Abstract
Social development is crucial in the ontogeny of animals living in complex societies and has lasting consequences in adulthood. Spotted hyenas (Crocuta crocuta) live in fission-fusion societies as complex as those of cercopithecine primates. The social positions adult hyenas hold within their groups are complex and varied, but little is known about how those positons emerge and change over the course of development. Using social network analysis (SNA), we tested predictions of hypotheses suggesting that sex and dominance status affect the social network positions of young hyenas across three stages of ontogeny: (1) while living at the communal den, (2) den independent but pre-reproductive, and (3) early adulthood. By examining rates at which hyenas were found alone and their association networks, aggression networks, and affiliation networks, we observed striking changes in individuals’ network positions across ontogeny, as well as pronounced sex differences. With the exception of rates at which individuals were found alone, which increased over ontogeny, most social network position metrics decreased greatly from infancy to adulthood. However, females showed considerably more rank-related variation in this trajectory than did males. Overall, social rank had stronger effects on the development of social network positions in females than males. Thus, females and males have different social development trajectories that appear to prepare them for their different respective futures of integrating into their natal clan or dispersing to a new one.
Significance statement
Social development is difficult to study, particularly in long-lived gregarious mammals, so little is known about the variables shaping the emergence during ontogeny of social roles played by adults. We used social network analysis to investigate how sex and rank affect the social positions of maturing spotted hyenas as their positions change across ontogenetic development. Females develop more complex social positions than males early in life, with strategies that vary with their dominance status. Although males are just as well connected to group-mates as females when they are cubs, they generally disengage from the natal clan as they mature; males clearly do not disperse because they are aggressively expelled from their natal group. Our data suggest for the first time that social development appears to prepare females and males for their alternative futures of philopatry or dispersal.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Just as the surroundings in which a child grows up are critical to its development, early environments are also important to nonhuman animals (Belsky et al. 1991; Moffitt et al. 1992; Belsky et al. 2007; McDonald 2007; Stanton and Mann 2012). For species living in highly complex societies like those of primates, bottlenose dolphins (Tursiops sp.), or elephants (Loxodonta africana), it is critical for individuals to develop the skills and relationships needed to function effectively as members of their social group and to maximize their fitness. An individual’s behavior and fitness appear to be strongly affected by both the structure of its social network and its position within that network, especially in complex societies (e.g., Silk 2003; Silk et al. 2010; Chiyo et al. 2012; Stanton and Mann 2012).
There is a long history in the primate literature, in particular, of documenting how and why early development is important to the long-term welfare of individual animals (e.g., Mason 1968). Robert Hinde found that proper social development was necessary for individual primates to become fully functioning adults in their social group (Hinde and Atkinson 1970; Hinde 1976). Hinde was one of the first primatologists to use social networks to describe the structure of animal groups and the positions of individuals within those groups, based on their interactions. However, since Hinde conducted his work in the 1960s and ‘70s, computational resources have improved tremendously, allowing us to use detailed network metrics to go beyond mere descriptions of association patterns or analysis of rates of interactions, as Hinde did, to document the positions or roles of individuals within their social networks. Behavioral biologists are now using social network analysis (SNA) to elucidate the roles of individuals in complex mammalian societies (e.g., Hock et al. 2010; Archie and Chiyo 2012; Wilson et al. 2013; Hobson and DeDeo 2015). Network analysis has a long history of use in disciplines ranging from mathematics to sociology but has only recently been utilized by behavioral biologists (Granovetter 1973; Barabasi and Albert 1999; Newman 2003; Croft et al. 2008). SNA enables us to document the ontogenetic development of the roles played by individual adults in their societies.
Many factors have been shown to affect behavioral development in general and social development in particular. For example, many primates show sexually dimorphic social roles in adulthood, but it is unclear how or why those patterns develop (Campbell et al. 2011). In both juvenile and adult bonnet macaques (Macaca radiata), sex, rank, and kinship differentially predict rates of affiliation and aggression interactions (Silk et al. 1981). By puberty, the social networks of rhesus macaques (Macaca mulatta) become sexually dimorphic, such that males play almost exclusively with other males and females with other females (Suomi 2005, but see Berman 1982). These sex-specific patterns of social interaction continue to diverge, setting males and females on different trajectories into the future (Suomi 2005).
Recent applications of SNA to bottlenose dolphins similarly show that males and females develop along different trajectories. Males tend to form the strongest social bonds with other young males, whereas females tend to develop the strongest bonds with their mothers (Stanton et al. 2011; Stanton and Mann 2012). These are among the few studies exploring early social position in wild mammals. To our knowledge, studies using SNA to examine social development in mammals with complex societies have been restricted to dolphins and primates, although social development can be crucial to long-term success in many other taxa as well (Makagon et al. 2012; Hobson et al. 2013; Pinter-Wollman et al. 2014).
Another factor that frequently affects the roles of individuals in complex hierarchical societies, such as those of cercopithecine primates, is social dominance status. High-ranking individuals may lead social lives that are fundamentally different from those of their group-mates because they enjoy priority of access to resources, and this has lasting consequences for their reproductive success, longevity, and stress levels (Ellis 1995; Holekamp et al. 1996; Silk 2003; Crockford et al. 2008). In baboons (Papio hamadryas ursinus), for instance, high-ranking females are groomed preferentially, whereas lower-ranking females are attacked more frequently, and this in turn affects the differential social stress they experience (Crockford et al. 2008).
Here we use SNA to test predictions of hypotheses suggesting that sex and dominance status affect patterns of social development in free-living spotted hyenas (Crocuta crocuta) as they do in primates. Spotted hyenas offer a particularly good model system in which to study effects of these variables on social development for two reasons. First, in contrast to primates, dolphins, and most other mammals living in complex societies, spotted hyenas develop by advancing through life-history stages that are clearly demarcated by such unambiguous milestones as cessation of dependence on dens for shelter (Holekamp and Smale 1998a). These milestones allow us to partition development into clear stages and compare effects of sex and rank among stages. Second, spotted hyenas live in societies, called clans, that are as large and complex as troops of baboons or macaques (Holekamp et al. 2015). Furthermore, as in these primates, dispersal behavior in hyenas is strongly sexually dimorphic, with most males emigrating to new groups shortly after reaching sexual maturity and females remaining for life in their natal groups (Smale et al. 1997). As in the societies of many cercopithecine primates, hyena social rank determines priority of access to key resources, such that it has profound effects on fitness (e.g., Holekamp et al. 1996; Swanson et al. 2011). However, in contrast to cercopithecine primates, spotted hyenas live in fission-fusion societies in which individuals are often found alone or with small subgroups of clan-mates (Kruuk 1972). The fission-fusion nature of hyena sociality allows us to complement social network metrics with measures of time spent alone and relationship strength among clan-mates, as reflected in association indices. Here, after assessing ontogenetic change in the percentage of their time individuals spent alone, we compare positions of high-, mid-, and low-ranking hyenas of both sexes in three types of ego networks across three life stages. We first assess association networks, which indicate the extent to which individuals in each sex and rank category spend time with particular clan-mates. We also assess aggression networks, which indicate which individuals are aggressors and which are targets during dyadic agonistic interactions. Finally, we assess affiliation networks based upon patterns of greeting behavior observed among clan-mates.
