaminase, which plays a role in the cross-linking of type 3 procollagen in wound matrices. The clinical effect from 50 mmol/l of putrescine in a eutectic vehicle (Fibrostat) examined in a phase 2 double-blind crossover study with 43 patients demonstrated significant improvement in hypertrophy. It is suggested that Fibrostat is a safe therapeutic agent for the treatment of hypertrophic scarring [61].
Cytotoxic agents also are being evaluated for their potential utility in the reduction of tissue bulk associated with these excessive scar states [135]. Cyclosporin could possibly be useful in prevention if T lymphocytes are proven to play a role (still experimental). Collagen inhibitors such as beta-aminoproprionitrile prevent cross-linking of collagen. Results are still awaited, but these preparations would not be used routinely in normal scar management [211]. Given the wide range of potential therapeutic agents, the future market for scar therapy remains highly promising [135].
Conclusion
The problematic nature of hypertrophic scarring combined with the lack of a consistently successful treatment regimen has led to much research into the biologic nature of scar formation [182]. Recent years have seen an increased understanding of the molecular and biologic mechanisms involved in keloidal and hypertrophic scar formation, allowing for the development of more specific therapeutic options for these lesions [45, 207], and the quest to identify conservative methods that successfully treat these lesions is ongoing [182]. Currently, the vast array of available methods for eradicating scars often are inconvenient, occasionally prove to be painful, and have unwanted side effects [9, 135]. Unfortunately, keloids and hypertrophic scars remain difficult to manage [207].
Many techniques for the management of hypertrophic scars and keloids have been proven through extensive use, but few have been supported by prospective studies with adequate control groups. Several new therapies have shown good results in small-scale trials, but these have not been repeated in larger trials with long-term follow-up evaluation [216]. Recommendations support a move to a more evidence-based approach to scar management. This approach highlights a primary role for silicon gel sheeting and intralesional corticosteroids in the management of a wide variety of abnormal scars. These are the only treatments for which sufficient evidence exists to make evidence-based recommendations.
A number of other therapies in common use have achieved acceptance as standard practice. However, it is highly desirable that many standard practices and new emerging therapies undergo large-scale studies with long-term follow-up evaluation before being recommended conclusively as alternative therapies for scar management [216]. Only recently have researchers begun to delineate the complex biochemical signaling pathways that regulate wound healing and scarring processes. Regulation of scar metabolism with regard to collagen and wound matrix degradation is likewise showing promise in generating alternate therapies for the treatment of abnormal scars. Understanding the exact process of normal and abnormal scar formation will help to define better ways for successfully managing and potentially preventing abnormal healing [193].
Treatment must be individualized depending on the distribution, size, thickness, and consistency of the lesions and the association of inflammation [37, 143]. It begins by educating the patient about the etiology of the scarring process [37]. There is a need for additional studies to understand the prevalence and risk factors for the development of hypertrophic scarring. The development of more reliable, valid, and objective measures for diagnosing and measuring the severity of hypertrophic scarring also is essential for further research in the area of prevention and treatment [64]. Currently, the combination approach with adjuvant therapy in addition to surgery, such as steroid injections and silicone materials, seems to be the best option [143, 194].
References
Acikel C, Ergun O, Ulkur E, Servet E, Celikoz B (2005) Camouflage of self-inflicted razor blade incision scars with carbon dioxide laser resurfacing and thin skin grafting. Plast Reconstr Surg 116:798
Akita S, Akino K, Imaizumi T, Tanaka K, et al. (2006) The quality of pediatric burn scars is improved by early administration of basic fibroblast growth factor. J Burn Care Res 27:333
Allison KP, Kiernan MN, Waters RA, Clement RM (2003) Pulsed dye laser treatment of burn scars: Alleviation or irritation? Burns 29:207
Apfelberg DB, Maser MR, White DN, Lash H (1989) Failure of carbon dioxide laser excision of keloids. Lasers Surg Med 9:382
Alster T (2003) Laser scar revision: Comparison study of 585-nm pulsed-dye laser with and without intralesional corticosteroids. Dermatol Surg 29:25
Alster TS (1997) Laser treatment of hypertrophic scars, keloids, and striae. Dermatol Clin 15:419
Alster TS, Handrick C (2000) Laser treatment of hypertrophic scars, keloids, and striae. Semin Cutan Med Surg 19:287
Alster TS, Nanni CA (1998) Pulsed-dye laser treatment of hypertrophic burn scars. Plast Reconstr Surg 102:2190
Alster TS, West TB (1997) Treatment of scars: A review. Ann Plast Surg 39:418
Amicucci G, Schietroma M, Rossi M, Mazzotta C (2005) Silicone occlusive sheeting vs silicone cushion for the treatment of hypertrophic and keloid scars: A prospective randomized study. Ann Ital Chir 76:79
Apikian M, Goodman G (2004) Intralesional 5-fluorouracil in the treatment of keloid scars. Australas J Dermatol 45:140
Asilian A, Darougheh A, Shariati F (2006) New combination of triamcinolone, 5-fluorouracil, and pulsed-dye laser for treatment of keloid and hypertrophic scars. Dermatol Surg 32:907
Atiyeh BS, Al-Amm CA, El-Musa K (2003) Improved scar quality following primary and secondary healing of cutaneous wounds. Aesth Plast Surg 27:411
Atiyeh BS, Al-Amm CA, El-Musa KA, Sawwaf A, Dham R (2003) Scar quality and physiologic barrier function restoration following moist and moist exposed dressings of partial thickness wounds. Dematol Surg 29:14
Atiyeh BS, Costagliola M, Hayek SN (2005) Keloid or hypertrophic scar: The controversy: Review of the Literature. Ann Plast Surg 54:676
Atiyeh BS, Ioannovich J, Al-Amm CA, El-Musa KA, Dham R (2002) Improving scar quality: A prospective clinical study. Aesth Plast Surg 26:470
Atkinson JA, McKenna KT, Barnett AG, McGrath DJ, Rudd MA (2005) Randomized, controlled trial to determine the efficacy of paper tape in preventing hypertrophic scar formation in surgical incisions that traverse Langer’s skin tension lines. Plast Reconstr Surg 116:1648
Baisch A, Riedel F (2006) Hyperplastic scars and keloids: Part I. Basics and prevention. HNO 54:893
Baum TM, Busuito MJ (1998) Use of a glycerin-based gel sheeting in scar management. Adv Wound Care 11:40
Baumann LS, Spencer J (1999) The effects of topical vitamin E on the cosmetic appearance of scars. Dermatol Surg 25:311
Bayat A, McGrouther DA (2005) Clinical management of skin scarring. Skinmed 4:165
Behroozan DS, Goldberg LH, Dai T, Geronemus RG, Friedman PM (2006) Fractional photothermolysis for the treatment of surgical scars: A case report. J Cosmet Laser Ther 8:35
Bellew SG, Weiss MA, Weiss RA (2005) Comparison of intense pulsed light to 595-nm long-pulsed pulsed dye laser for treatment of hypertrophic surgical scars: A pilot study. J Drugs Dermatol 4:448
Berman B, Dunan MR (1989) Short-term keloid treatment in vivo with human interferon α2b results in a selective and persistent normalization of keloid fibroblast collagen, glycosaminoglycan, and collagenase production in vitro. J Am Acad Dermatol 21:694
Berman B, Flores F (1999) Comparison of a silicone gel-filled cushion and silicon gel sheeting for the treatment of hypertrophic or keloid scars. Dermatol Surg 25:484
Berman B, Flores F (1997) Recurrence rates of excised keloids treated with postoperative triamcinolone acetonide injections or interferon alfa-2b injections. J Am Acad Dermatol 37:755
Berman B, Flores F (1998) The treatment of hypertrophic scars and keloids. Eur J Dermatol 8:591
Beuth J, Hunzelmann N, Van Leendert R, Basten R, et al. (2006) Safety and efficacy of local administration of contractubex to hypertrophic scars in comparison to corticosteroid treatment: Results of a multicenter, comparative epidemiological cohort study in Germany. In Vivo 20:277
Bock O, Schmid-Ott G, Malewski P, Mrowietz U (2006) Quality of life of patients with keloid and hypertrophic scarring. Arch Dermatol Res 297:433
Bombaro KM, Engrav LH, Carrougher GJ, Wiechman SA, et al. (2003) What is the prevalence of hypertrophic scarring following burns? Burns 29:299
Bonte F, Dumas M, Chaudagne C, Meybeck A (1995) Asiaticoside and madecassoside activity on human fibroblast type I and III collagen. Ann Pharm Franc 53:38
Borgognoni L, Reali UM (1997) Intralesional hyaluronic acid treatment of pathological scars. Ann Plast Surg 38:308
Bosse JP, Papillon J, Frenette G, Dansereau J, et al. (1979) Clinical study of a new antikeloid agent. Ann Plast Surg 3:13
Boutli-Kasapidou F, Tsakiri A, Anagnostou E, Mourellou O (2005) Hypertrophic and keloidal scars: An approach to polytherapy. Int J Dermatol 44:324