Methods
Study site and subject animals
This study took place in the Masai Mara National Reserve, Kenya. Subjects were members of a single large clan of spotted hyenas that defend a group territory in the Talek region (Boydston et al. 2001). Detailed behavioral data (described in detail below) were collected via daily, year-round monitoring from June 1988 through September 2013. We identified individual hyenas by their unique spots, determined the sex of each animal based on its phallic morphology (Frank et al. 1991), and determined its birthdate to ± 7 days based on its appearance when first observed (Holekamp et al. 1996). Because our study involved focal animals in the field, it was not possible to record data blindly.
Spotted hyenas live in mixed-sex, matrilineal societies organized by linear dominance hierarchies (Frank 1986; Holekamp et al. 2012), and they acquire their social ranks via a learning process typical of old-world monkeys called “maternal rank inheritance” (Holekamp and Smale 1991, 1993). Young hyenas of both sexes acquire social ranks immediately below those of their mothers during the first 2 years of life. Clans contain multiple matrilines of adult natal females and their young, but most breeding males are immigrants born elsewhere. Females are socially dominant to males among adults because males enter the hierarchy of their new clan at the bottom when they immigrate to join a new social group (East and Hofer 2001). Adult females and their young tend to be core figures in hyena societies, but adult males are more peripheral (Kruuk 1972; Holekamp et al. 1997; Szykman et al. 2001). Rank relationships among adult females are quite stable over long periods (Holekamp et al. 2012). High-ranking females enjoy significantly greater reproductive success than do low-ranking hyenas (Holekamp et al. 1996; Hofer and East 2003; Swanson et al. 2011), which are also more strongly affected by fluctuations in prey abundance than are high-ranking hyenas (Holekamp et al. 1996). Here we assigned each individual a social rank based on its wins and losses in dyadic agonistic interactions, then we further assigned each subject to one of three rank categories by standardizing the ranks from − 1 to 1 (lowest rank to highest rank) in each year of our study, and categorizing any ranks − 1 to − 0.33 as low-ranking, − 0.33 to 0.33 as mid-ranking, and 0.33 to 1 as high-ranking. Until cubs acquired their own ranks, they were assigned the social ranks of their mothers.
To explore the social development of our subjects, we used the distinctive milestone markers in hyena development to partition ontogenetic development into three stages (Table 1). Young hyenas in our study area live at a communal den with other members of their cohort until they are 9–10 months old. During this stage, social interactions are more limited than during later stages because cubs’ choices of social partners are restricted to members of their cohorts and whichever den independent hyenas choose to visit the den. Thus, the first stage of development on which we focus in this paper was the communal den stage (CD), lasting from the date on which each cub was first seen until its date of den independence. All subjects were first seen within the first 3 months of life and were restricted to animals with known dates at which they became independent of the communal den. We determined that a juvenile was independent of the den when it was found away from the den on at least three consecutive occasions.
During the second stage of development, juveniles are independent of the den, and potentially able to interact with all their clan-mates, but they remain dependent on their mothers for food until they wean, on average, at 14 months of age (Holekamp and Smale 1998a). During this second stage of development, juveniles learn their ranks in relation to clan members with whom they did not interact at the communal den. The second stage of development here was thus the stage of den independence (DI), defined for each individual to start when the cub became den independent and to be equal in length to the length of its CD stage for SNA. Although juveniles could be weaned at any time during the DI stage, and although this might conceivably influence network metrics during this stage, youngsters continue to rely heavily on their mothers for assistance in feeding throughout the DI stage because their skulls and skull musculature are far from fully developed (Tanner et al. 2009; Watts et al. 2009; Swanson et al. 2013). Thus, it seemed unlikely that DI metrics would be strongly affected by variable weaning ages among juveniles. Furthermore, our analyses required that all three developmental stages be of the same duration within each juvenile’s ontogeny, so instead of ending the DI stage at weaning, we made the DI stage the same length as the CD stage.
Male and female spotted hyenas both reach sexual maturity at approximately 24 months of age; males disperse to new clans 1–76 months after reaching sexual maturity, whereas females remain in their natal clans for life (Smale et al. 1997; Van Horn et al. 2003; Höner et al. 2010). Here, all natal animals under 24 months old were considered juveniles, and older animals were considered adults. We defined the adult stage of development as starting on the day on which an individual reached 24 months of age and extending from that date for a period equal in length to that of its CD stage. The adult stage for each hyena was thus standardized to represent its adult social world before it died or dispersed; all subjects had disappearance or dispersal dates after the end of their adult stages, as defined here. Within individuals, all three stages of development were of the same length for consistent comparisons, and each individual subject was observed during all three stages of development, so sample sizes were the same across all developmental stages. Mean stage length was 7.17 ± 0.13 months for females (n = 108; 52 high-ranking, 34 mid-ranking, 22 low-ranking) and 7.45 ± 0.22 months for males (n = 109; 52 high-ranking, 28 mid-ranking, 29 low-ranking).
Behavioral data collection
Behavioral observations were conducted year-round over the 25-year study period, from our vehicles, which we used as mobile blinds. Observations were made daily between 0530 and 0900 h and again between 1700 and 2000 h. Each observation session was initiated when we found one or more hyenas separated from others by at least 200 m and terminated when we left that individual or group. In the absence of vocal communication, hyenas appear to be completely unaware of one another when separated by more than 200 m (Smith et al. 2008). Although no focal hyenas were radio-collared here, subgroups of hyenas were located either via use of radio telemetry or while observers drove daily circuits in which all high points within the study clan’s home range were visited. By making 360° visual scans with binoculars from each high point, we were able to sample all parts of the clan’s territory every day for presence of subgroups of hyenas. Each subgroup sighted or found via telemetry was then visited to determine its composition. On average, subgroups were found 1.11 ± 0.03 km apart (N = 1291 distances, ranging from 201 m to 9.8 km) (Smith et al. 2008).
To maximize independence of observations, we used only the first session in which an individual was seen during morning or evening observation periods. At the beginning of each observation session and subsequently at 15–20-min intervals, we performed scan samples in which we recorded all individuals present (Altmann 1974). From our session data, we determined association patterns based on the twice-weight index of association (Cairns and Schwager 1987), as used previously (Holekamp et al. 1997, 2012; Szykman et al. 2001). Because some subgroups were easier to find than others, given their size or the presence of one or more individuals fitted with radio collars, the twice-weight index was the association index most appropriate for our sampling methods (Cairns and Schwager 1987); dyadic twice-weight association indices are robust and accurate indicators of social bond strength in spotted hyenas (Holekamp et al. 1997, 2012; Smith et al. 2007). We also used all-occurrence sampling (Altmann 1974) to record all agonistic and affiliation interactions. We built networks based only upon aggressive interactions in which we could see which individual initiated the attack and which individual was its recipient. Because greeting ceremonies, in which individuals raise their legs to one another to sniff the other’s anogenital region, were previously shown to promote and maintain social bonds (Smith et al. 2011), we used greeting behaviors to construct affiliation networks. We created affiliation networks using only interactions in which we knew which individual initiated each greeting by lifting its leg first.
Network construction
Social networks consist of groups of more than two individual animals (nodes) connected by behavioral interactions or co-occurrences in space (ties or links) and portrayed as graphs or matrices. Ties can be directed if the behavior has an initiator and a receiver or undirected when there is no clear direction in the relationship. Here networks based on associations were undirected, as they indicated co-occurrence, but both aggression and affiliation networks were directed. We used only ego networks in this study; these are networks containing only those individuals directly connected to a focal animal.
We used the R package, “network” version 1.13.0 (Butts 2008, 2015) to build three social networks per subject per network type, each based on data collected during one of the three stages of development. The focal individual had to be seen at least ten times during each developmental stage for its network to be calculated, and each of its partners also had to be seen at least ten times during a particular stage to be included in the ego network. Each tie in each association network occurred when individuals meeting our selection criteria were seen together in one or more observation sessions. Ties in aggression networks occurred when an individual initiated, or received an aggression from, a group-mate during the developmental stage in question. Similarly, ties in affiliation networks were added when an individual initiated or received a greeting ceremony from a group-mate during that developmental stage. In this study, we chose to use binary networks with strong criteria for inclusion so that we could make a robust baseline assessment of social position development.
Network metrics and statistical analysis
For each focal individual, during each stage of development (CD, DI, and adulthood), for each network type, we calculated several measures of social network position. First, we calculated the proportion of observation sessions in which the focal individual was found alone, when it clearly could not be interacting with other animals, as the number of sessions in which the individual was seen alone divided by the total number of sessions in which the individual was observed during that developmental stage. We next calculated degree centrality, here called “degree,” which is the number of other individuals to which the focal individual is connected. Degree is an important metric in social networks, as having a higher degree can indicate that an individual is more of a social hub, which in turn can affect its fitness and its exposure to both information and pathogens (e.g., Hamede et al. 2009; Barocas et al. 2011; Royle et al. 2012). In directed networks, we calculated both in-degree centrality, which represents the number of individuals that directed actions at the focal individual, and out-degree centrality, representing the number of individuals with which the focal animal initiated interactions. We also determined ego density, which is the number of ties that occur in the ego network, excluding the ego, out of all possible ties that could potentially be formed with all hyenas in the ego network. In ego networks, density indicates how much the individuals in the focal animal’s network interact with one another; this can be important for learning and disease exposure in animal societies, as more dense networks enable more efficient information flow, and spread of pathogens, within the group (Hanneman and Riddle 2005; Croft et al. 2008; Kurvers et al. 2011). Social network metrics were calculated using the “statnet” package (v.2016.4) in R (Handcock et al. 2008).
Once the network metrics were calculated, we then ran each metric in its own mixed effects model using R (v. 3.2.3) and glmmADMB (Fournier et al. 2012; Skaug et al. 2016). For all degree metrics, we used a Poisson error distribution, and for alone rates and density metrics, we used a logistic error distribution. Because the metrics calculated for one individual in a network are dependent on the other animals in the network, network data often violate assumptions of independence, requiring randomization tests to generate p values that reflect independence among subjects. However, that was unnecessary here because we were working with 1953 different ego networks, with only 7% structural and 9.6% temporal overlap among networks. Three different ego networks were calculated for each of 108 females and 109 male subjects in each of three different developmental stages, drawing from hyenas studied over a period of 25 years. Thus, the ego networks studied here were almost entirely independent of one another due to rare overlap with respect to both the periods during which individuals were observed and the individuals comprising the networks.
To test our hypotheses that sex and rank affect network position over ontogeny, we predicted each metric with sex, developmental stage, rank, and specific interactions among these three key variables.We have previously documented sexually dimorphic development in other aspects of the behavior of spotted hyenas (e.g., Boydston et al. 2005), and we expected that rank might have different effects on females and males here because females retain their rank by remaining in their natal clan whereas males lose their natal rank when they disperse. Therefore, we included two-way interactions between (sex and developmental stage) and (sex and rank), as well as the three-way interaction of (sex, developmental stage, and rank). Each model also included the identity of the focal individual as a random effect. During the periods of study, clan size ranged from 36 to 125 and contained, on average, 77.31 ± 0.57 hyenas. Therefore, we included an offset for clan size during the stage in question; this value was log transformed to make the scale more closely comparable to our response measures. Group size is known to affect network metrics because it limits the number of individuals with which a focal animal can interact. Thus, the equation for each GLMM run was:
We considered differences among groups to be significant when P < 0.05. All reported mean values are shown ±SE.
Data availability statement
Although still actively being explored as part of Turner’s dissertation work, starting in 2018, the datasets generated during and/or analyzed during the current study will be available from the corresponding author on request.