Boyce DE, Bantick G, Murison MS (2000) The use of ADCON-T/N glycosaminoglycan gel in the revision of tethered scars. Br J Plast Surg 53:403
Branagan M, Chenery DH, Nicholson S (2000) Use of the infrared attenuated total reflectance spectroscopy for the in vivo measurement of hydration level and silicone distribution in the stratum corneum following skin coverage by polymeric dressings. Skin Pharmacol Appl Skin Physiol 13:157
Brissett AE, Sherris DA (2001) Scar contractures, hypertrophic scars, and keloids. Facial Plast Surg 17:263
Brown CA (2001) A comparison of the outcomes of two clinical audits of burn pressure garment satisfaction and compliance in Saudi Arabia. Burns 27:342
Burd A, Huang L (2005) Hypertrophic response and keloid diathesis: Two very different forms of scar. Plast Reconstr Surg 116:150e
Carney SA, Cason CG, Gowar JP, Stevenson JH, et al. (1994) Cica care gel sheeting in the management of hypertrophic scarring. Burns 20:163
Chan HH, Wong DS, Ho WS, Lam LK, Wei W (2004) The use of pulsed-dye laser for the prevention and treatment of hypertrophic scars in Chinese persons. Dermatol Surg 30:987
Chan KY, Lau CL, Adeeb SM, Somasundaram S, Nasir-Zahari M (2005) A randomized, placebo-controlled, double-blind, prospective clinical trial of silicone gel in prevention of hypertrophic scar development in median sternotomy wound. Plast Reconstr Surg 116:1013
Chang CC, Kuo YF, Chiu H, Lee JL, et al. (1995) Hydration, not silicone, modulates the effects of keratinocytes on fibroblasts. J Surg Res 59:705
Chang P, Laubenthal KN, Lewis RW, Rosenquist M, et al. (1995) Prospective, randomized study of the efficacy of pressure garment therapy in patients with burns. J Burn Care Rehabil 16:473
Chen MA, Davidson TM (2005) Scar management: Prevention and treatment strategies. Curr Opinion Otolaryngol Head Neck Surg 13:242
Cheng JCY, Evans JH, Leung KS, Clark JA, et al. (1984) Pressure therapy in the treatment of postburn hypertrophic scar: A clinical look into its usefulness and fallacies by pressure monitoring. Burns 10:154
Chowdri NA, Masarat M, Mattoo A, Darzi MA (1999) Keloids and hypertrophic scars: Results with intraoperative and serial postoperative corticosteroid injection therapy. Aust N Z J Surg 69:655
Chung VQ, Kelley L, Marra D, Jiang SB (2006) Onion extract gel versus petrolatum emollient on new surgical scars: A prospective double-blinded study. Dermatol Surg 32:193
Collins J (1992) Pressure techniques for the prevention of hypertrophic scar. Clin Plast Surg 19:733
Copcu E, Sivrioglu N, Oztan Y (2004) Combination of surgery and intralesional verapamil injection in the treatment of the keloid. J Burn Care Rehab 25:1
Costa AM, Peyrol S, Porto LC, Comparin JP, et al. (1999) Mechanical forces induce scar remodeling study in non–pressure-treated versus pressure-treated hypertrophic scars. Am J Pathol 155:1671
Costagliola M, Rougé D, Gavroy JP, Ster F (1994) Quantitative and qualitative aspect of the vitro pressure test. 9ème Congrés de l’International Society for Burn Injury, Paris
Cruz-Korchin NI (1996) Effectiveness of silicone sheets in the prevention of hypertrophic breast scars. Ann Plast Surg 37:345
Davey RB, Wallis KA, Bowering K (1991) Adhesive contact media: An update on graft fixation and burn scar management. Burns 17:313
De Cubber J (1998) A silicone elastomer manipulation technique for the production of medical devices. J Facial Somato Prosthetics 4:15
De Cuyper C (2006) Permanent make-up: Indications and complications. Kosmet Med 27:56
De Groot AC, Berretty PMJ, Ginkel GJW, den Henjst CW, et al. (1991) Allergic contact dermatitis from tocopheryl acetate in cosmetic creams. Contact Dermatitis 25:302
De Oliveira GV, Nunes TA, Magna LA, Cintra ML, et al. (2001) Silicone versus nonsilicone gel dressings: A controlled trial. Dermatol Surg 27:721
Desmoulière A, Regard M, Darby I, Gabbiani G (1995) Apoptosis mediates the decrease in cellularity during the transition between granulation tissue and scar. Am J Pathol 146:56
Dinh Q, Veness M, Richards S (2004) Role of adjuvant radiotherapy in recurrent earlobe keloids. Australas J Dermatol 45:162
Dolynchuk KN, Ziesmann M, Serletti JM (1996) Topical putrescine (Fibrostat) in treatment of hypertrophic scars: Phase II study. Plast Reconstr Surg 97:117
Eberlein A, Schepler H, Spilker G, Altmeyer P, Hartmann B (2005) Erbium:YAG laser treatment of post-burn scars: Potentials and limitations. Burns 31:15
Espana A, Solano T, Quintanilla E (2001) Bleomycin in the treatment of keloids and hypertrophic scars by multiple needle punctures. Dermatol Surg 27:23–27