Results
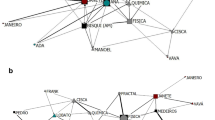
Data describing observations per individual and interaction frequency for focal hyenas in each stage of development are summarized in Table S1.The social network metrics of individuals exhibited striking variation over the course of ontogeny, generally decreasing with age and social rank (Table 3). Figure 1 shows representative examples of affiliation networks for one high-ranking, one mid-ranking, and one low-ranking female across our three ontogenetic stages; metrics calculated for each of these networks are shown in Table S2. Figure 1 clearly shows that the high-ranking female had more individuals and interactions in her networks than did the mid- or low-ranking females. However, we observed considerable variation among network types, and not all of them looked exactly like these.
Examples of typical affiliation ego networks of a high-ranking (ego=GIL), b mid-ranking (ego=HML), and c low-ranking (ego=BERN) females across ontogeny (see Table S1 for the specific network metrics of each ego)
Changes in network position across developmental stages
Although cubs were rarely seen alone during the CD stage, hyenas of both sexes were seen alone significantly more often as they matured (Table 3 and Fig. 2).In association networks, both degree centrality and density decreased significantly between the CD stage and adulthood (Table 3 and Figs. 3 and S1). In aggression networks, we observed that in-degree, out-degree, and density of individuals’ ego networks significantly decreased over ontogeny (Table 3 and Figs. 5 and S2). As with most other network metrics, greeting in-degree and out-degree also decreased significantly over the course of development in affiliation networks (Table 3 and Fig. 6). Similarly, we found that affiliation densities also decreased significantly over the course of ontogeny (Table 3 and Fig. S3). Note that our analyses of degree and density, which were unweighted, assumed that a pair of individuals found together once during a developmental stage had the same value as a pair often found together. However, given the rigor of our criteria for inclusion of individuals in these analyses, as described above, we are nevertheless confident that our results are robust.
Effects of social rank on proportion of time found alone. Mean proportion of observation sessions in which animals were found alone, based on rank level for a females and b males across three developmental stages: communal den (CD), den independent (DI), and adulthood. Sample sizes were as follows:high-rank (black circle, f = 52, m = 52), mid-rank (white circle, f = 34, m = 28), and low-rank (black square, f = 22, m = 29). Sample sizes represent numbers of focal individuals and error bars indicate ±SE
Effects of social rank on association network metrics. Mean degree in the association networks of a females and b males by rank level across developmental stages: communal den (CD), den independent (DI), and adulthood. Degree indicates the number of other individuals in the focal animal’s network. Sample sizes and error bars are as in Fig. 2
Effects of sex on network position
We found no significant differences between males and females with respect to the proportion of time spent alone (Table 3 and Fig. 2). We did however observe a significant interaction between sex and developmental stage, with males being seen alone more than females by the time they reached adulthood (Table S3).
Degree centrality of association networks was significantly higher for females than males during the adult stage; thus, females associated with more individuals than did males by adulthood (Tables 3 and S3 and Fig. 3), which supports our hypothesis that sex affects social network positions. The mean ego density of association networks for both males and females also generally declined across ontogeny (Table 2), but sex had no significant effect on this metric (Table 3 and Fig. S1).
In aggression networks, the degree centrality of each focal animal indicated the total number of individuals in its aggression ego network, which generally declined differently over time between the sexes (Table 2). By the time hyenas reached adulthood, the number of individuals aggressing upon them was significantly higher for females than males, even though in-degree still generally decreased over development in both sexes (Tables 3 and S3 and Fig. 4a). There was no overall sex difference in mean out-degree in aggression networks, but the average out-degree centrality among females was significantly higher than that among males during adulthood. The mean number of individuals aggressed upon by males declined across ontogeny, but this was not true for females, in which this metric remained relatively constant over development (Tables 3 and S3 and Fig. 4b). The average aggression network density, indicating how connected all animals were within the ego network, declined over ontogeny, but we observed no significant difference in this between the sexes (Tables 2 and 3 and Fig. S2).
Effects of sex on aggression network metrics: Mean a in-degree vs. b out-degree in the aggression networks of female and male spotted hyenas across developmental stages: communal den (CD), den independent (DI), and adulthood. Sample sizes were as follows: females (black circle, n = 108), males (white circle, n = 109). Sample sizes represent numbers of focal individuals and error bars indicate ±SE
In affiliation networks, the decreasing in-degree over the course of ontogeny appeared to be largely driven by values for males declining more steeply than those for females, whose values varied more during ontogeny (Table 2); here, we observed a significant effect of the interaction between sex and developmental stage on in-degree centrality (Tables 3 and S3 and Fig. 6a). We also found an interaction between sex and developmental stage in which females had higher mean affiliation out-degree than males during DI and adult stages (Tables 3 and S3 and Fig. 6b). Furthermore, similar to the patterns seen in aggression networks, out-degree in the greeting networks of females remained relatively consistent as they matured. By contrast, males greeted with significantly more individuals than did females during the CD stage but with relatively few clan-mates during adulthood (Fig. 6b). Affiliation network densities decreased over the course of development in both sexes (Table 2), although network densities decreased less sharply for females than did those for males, and females tended to have higher densities in general (Tables 3 and S3 and Fig. S3).
Effects of rank on network position
In support of the hypothesis that dominance status affected network positions, we observed a significant interaction in females between rank and developmental stage where, by adulthood, mid-ranking females tended to be alone more often than were either high- or low-ranking females (Table S4). Social rank did not significantly affect degree centrality or density for either females or males in their association networks (Tables 3 and S4 and Figs. 3 and S1). However, in aggression networks, mid-ranking hyenas had significantly higher in-degree centrality than did either high- or low-ranking individuals, indicating that the former were the targets of more aggression (Table 3). The in-degree of mid-ranking females was higher during the DI stage than during either CD or adult stages (Fig. 5a and Table S4). Additionally, there was a significant interaction in males between developmental stage and rank, in which low-ranking males had lower in-degree centrality than did males in other rank categories during adulthood (Fig. 5b and Table S4). High-ranking females had a significantly higher out-degree than mid-ranking and low-ranking females, indicating that higher-ranking females aggressed on more individuals than did lower-ranked female hyenas, particularly in adulthood (Table S4). On the other hand, high- and mid-ranking males aggressed on more individuals than did low-ranking males, as indicated by out-degree measures, but this difference was only significant in adulthood (Table S4). Social rank had no significant effect on the aggression network densities of focal individuals, and we observed no significant interactions among any of the factors (Table 3 and Fig. S2).
Effects of social rank on aggression networks metrics: Mean in-degree in the aggression networks of a females and b males by rank level across developmental stages: communal den (CD), den independent (DI), and adulthood. Sample sizes and error bars are as in Fig. 2
Social rank did not significantly affect affiliation network in-degree in either sex, but there was a significant interaction between rank and developmental stage. That is, significantly fewer clan-mates initiated greetings with mid- and low-ranking females than with high-ranking females except during the CD stage (Fig. 7 and Table S4). Low-ranking individuals, however, initiated greetings with significantly more individuals than did higher-ranking individuals (Table 3). The significant interaction between sex and rank indicated that mid-ranking females had lower out-degree centrality than did mid-ranking males (Table S3). Low-ranking males also initiated greetings with significantly fewer individuals than did high- or mid-ranking males in adulthood (Table S4).
Lastly, mid-ranking individuals tended to have higher affiliation densities than did hyenas in other rank categories, but this appeared to be driven partially by mid-ranking males, who had higher network densities than high- or low-ranking males during the CD stage, but whose densities then dropped below values for the other rank groups during the DI stage (Tables 3 and S4 and Fig. S3). Mid-ranking males also had significantly higher densities than did mid-ranking females, but this also appeared to be driven mainly by interactions during the CD stage (Table S3 and Fig. S3).
Discussion
Changes in network position across developmental stages
Hyenas generally spent more time alone, and their social interactions diminished overall, as they matured. Because the communal den is the clan’s social hub, cubs living there rarely have opportunities to be alone, and they tend to have more individuals with which to interact, and more often, than during later stages of development. Most females with den-dwelling cubs visit daily to nurse them, older siblings come to visit their mothers and younger siblings, and other clan members come to socialize and investigate their new clan-mates (Holekamp et al. 1997). Once youngsters were no longer dependent on dens here, they were seen alone more often, their social interactions diminished, and this trend increased even further in adulthood, as the fission-fusion character of the hyenas’ society became more fully apparent.
The numbers of individuals toward which females directed aggressive and affiliative acts, and from which they received such acts, did not change significantly across ontogeny. There was, however, a great deal of variation within these metrics, suggesting that individual personality traits might affect network positions, as also occurs among Trinidadian guppies (Poecilia reticulata) (Croft et al. 2009), great tits (Parus major) (Aplin et al. 2013), rhesus macaques (M. mulatta) (McCowan et al. 2011), chimpanzees (Pan troglodites) (Massen and Koski 2014), and other species in which personality and network position are related.
Effects of sex on network position
An individual’s sex only directly affected affiliation density (Table 3), but sex interacted significantly with developmental stage to affect several other network metrics (Table 3), supporting our hypothesis that sex affects the development of social network position. Even though the two sexes exhibited similar values early in life, females had larger, more dense association and aggression networks than males by adulthood, indicating that they were more central and better connected than maturing males. This pattern is also seen in primates, although SNA has seldom been used to document these changes (Pereira 1988; Lehmann and Ross 2011). It is interesting that male and female hyenas did not differ with respect either to the proportion of time spent alone or in their positions in affiliation networks. Frequently in matriarchal mammalian societies, males are alone more often and have different, usually smaller, less connected affiliation network positions than do females (e.g., Evans and Harris 2008; Onyango et al. 2013); this pattern only appeared in adulthood among hyenas and was not observed among juveniles. Juvenile patterns tend to be understudied in primates and other mammals growing up in complex societies.
In contrast to those of female hyenas, male social position metrics in directed networks showed a consistent pattern of decreasing significantly across the course of ontogeny. As seen in both in-degree and out-degree centrality metrics in both types of directed networks, males were both initiating interactions with fewer clan-mates and receiving interactions from fewer clan-mates than were their female peers (Figs. 4 and 6). As they mature, females are more interactive and involved in more agonistic interactions than males, as both attackers and recipients of attacks. By contrast, females are relatively stable in their levels of affiliation compared to males, whose values start higher than females during the CD stage but then drop significantly by adulthood. Male ego networks were also frequently less dense than those of females, indicating that males were interacting with other individuals that interacted with one another less than did females. Anecdotally, in the field, we observe males nearing dispersal age tending to associate almost exclusively with immigrant males or other maturing males who are also about to disperse. This pattern has also been seen in primates (Onyango et al. 2012, 2013).
Interestingly, our data clearly demonstrate that male spotted hyenas are not forced out of their natal clan at dispersal (Kruuk 1972; Smale et al. 1997; Holekamp and Smale 1998b; Van Horn et al. 2003). Numbers of clan-mates directing aggressive acts toward males decreased greatly as they reached adulthood, which would not be expected if males were aggressively expelled from their natal groups (Figs. 4a and 5b). Instead, we saw that males generally spontaneously disengaged from interactions within the natal clan as they prepared to disperse.
Effects of rank on network position
We observed what appears to be the emergence during ontogeny of different social strategies based on rank among females but not among males (e.g., Figs. 2, 5, and 7), which supports hypotheses suggesting that both sex and dominance status affect the network positions of individual hyenas as they develop. Males mainly only showed rank-related variation in their affiliation networks, with high-ranking individuals initiating greetings with more individuals, and having higher affiliation network densities, by adulthood than did lower-ranking males (Table 3). These rank differences among young adult natal males may be related to the rank-related variation documented in the success enjoyed by males in their new clans after dispersal; sons of high-ranking females enjoy greater reproductive success in their new clans than do lower-ranking males (Höner et al. 2010).
Females showed a much more complicated pattern than males of rank-related variation across network types. Generally high- or low-ranking females each adopted a particular strategy, with the pattern observed among mid-ranking individuals resembling that of one of those other rank classes, depending on the metric being assessed. However, mid-ranking females were the only ones who had significantly more individuals aggressing upon them after the CD stage than did either high- or low-ranking females, whose in-degree values decreased over ontogeny (Fig. 5). Furthermore, mid-ranking females strongly tended to be alone more often than did either high- or low-ranking females as they matured (Fig. 2a). Mid-ranking hyenas must remember more individuals both above and below their own rank positions than either high-ranking females, who know that most others are ranked below them, or low-ranking females, who know that most clan-mates out-rank them. This middle position in the hierarchy may be why mid-ranking females are aggressed upon more than other ranks, as they may have a harder time learning their ranks; this might explain why they tend to be seen alone more often by adulthood. Uncertainty in the status of mid-ranking individuals can have adverse health effects, as seen in rhesus macaques (Vandeleest et al. 2016). Furthermore, primates have been shown to utilize different social strategies to compensate for rank-related disadvantages to gain better access to resources and improve reproductive success (Anderson and Mason 1978; Bercovitch 1991), which may be occurring here, although we would need to look at later adulthood to determine whether the tendency to be alone more often strengthens over time.