Esselman PC, Thombs BD, Magyar-Russell G, Fauerbach JA (2006) Burn rehabilitation: State of the science. Am J Phys Med Rehabil 85:383
Feng X, Tan J, Pan Y, Wu Q, et al. (2006) Control of hypertrophic scar from inception by using xenogenic (porcine) acellular dermal matrix (ADM) to cover deep second-degree burn. Burns 32:293
Field T, Peck M, Hernandez-Rief M, Krugman S, et al. (2000) Postburn itching, pain, and psychological symptoms are reduced with massage therapy. J Burn Care Rehabil 21:189
Field T, Peck Krugman S, Tuchel T, Schanberg S, et al. (1998) Burn injuries benefit from massage therapy. J Burn Care Rehabil 19:241
Fitzpatrick RE (1999) Treatment of inflamed hypertrophic scars using intralesional 5-FU. Dermatol Surg 25:224
Fricke NB, Omnell ML, Dutcher KA, Hollender LG, Engrav LH (1999) Skeletal and dental disturbances in children after facial burns and pressure garment use: A 4 year follow up. J Burn Care Rehabil 20:239
Fulton JE Jr (1995) Silicone gel sheeting for the prevention and management of evolving hypertrophic and keloid scars. Dermatol Surg 21:947
Gailloud-Matthieu MC, Raffoul W, Egloff DV (1999) Hypertrophic scars and keloids: Which therapeutic options today? Rev Med Suisse Romande 119:721
George WM (1999) Linear lymphatic hypopigmentation after intralesional corticosteroid injection: Report of two cases. Cutis 64:61
Gerasimenko MIu, Iusova ZhIu, Zenger VG, Kazantseva IA (2005) Differentiated approach to medicine phonophoresis in complex treatment of cicatrical deformations. Vestn Ross Akad Med Nauk 6:29
Gerow FJ, Hardy SB, Spira M, Law SW (1963) Immersion treatment for burns: An experimental study. Surg Forum 14:32
Ghahary A, Shen YJ, Scott PG, Tredget EE (1995) Immunolocalization of TGF-beta 1 in human hypertrophic scar and normal dermal tissues. Cytokine 7:184
Gibbons M, Zuker R, Brown M, Candlish S, et al. (1994) Experience with silastic gel sheeting in pediatric scarring. J Burn Care Rehabil 15:69
Gilman TH (2003) Silicone sheet for treatment and prevention of hypertrophic scar: A new proposal for the mechanism of efficacy. Wound Rep Reg 11:235–236
Giele HP, Liddiard K, Currie K, Wood FM (1997) Direct measurement of cutaneous pressures generated by pressure garments. Burns 23:137
Giele H, Liddiard K, Booth K, Wood F (1998) Anatomical variations in pressures generated by pressure garments. Plast Reconstr Surg 101:399
Giugliano G, Pasquali D, Notaro A, Brongo S, et al. (2003) Verapamil inhibits interleukin-6 and vascular endothelial growth factor production in primary cultures of keloid fibroblasts. Br J Plast Surg 56:804
Gold MH (1994) A controlled clinical trail of topical silicone gel sheeting in the treatment of hypertrophic scars and keloids. J Am Acad Dermatol 30:506
Gold MH, Foster TD, Adair MA, Burlison K, Lewis T (2001) Prevention of hypertrophic scars and keloids by the prophylactic use of topical silicone gel sheets following a surgical procedure in an office setting. Dermatol Surg 27:641
Gopinath D, Ahmed MR, Gomathi K (2004) Dermal wound healing processes with curcumin induced collagen films. Biomaterials 25:1911
Govindarajan R, Vijayakumar M, Rao C, Shirwaikar A, et al. (2004) Healing potential of Anogeissus latifolia for dermal wounds in rats. Acta Pharm 54:331
Hamanova H, Broz L (2002) Topigel in the treatment of hypertrophic scars after burn injuries. Acta Chir Plast 44:18
Hambleton J, Shakespeare P, Pratt B (1992) The progress of hypertrophic scars monitored by ultrasound measurements of thickness. Burns 18:301
Hanasono MM, Lum J, Carroll LA, Mikulec AA, Koch RJ (2004) The effect of silicone gel on basic fibroblast growth factor levels in fibroblast cell culture. Arch Facial Plast Surg 6:88
Har-Shai Y, Amar M, Sabo E (2003) Intralesional cryotherapy for enhancing the involution of hypertrophic scars and keloids. Plast Reconstr Surg 111:1841
Har-Shai Y, Lindenbaum E, Tendler M, Gamliel-Lazarovich A, et al. (1999) Negatively charged static electricity stimulation as a possible mechanism for enhancing the involution of hypertrophic and keloid scars. Isr Med Assoc J 1:203
Harumi M, Miyuki N, Hideo M, Kiyokazu K (2001) Effects of clothing pressure exerted on a trunk on heart rate, blood pressure, skin blood flow, and respiratory function. J Textile Mach Soc Jpn 54:57
Havlik RJ (1997) Vitamin E and wound healing: Safety and efficacy reports. Plast Reconstr Surg 100:1901
Hirshowitz B, Ullmann Y, Har-Shai Y, Vilenski A, Peled IJ (1993) Silicone occlusive sheeting (SOS) in the management of hypertrophic scarring, including the possible mode of action of silicone, by static electricity. Eur J Plast Surg 16:5