Our findings also highlight the fact that high-ranking females lead strikingly different social lives from those of either low- or mid-ranking females in this species, starting early in ontogeny. In the field, we often see that high-ranking females are accompanied by an entourage of “groupies” (Smith et al. 2007). Although we found no significant effects of female rank on position in association networks, which would be expected because lower-ranking “groupies” are associating with higher-ranking females (Smith et al. 2007), high-ranking females did appear to be more directly socially active, indicating that they were developing into social hubs in their networks. High-ranking females initiated more aggressive acts and received more affiliative acts than did females of other ranks (Fig. 7 and Table S4). Many other mammals, especially primates, also have societies in which high-ranking individuals function as social hubs (Pereira 1988; Smith et al. 2007; Silk et al. 2012). However, high-ranking female hyenas did not initiate greetings with more individuals than did mid- or low-ranking females, perhaps because it is equally important for females of all ranks to help maintain social cohesion within the clan (Smith et al. 2011).
Effects of sex on affiliation network metrics: Mean a in-degree vs. b out-degree in the affiliation networks of female and male spotted hyenas across developmental stages: communal den (CD), den independent (DI), and adulthood. Sample sizes and error bars are as in Fig. 4
Low-ranking female hyenas may cultivate fewer, but nonetheless important, direct ties with specific clan-mates rather than maintaining relationships with many different conspecifics, as high-ranking females appear to do. Here high- and low-ranking females spent similar amounts of time alone, and they had similar association patterns.Previous research has shown that high- and low-ranking hyenas have significantly different association patterns (Holekamp et al. 1997), but earlier workers used mean association indices in their analyses whereas we used the social network positions of degree centrality and density, which account for numbers of associates and connectedness within networks.Together, our findings indicate that, although their positons in the network have similar structures, low-ranking females have weaker associations overall than do high-ranking females (Holekamp et al. 1997). Furthermore, with the sole exception of aggressive acts directed toward them, in social network position metrics where we observed significant rank effects (aggressions directed by and at them; affiliations directed by and at them), low-ranking female hyenas exhibited lower values than did high-ranked individuals, on average (Table 2c and Figs. 5a and 7a). The fact that low-ranking females directly interacted with fewer individuals than the number with which they associated suggests that they may be actively choosing their preferred social partners while associating less strongly with, or actively avoiding, other group-mates.
Effects of social rank on affiliation network metrics: Mean in-degree in the affiliation networks of a females and b males by rank level across developmental stages: communal den (CD), den independent (DI), and adulthood. Sample sizes and error bars are as in Fig. 2
Conclusions
Our results suggest that spotted hyenas adopt social strategies during development that vary with their age, sex, and rank. Such strategies have previously been most reported in primates. For instance, although high rank is frequently hypothesized to enhance reproductive success, some have found that primates may use other social skills and strategies to circumvent their rank limitations and manage to achieve high reproductive success despite those limitations (reviewed in Bercovitch 1991).
The prolonged juvenile period in primates is known to be driven in part by the time needed to learn how to live in their complex societies (Joffe 1997; Walker et al. 2006). Our data suggest that, even though the prolonged juvenile stage in spotted hyenas is required largely by the time needed to develop a feeding morphology specialized for bone-cracking (Watts et al. 2009; Holekamp et al. 2013), it also provides spotted hyenas with opportunities to learn to live in their primate-like social groups. However, social development is a largely unexplored aspect of the behavior of non-primate mammals. There is a pressing need to explore social development in other taxa, and this paper represents a first step toward elucidating social development in a highly gregarious carnivore. The findings presented here raise many new questions. For example, we will be fascinated to see whether the different social positions and strategies indicated by our data have fitness consequences for individuals, as have been documented in adult primates (Silk 2003; Silk et al. 2010; Archie et al. 2014; Brent et al. 2017). It will also be critical to determine whether there are sensitive periods in social development that are crucial to becoming a functioning member of society and affect future longevity or reproductive success.
References
Altmann J (1974) Observational study of behavior: sampling methods. Behaviour 49(3):227–267. https://doi.org/10.1163/156853974X00534
Anderson CO, Mason WA (1978) Competitive social strategies in groups of deprived and experienced rhesus monkeys. Dev Psychobiol 11(4):289–299. https://doi.org/10.1002/dev.420110402
Aplin LM, Farine DR, Morand-Ferron J, Cole EF, Cockburn A, Sheldon BC (2013) Individual personalities predict social behaviour in wild networks of great tits (Parus major). Ecol Lett 16(11):1365–1372. https://doi.org/10.1111/ele.12181
Archie EA, Chiyo PI (2012) Elephant behaviour and conservation: social relationships, the effects of poaching, and genetic tools for management. Mol Ecol 21(3):765–778. https://doi.org/10.1111/j.1365-294X.2011.05237.x
Archie EA, Tung J, Clark M, Altmann J, Alberts SC (2014) Social affiliation matters: both same-sex and opposite-sex relationships predict survival in wild female baboons. Proc R Soc B 281(1793):20141261. https://doi.org/10.1098/rspb.2014.1261