Ho WS, Ying SY, Chan PC, Chan HH (2006) Use of onion extract, heparin, allantoin gel in prevention of scarring in Chinese patients having laser removal of tattoos: A prospective randomized controlled trial. Dermatol Surg 32:891
Holme SA, Beattie PE, Fleming CJ (2002) Cosmetic camouflage advice improves quality of life. Br J Dermatol 147:946
Hulsbergen-Henning JP, Roskam Y, van Gemert MJ (1986) Treatment of keloids and hypertrophic scars with an argon laser. Lasers Surg Med 6:72
Hubbard M, Masters IB, Williams GR, Chang AB (2000) Severe obstructive sleep apnea secondary to pressure garments used in the treatment of hypertrophic burn scars. Eur Respir J 16:1205
Jackson BA, Shelton AJ, McDaniel DH (1999) Pilot study evaluating topical onion extract as treatment for postsurgical scars. Dermatol Surg 25:267
Jaros E, Priborsky J, Klein L (1999) Treatment of keloids and hypertrophic scars with cryotherapy. Acta Medica (Hradec Kralove) Suppl 42:61
Jenkins M, Alexander JW, Mac Millian BG (1986) Failure of topical steroids and vitamin E to reduce postoperative scar formation following reconstructive surgery. J Burn Care Rehabil 7:309
Johnson J, Greenspan B, Gorga D, Nagler W, Goodwin C (1994) Compliance with pressure garment use in burn rehabilitation. J Burn Care Rehabil 15:181
Jurjus A, Atiyeh BS, Abdallah IM, Jurjus RA, et al. Pharmacological modulation of wound healing in experimental burns. Burns 2007 in press
Kang N, Sivakumar B, Sanders R, Nduka C, Gault D (2006) Intralesional injections of collagenase are ineffective in the treatment of keloid and hypertrophic scars. J Plast Reconstr Aesthet Surg 59:693
Kapoor M, Howard R, Hall I, Appleton I (2004) Effects of epicatechin gallate on wound healing and scar formation in a full thickness incisional wound healing model in rats. Am J Pathol 165:299
Katz BE (1995) Silicone gel sheeting in scar therapy. Cutis 56:65
Khoosal D, Goldman RD (2006) Vitamin E for treating children’s scars: Does it help reduce scarring? Can Family Physician 52:855
Kono T, Ercocen AR, Nakazawa H, Nozaki M (2005) Treatment of hypertrophic scars using a long-pulsed dye laser with cryogen-spray cooling. Ann Plast Surg 54:487
Kopp J, Wang GY, Kulmburg P, Schultze-Mosgau S, et al. (2004) Accelerated wound healing by in vivo application of keratinocytes overexpressing KGF. Mol Ther 10:86
Kosaka M (2004) The current therapy and prevention of hypertrophic scars and keloids. Skin Res 3:628
Kosaka M, Kamiishi H (2001) New concept of balloon-compression wear for the treatment of keloids and hypertrophic scars. Plast Reconstr Surg 108:1454
Kuhn MA, Moffit MR, Smith PD, Lyle WG, et al. (2001) Silicone sheeting decreases fibroblast activity and downregulates TGFbeta2 in hypertrophic scar model. Int J Surg Invest 2:467
Larrabee WF Jr, East CA, Jaffe HS, Stephenson C, Petrson KE (1990) Intralesional interferon [gamma] treatment for keloids and hypertrophic scars. Arch Otolaryngol Head Neck Surg 116:1159
Larson DL, Abston S, Evans EB, Dobrkovsky M, Linares HA (1971) Techniques for decreasing scar formation and contractures in the burned patient. J Trauma 11:807
Larson DL, Abston S, Willis B, Linares HA, et al. (1974) Contracture and scar formation in the burned patient. Clin Plast Surg 1:653
Lebwohl M (2000) From the literature: Intralesional 5-FU in the treatment of hypertrophic scars and keloids: Clinical experience. J Am Acad Dermatol 42:677
Lee DK, Serkin AL (2004) Carbon dioxide laser and Apligraf for a painful plantar hypertrophic scar. J Am Podiatr Med Assoc 94:61
Lee JP, Jalili RB, Tredget EE, Demare JR, Ghahary A (2005) Antifibrogenic effects of liposome-encapsulated IFN-alpha2b cream on skin wounds in a fibrotic rabbit ear model. J Interferon Cytokine Res 25:627
Lee SM, Ngim CK, Chan YY, Ho MJ (1996) A comparison of Sil-K and Epiderm in scar management. Burns 22:483
LeVier RR, Harrison MC, Cook RR, Lane TH (1993) What is silicone? Plast Reconstr Surg 92:163
Leung K, Cheng J, Ma G, Clark J, Leung P (1984) Complications of pressure therapy for postburn hypertrophic scar. Burns 10:434
Leung PC, Ng M (1980) Pressure therapy for hypertrophic scars resulting from burns. Burns 6:244
Leung P, Ng M (1980) Pressure treatment for hypertrophic scars resulting from burns. Burns 6:244
Linares HA (1996) From wound to scar. Burns 22:339
Linares HA, Larson DL, Willis-Galstaun BA (1993) Historical notes on the use of pressure in the treatment of hypertrophic scars or keloids. Burns 19:17
Loladze M, Alibegashvili M, Turmanidze TS, Iashvili B, et al. (2005) Use of bilidase for the treatment of experimental hypertrophic postburn cicatrices. Bull Exp Biol Med 139:98