Barabasi A, Albert R (1999) Emergence of scaling in random networks. Science 286(5439):509–512
Barocas A, Ilany A, Koren L, Kam M, Geffen E (2011) Variance in centrality within rock hyrax social networks predicts adult longevity. PLoS One 6(7):e22375. https://doi.org/10.1371/journal.pone.0022375
Belsky J, Steinberg L, Draper P (1991) Childhood experience, interpersonal development, and reproductive strategy: an evolutionary theory of socialization. Child Dev 62(4):647–670. https://doi.org/10.2307/1131166
Belsky J, Vandell DL, Burchinal M, Clarke-Stewart KA, McCartney K, Owen MT, The NICHD Early Child Care Research Network (2007) Are there long-term effects of early child care? Child Dev 78(2):681–701. https://doi.org/10.1111/j.1467-8624.2007.01021.x
Bercovitch FB (1991) Social stratification, social strategies, and reproductive success in primates. Ethol Sociobiol 12(4):315–333. https://doi.org/10.1016/0162-3095(91)90023-J
Berman CM (1982) The ontogeny of social relationships with group companions among free-ranging infant rhesus monkeys. II. Differentiation and attractiveness. Anim Behav 30(1):149–162. https://doi.org/10.1016/S0003-3472(82)80250-9
Boydston EE, Morelli TL, Holekamp KE (2001) Sex differences in territorial behavior exhibited by the spotted hyena (Hyaenidae, Crocuta crocuta). Ethology 107(5):369–385. https://doi.org/10.1046/j.1439-0310.2001.00672.x
Boydston EE, Kapheim KM, Van Horn RC, Smale L, Holekamp KE (2005) Sexually dimorphic patterns of space use throughout ontogeny in the spotted hyena (Crocuta crocuta). J Zool 267(03):271–281. https://doi.org/10.1017/S0952836905007478
Brent LJN, Ruiz-Lambides A, Platt ML (2017) Family network size and survival across the lifespan of female macaques. Proc R Soc B 284(1854):20170515. https://doi.org/10.1098/rspb.2017.0515
Butts CT (2008) Network: a package for managing relational data in R. J Stat Softw 24:1–36
Butts CT (2015) Network: classes for relational data. https://cran.r-project.org/web/packages/network/vignettes/networkVignette.pdf
Cairns SJ, Schwager SJ (1987) A comparison of association indices. Anim Behav 35(5):1454–1469. https://doi.org/10.1016/S0003-3472(87)80018-0
Campbell CJ, Fuentes A, MacKinnon K, Bearder SK, Stumpf RM (2011) Primates in perspective. Oxford University Press, New York
Chiyo PI, Moss CJ, Alberts SC (2012) The influence of life history milestones and association networks on crop-raiding behavior in male African elephants. PLoS One 7(2):e31382. https://doi.org/10.1371/journal.pone.0031382
Crockford C, Wittig RM, Whitten PL, Seyfarth RM, Cheney DL (2008) Social stressors and coping mechanisms in wild female baboons (Papio hamadryas ursinus). Horm Behav 53(1):254–265. https://doi.org/10.1016/j.yhbeh.2007.10.007
Croft DP, James R, Krause J (2008) Exploring animal social networks. Princeton University Press, Princeton. https://doi.org/10.1515/9781400837762
Croft DP, Krause J, Darden SK (2009) Behavioural trait assortment in a social network: patterns and implications. Behav Ecol Sociobiol 63(10):1495–1503. https://doi.org/10.1007/s00265-009-0802-x
East ML, Hofer H (2001) Male spotted hyenas (Crocuta crocuta) queue for status in social groups dominated by females. Behav Ecol 12(5):558–568. https://doi.org/10.1093/beheco/12.5.558
Ellis L (1995) Dominance and reproductive success among nonhuman animals: a cross-species comparison. Ethol Sociobiol 16(4):257–333. https://doi.org/10.1016/0162-3095(95)00050-U
Evans KE, Harris S (2008) Adolescence in male African elephants, Loxodonta africana, and the importance of sociality. Anim Behav 76(3):779–787. https://doi.org/10.1016/j.anbehav.2008.03.019
Fournier DA, Skaug HJ, Ancheta J, Ianelli J, Magnusson A, Maunder MN, Nielsen A, Sibert J (2012) AD Model Builder: using automatic differentiation for statistical inference of highly parameterized complex nonlinear models. Optim Method Softw 27(2):233–249. https://doi.org/10.1080/10556788.2011.597854
Frank LG (1986) Social organization of the spotted hyaena (Crocuta crocuta). I. Demography. Anim Behav 34(5):1500–1509. https://doi.org/10.1016/S0003-3472(86)80220-2
Frank LG, Glickman SE, Licht P (1991) Fatal sibling aggression, precocial development, and androgens in neonatal spotted hyenas. Science 252(5006):702–704. https://doi.org/10.1126/science.2024122
Granovetter MS (1973) The strength of weak ties. Am J Sociol 78(6):1360–1380. https://doi.org/10.1086/225469
Hamede RK, Bashford J, McCallum H, Jones M (2009) Contact networks in a wild Tasmanian devil (Sarcophilus harrisii) population: using social network analysis to reveal seasonal variability in social behaviour and its implications for transmission of devil facial tumour disease. Ecol Lett 12(11):1147–1157. https://doi.org/10.1111/j.1461-0248.2009.01370.x
Handcock MS, Hunter DR, Butts CT, Goodreau SM, Morris M (2008) Statnet: software tools for the representation, visualization, analysis and simulation of network data. J Stat Softw 24(1):1548–7660
Hanneman RA, Riddle M (2005) Introduction to social network methods. University of California, Riverside
Hinde RA (1976) Interactions, relationships and social structure. Man 11(1):1–17. https://doi.org/10.2307/2800384
Hinde RA, Atkinson S (1970) Assessing the roles of social partners in maintaining mutual proximity, as exemplified by mother-infant relations in rhesus monkeys. Anim Behav 18:169–176. https://doi.org/10.1016/0003-3472(70)90087-4
Hobson EA, DeDeo S (2015) Social feedback and the emergence of rank in animal society. PLoS Comput Biol 11(9):e1004411. https://doi.org/10.1371/journal.pcbi.1004411
Hobson EA, Avery ML, Wright TF (2013) An analytical framework for quantifying and testing patterns of temporal dynamics in social networks. Anim Behav 85(1):83–96. https://doi.org/10.1016/j.anbehav.2012.10.010
Hock K, Ng KL, Fefferman NH (2010) Systems approach to studying animal sociality: individual position versus group organization in dynamic social network models. PLoS One 5(12):e15789. https://doi.org/10.1371/journal.pone.0015789