Lu L, Saulis AS, Liu WR, Roy NK, et al. (2005) The temporal effects of anti-TGF-beta1, -2, and -3 monoclonal antibody on wound healing and hypertrophic scar formation. J Am College Surg 201:391
Lupton JR, Alster TS (2002) Laser scar revision. Dermatol Clin 20:55
Macintyre L, Baird M (2006) Pressure garments for use in the treatment of hypertrophic scars: A review of the problems associated with their use. Burns 32:10
Magliaro A, Gianfaldoni R, Cervadoro G (1999) Treatment of burn scars with a gel based on allantoin and sulfomucopolysaccharides. G Ital Dermatol Venereol 134:153
Majan JI (2006) Evaluation of a self-adherent soft silicone dressing for the treatment of hypertrophic postoperative scars. J Wound Care 15:193
Malaker K, Vijayraghavan K, Hodson I, Al Yafi T (2004) Retrospective analysis of treatment of unresectable keloid with primary radiation over 25 years. Clin Oncol (R Coll Radiol) 16:290
Malick MH, Carr JA (1980) Flexible elastomer molds in burn scar control. Am J Occup Ther 34:603
Manuskiatti W, Fitzpatrick RE (2002) Treatment response of keloidal and hypertrophic sternotomy scars: Comparison among intralesional corticosteroid, 5-fluorouracil, and 585-nm flashlamp-pumped pulsed-dye laser treatments. Arch Dermatol 138:1149
Martin A (1996) The use of antioxidants in healing. Dermatol Surg 22:156
McCraw JB, McCraw JA, McMellin A, Bettencourt N (1999) Prevention of unfavorable scars using early pulse-dye laser treatments: A preliminary report. Ann Plast Surg 42:7
Meier K, Nanney LB (2006) Emerging new drugs for scar reduction. Expert Opin Emerging Drugs 11:39
Meshkinpour A, Ghasri P, Pope K, Lyubovitsky JG, et al. (2005) Treatment of hypertrophic scars and keloids with a radiofrequency device: A study of collagen effects. Lasers Surg Med 37:343
Miller J, Hardy SB, Spira M (1965) Treatment of burns of the hand with silicone dressing and early motion: Preliminary report. J Bone Joint Surg 47:938
Miller MC, Nanchahal J (2005) Advances in the modulation of cutaneous wound healing and scarring. BioDrugs 19:363
Mizutani H, Yoshida T, Nouchi N, Hamanaka H, Shimizu M (1999) Topical tocoretinate improved hypertrophic scar, skin sclerosis in systemic sclerosis, and morphea. J Dermatol 26:11–17
Murison M, James W (2006) Preliminary evaluation of the efficacy of Dermatix silicone gel in the reduction of scar elevation and pigmentation. J Plast Reconstr Aesthetic Surg 59:437
Musgrave M, Umraw N, Fish J, Gomez M, et al. (2002) The effect of silicone gel sheets on perfusion of hypertrophic burn scars. J Burn Care Rehab 23:208
Mustoe TA, Cooter RD, Gold MH, Hobbs FD, et al. (2002) International clinical recommendations on scar management. Plast Reconstr Surg 110:560
Mutalik S (2005) Treatment of keloids and hypertrophic scars. Indian J Dermatol Venereol Leprol 71:3
Nachbar F, Korting HC (1995) The role of vitamin E in normal and damaged skin. J Mol Med 73:7
Naeini FF, Najafian J, Ahmadpour K (2006) Bleomycin tattooing as a promising therapeutic modality in large keloids and hypertrophic scars. Dermatol Surg 32:1023
Nakaoka H, Miyauchi S, Miki Y (1995) Proliferating activity of dermal fibroblasts in keloids and hypertrophic scars. Acta Derm Venereol 75:102
Nedelec B, Ghahary A, Scott P, Tredget E (2000) Control of wound contraction: Basic and clinical features. Hand Clin 16:289
Niessen F, Spauwen P, Robinson P, Fidler V, Kon M (1998) The use of silicone occlusive sheeting (Sil-K) and silicone occlusive gel (Epiderm) in the prevention of hypertrophic scar formation. Plast Reconstr Surg 102:1962
Niessen FB, Spauwen PHM, Schalkwijk J, Kon M (1999) On the nature of hypertrophic scars and keloids: A review. Plast Reconstr Surg 104:1435
Nikkonen MM, Pitkanen JM, Al-Qattan MM (2001) Problems associated with the use of silicone gel sheeting for hypertrophic scars in the hot climate of Saudi Arabia. Burns 27:498
Norris JE (1991) The effect of carbon dioxide laser surgery on the recurrence of keloids. Plast Reconstr Surg 87:44
Norris JEC (1995) Superficial X-ray therapy in keloid management: A retrospective study of 24 cases and literature review. Plast Reconstr Surg 95:1051
Nouri K, Vidulich K, Rivas MP (2006) Lasers for scars: A review. J Cosmet Dermatol 5:14
O’Brien L, Pandit A (2006) Silicon gel sheeting for preventing and treating hypertrophic and keloid scars. Cochrane Database Syst Rev 25(1):CD003826
Ogawa R, Mitsuhashi K, Hyakusoku H, Miyashita T (2003) Postoperative electron-beam irradiation therapy for keloids and hypertrophic scars: Retrospective study of 147 cases followed for more than 18 months. Plast Reconstr Surg 111:547
Panin G, Strumia R, Ursini F (2004) Topical alpha-tocopherol acetate in the bulk phase: Eight years of experience in skin treatment. Ann N Y Acad Sci 1031:443
Patino O, Novick C, Merlo A, Benaim F (1999) Massage in hypertrophic scars. J Burn Care Rehabil 20:268
Patterson RP, Fisher SV (1979) The accuracy of electrical transducers for the measurement of pressure applied to the skin. IEEE Trans Biomed Eng 26:450
Peacock EE, Madden JW, Trier WC (1970) Biologic basis for treatment of keloids and hypertrophic scar. South Med J 63:755
Pekins K, Davey RB, Wallis KA (1982) Silicone gel: A new treatment for burn scars and contractures. Burns 9:201
Phan TT, Lim IJ, Chan SY, Tan EK, et al. (2004) Signaling in keloid-derived fibroblasts by quercetin/smad signaling in keloid-derived fibroblasts by quercetin: Implications for the treatment of excessive scars. J Trauma 57:1032
Poveda A, Campech M, Gavroy JP, Ster F (1998) Le massage «en brûlure»: Formes topographiques et chronologiques. 18 Congrés de la Société Française d’Etude et de Traitement de la Brûlure, SFETB, Saint Gervais
Puzey G (2002) The use of pressure garments on hypertrophic scars. J Tissue Viability 12:11
Quinn KJ, Evans JH, Courtney JM, Gaylor JD, Reid WH (1985) Nonpressure treatment of hypertrophic scars. Burns Incl Therm Inj 12:102
Rawlins JM, Lam WL, Karoo RO, Naylor IL, Sharpe DT (2006) Pentoxifylline inhibits mature burn scar fibroblasts in culture. Burns 32:42
Rayner K (2000) The use of pressure therapy to treat hypertrophic scarring. J Wound Care 9:151
Reid WH, Evans JH, Naismith RS, Tully AE, Sherwin S (1987) Hypertrophic scarring and pressure therapy. Burns 13:S29
Reno F, Sabbatini M, Stella M, Magliacani G, Cannas M (2005) Effect of in vitro mechanical compression on Epilysin (matrix metalloproteinase-28) expression in hypertrophic scars. Wound Repair Regen 13:255
Reiken SR, Wolfort SF, Berthiaume F, Compton C, et al. (1997) Control of hypertrophic scar growth using selective photothermolysis. Lasers Surg Med 21:7
Reiffel RS (1995) Prevention of hypertrophic scars by long-term paper tape application. Plast Reconstr Surg 96:1715
Remlinger KA (2003) Cutaneous reactions to chemotherapy drugs: The art of consultation. Arch Dermatol 139:77
Reno F, Grazianetti P, Cannas M (2001) Effects of mechanical compression on hypertrophic scars: Prostaglandin E2 release. Burns 27:215
Reno F, Sabbatini M, Lombardi F, Stella M, et al. (2003) In vitro mechanical compression induces apoptosis and regulates cytokines release in hypertrophic scars. Wound Rep Reg 11:331
Ricketts CH, Martin L, Faria DT, Saed GM, Fivenson DP (1996) Cytokine mRNA changes during the treatment of hypertrophic scars with silicone and nonsilicone gel dressings. Dermatol Surg 22:955
Rockwell WB, Cohen IK, Ehrlich HP (1989) Keloids and hypertrophic scars: A comprehensive review. Plast Reconstr Surg 84:827
Roques C (2002) Massage applied to scars. Wound Repair Regen 10:126
Rose MP, Deitch EA (1985) The clinical use of a tubular compression bandage, Tubigrip, for burn-scar therapy: A critical analysis. Burns 12:58
Roseborough IE, Grevious MA, Lee RC (2004) Prevention and treatment of excessive dermal scarring. J Natl Med Assoc 96:108
Rusciani L, Rossi G, Bono R (1993) Use of cryotherapy in the treatment of keloids. J Dermatol Surg Oncol 19:529
Sanders KW, Gage-White L, Stucker FJ (2005) Topical mitomycin C in the prevention of keloid scar recurrence. Arch Facial Plast Surg 7:172
Saray Y, Gulec AT (2005) Treatment of keloids and hypertrophic scars with dermojet injections of bleomycin: A preliminary study. Int J Dermatol 44:777
Saulis A, Mogford JH, Mustoe TA (2002) Effect of Mederma on hypertrophic scarring in the rabbit ear model. Plast Reconstr Surg 110:177
Sawada Y, Urushidate S, Nihei Y (1998) Hydration and occlusive treatment of a sutured wound. Ann Plast Surg 41:508
Sawada Y, Sone K (1990) Treatment of scars and keloids with a cream containing silicone oil. Br J Plast Surg 43:683
Sawada Y, Sone K (1992) Hydration and occlusion treatment for hypertrophic scars and keloids. Br J Plast Surg 45:599
Schmidt A, Gassmueller J, Hughes-Formella B, Bielfeldt S (2001) Treating hypertrophic scars for 12 or 24 hours with a self-adhesive hydroactive polyurethane dressing. J Wound Care 10:149
Schultze-Mosgau S, Blaese MA, Grabenbauer G, Wehrhan F, et al. (2004) Smad-3 and Smad-7 expression following anti-transforming growth factor beta 1 (TGFbeta1)-treatment in irradiated rat tissue. Radiother Oncol 70:249
Shakespeare PG, van Reeterghem L (1985) Some observations on the surface structure of collagen in hypertrophic scars. Burns 11:175
Sheridan RL, MacMillan K, Donelan M, Choucair R, et al. (1997) Tunable dye laser neovessel ablation as an adjunct to the management of hypertrophic scarring in burned children: Pilot trial to establish safety. J Burn Care Rehabil 18:317
Sherris DA, Larrabee WF Jr, Murakami CS (1995) Management of scar contractures, hypertrophic scars, and keloids. Otolaryngol Clin North Am 28:1057
Shmoilov AM, Rudenskaya GN, Isaev VA, Baydakov AV, et al. (2006) A comparative study of collagenase complex and new homogeneous collagenase preparations for scar treatment. J Drug Deliv Sci Technol 16:285
Silfen R, Amir A, Hauben DJ, Calderon S (2001) Effect of facial pressure garments for burn injury in adult patients after orthodontic treatment. Burns 27:409
Slemp AE, Kirschner RE (2006) Keloids and scars: A review of keloids and scars, their pathogenesis, risk factors, and management. Curr Opin Pedatr 18:396
Smeets L, Grandjean F-X, Nickers P, Deleuze J-P, et al. (2006) How I treat fibroproliferative scars. Rev Med Liege 61:11
So K, Umraw N, Scott J, Campbell K, et al. (2003) Effects of enhanced patient education on compliance with silicone gel sheeting and burn scar outcome: A randomized prospective study. J Burn Care Rehabil 24:411
Sproat JE, Dalcin A, Weitauer N, Roberts RS (1992) Hypertrophic sternal scars: Silicone gel sheeting versus kenalog injection treatment. Plast Reconstr Surg 90:988
Staley MJ, Richard RL (1997) Use of pressure to treat hypertrophic burn scars. Adv Wound Care 10:44
Stewart R, Bhagwanjee AM, Mbakaza Y, Binase T (2000) Pressure garment adherence in adult patients with burn injuries: An analysis of patient and clinician perceptions. Am J Occup Ther 54:598
Su CW, Alizadeh K, Boddie A, Lee RC (1998) The problem scar. Clin Plast Surg 25:451
Suetak T, Sasai S, Zhen YX, Tagami H (2000) Effects of silicone gel sheet on the stratum corneum hydration. Br J Plast Surg 53:503
Sumitra M, Manikandau P, Suguna L (2005) Efficacy of Butea monosperma on dermal wounds in rats. Int J Biochem Cell Biol 37:566
Tredget EE, Nedelec B, Scott PG, Ghahary A (1997) Hypertrophic scars, keloids, and contractures: The cellular and molecular basis for therapy. Surg Clin North Am 77:701
Tredget EE, Shankowsky HA, Pannu R, Nedelec B, et al. (1998) Transforming growth factor-beta in thermally injured patients with hypertrophic scars: Effects of interferon alpha-2b. Plast Reconstr Surg 102:1317
Tredget EE, Wang R, Shen Q, Scott PG, Ghahary A (2000) Transforming growth factor-beta mRNA and protein in hypertrophic scar tissues and fibroblasts: Antagonism by IFN-alpha and IFN-gamma in vitro and in vivo. J Interferon Cytokine Res 20:143
Tsubota A, Chayama K, Ikeda K, Yasuji A, et al. (1994) Factors predictive of response to interferon-alpha therapy in hepatitis C virus infection. Hepatology 19:1080
Tuan T, Nichter LS (1998) The molecular basis of keloid and hypertrophic scar formation. Mol Med Today 1:19
Urioste SS, Arndt KA, Dover JS (1999) Keloids and hypertrophic scars: Review and treatment strategies. Semin Cutan Med Surg 18:159
Van den Kerckhove E, Stappaerts K, Boeckx W, Van den Hof B, et al. (2001) Silicones in the rehabilitation of burns: A review and overview. Burns 27:205
Van den Kerckhove E, Stappaerts K, Fieuws S, Laperre J, et al. (2005) The assessment of erythema and thickness on burn related scars during pressure garment therapy as a preventive measure for hypertrophic scarring. Burns 31:696
Ward RS (1991) Pressure therapy for the control of hypertrophic scar formation after burn injury: A history and review. J Burn Care Rehabil 12:257
Widgerow AD, Chait LA, Stals R, Stals PJ (2000) New innovations in scar management. Aesth Plast Surg 24:227
Wu J-X, Liu Q-S, Zhou X-L, Qin W, et al. (2005) Primary observation of therapeutic effect of collagenase on hypertrophic scar. Chin J Clin Rehab 38:168
Yamamoto T (2006) Bleomycin and the skin. Br J Dermatol 155:869
Yii NW, Frame JD (1996) Evaluation of cynthaskin and topical steroid in the treatment of hypertrophic scars and keloids. Eur J Plast Surg 19:162
Yosipovitch G, Widijanti Sugeng M, Goon A (2001) A comparison of the combined effect of cryotherapy and corticosteroid injections versus corticosteroids and cryotherapy on keloids: A controlled study. J Dermatol Treat 12:87
Ziegler UE (2004) International clinical recommendations on scar management. Zentralbl. Chir 129:296
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Atiyeh, B.S. Nonsurgical Management of Hypertrophic Scars: Evidence-Based Therapies, Standard Practices, and Emerging Methods. Aesth Plast Surg 31, 468–492 (2007). https://doi.org/10.1007/s00266-006-0253-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00266-006-0253-y