Hofer H, East ML (2003) Behavioral processes and costs of co-existence in female spotted hyenas: a life history perspective. Evol Ecol 17(4):315–331. https://doi.org/10.1023/A:1027352517231
Holekamp KE, Smale L (1991) Dominance acquisition during mammalian social development: the “inheritance” of maternal rank. Am Zool 31(2):306–317. https://doi.org/10.1093/icb/31.2.306
Holekamp KE, Smale L (1993) Ontogeny of dominance in free-living spotted hyaenas: juvenile rank relations with other immature individuals. Anim Behav 46(3):451–466. https://doi.org/10.1006/anbe.1993.1214
Holekamp KE, Smale L (1998a) Behavioral development in the spotted hyena. Bioscience 48(12):997–1005. https://doi.org/10.2307/1313456
Holekamp KE, Smale L (1998b) Dispersal status influences hormones and behavior in the male spotted hyena. Horm Behav 33(3):205–216. https://doi.org/10.1006/hbeh.1998.1450
Holekamp KE, Smale L, Szykman M (1996) Rank and reproduction in the female spotted hyaena. J Reprod Fertil 108(2):229–237. https://doi.org/10.1530/jrf.0.1080229
Holekamp KE, Cooper SM, Katona CI, Berry NA, Frank LG, Smale L (1997) Patterns of association among female spotted hyenas (Crocuta crocuta). J Mammal 78(1):55–64. https://doi.org/10.2307/1382638
Holekamp KE, Smith JE, Strelioff CC, Van Horn RC, Watts HE (2012) Society, demography and genetic structure in the spotted hyena. Mol Ecol 21(3):613–632. https://doi.org/10.1111/j.1365-294X.2011.05240.x
Holekamp KE, Swanson EM, Van Meter PE (2013) Developmental constraints on behavioural flexibility. Philos T Roy Soc B 368(1618):20120350. https://doi.org/10.1098/rstb.2012.0350
Holekamp KE, Dantzer B, Stricker G, Yoshida KCS, Benson-Amram S (2015) Brains, brawn and sociality: a hyaena’s tale. Anim Behav 103:237–248. https://doi.org/10.1016/j.anbehav.2015.01.023
Höner OP, Wachter B, Hofer H, Wilhelm K, Thierer D, Trillmich F, Burke T, East ML (2010) The fitness of dispersing spotted hyaena sons is influenced by maternal social status. Nat Commun 1:60–67
Joffe TH (1997) Social pressures have selected for an extended juvenile period in primates. J Hum Evol 32(6):593–605. https://doi.org/10.1006/jhev.1997.0140
Kruuk H (1972) The spotted hyena: a study of predation and social behavior. University of Chicago Press, Chicago
Kurvers RHJM, Adamczyk VMAP, van Wieren SE, Prins HHT (2011) The effect of boldness on decision-making in barnacle geese is group-size-dependent. Proc R Soc Lond B 278(1714):2018–2024. https://doi.org/10.1098/rspb.2010.2266
Lehmann J, Ross C (2011) Baboon (Papio anubis) social complexity—a network approach. Am J Primatol 73(8):775–789. https://doi.org/10.1002/ajp.20967
Makagon MM, McCowan B, Mench JA (2012) How can social network analysis contribute to social behavior research in applied ethology? Appl Anim Behav Sci 138(3-4):152–161. https://doi.org/10.1016/j.applanim.2012.02.003
Mason WA (1968) Early social deprivation in nonhuman primates: implications for human behavior. In: Glass DS (ed) Environmental influences. Rockefeller University Press, New York, pp 70–101
Massen JJM, Koski SE (2014) Chimps of a feather sit together: chimpanzee friendships are based on homophily in personality. Evol Hum Behav 35(1):1–8. https://doi.org/10.1016/j.evolhumbehav.2013.08.008
McCowan B, Beisner BA, Capitanio JP, Jackson ME, Cameron AN, Seil S, Atwill ER, Hsieh F (2011) Network stability is a balancing act of personality, power, and conflict dynamics in rhesus macaque societies. PLoS One 6(8):e22350. https://doi.org/10.1371/journal.pone.0022350
McDonald DB (2007) Predicting fate from early connectivity in a social network. Proc Natl Acad Sci USA 104(26):10910–10914. https://doi.org/10.1073/pnas.0701159104
Moffitt TE, Caspi A, Belsky J, Silva PA (1992) Childhood experience and the onset of menarche: a test of a sociobiological model. Child Dev 63(1):47–58. https://doi.org/10.2307/1130900
Newman MEJ (2003) The structure and function of complex networks. SIAM Rev 45(2):167–256. https://doi.org/10.1137/S003614450342480
Onyango PO, Gesquiere LR, Altmann J, Alberts SC (2012) Testosterone positively associated with both male mating effort and paternal behavior in savanna baboons (Papio cynocephalus). Horm Behav 63:430–436
Onyango PO, Gesquiere LR, Altmann J, Alberts SC (2013) Puberty and dispersal in a wild primate population. Horm Behav 64(2):240–249. https://doi.org/10.1016/j.yhbeh.2013.02.014
Pereira ME (1988) Effects of age and sex on intra-group spacing behaviour in juvenile savannah baboons, Papio cynocephalus cynocephalus. Anim Behav 36(1):184–204. https://doi.org/10.1016/S0003-3472(88)80262-8
Pinter-Wollman N, Hobson EA, Smith JE, Edelman AJ, Shizuka D, de Silva S, Waters JS, Prager SD, Sasaki T, Wittemyer G, Fewell J, McDonald DB (2014) The dynamics of animal social networks: analytical, conceptual, and theoretical advances. Behav Ecol 25(2):242–255. https://doi.org/10.1093/beheco/art047
Royle NJ, Pike TW, Heeb P, Richner H, Kölliker M (2012) Offspring social network structure predicts fitness in families. Proc R Soc Lond B 279(1749):4914–4922. https://doi.org/10.1098/rspb.2012.1701
Silk JB (2003) Social bonds of female baboons enhance infant survival. Science 302(5648):1231–1234. https://doi.org/10.1126/science.1088580
Silk JB, Samuels A, Rodman PS (1981) The influence of kinship, rank, and sex on affiliation and aggression between adult female and immature bonnet macaques (Macaca radiata). Behaviour 78(1):111–137. https://doi.org/10.1163/156853981X00284
Silk JB, Beehner JC, Bergman TJ, Crockford C, Engh AL, Moscovice LR, Wittig RM, Seyfarth RM, Cheney DL (2010) Strong and consistent social bonds enhance the longevity of female baboons. Curr Biol 20(15):1359–1361. https://doi.org/10.1016/j.cub.2010.05.067
Silk JB, Alberts SC, Altmann J, Cheney DL, Seyfarth RM (2012) Stability of partner choice among female baboons. Anim Behav 83(6):1511–1518. https://doi.org/10.1016/j.anbehav.2012.03.028
Skaug H, Fournier D, Bolker BM, Nielsen A, Magnusson A (2016) Generalized linear mixed models using AD model builder. https://rdrr.io/rforge/glmmADMB/
Smale L, Nunes S, Holekamp KE (1997) Sexually dimorphic dispersal in mammals: patterns, causes, and consequences. Adv Stud Behav 26:181–250. https://doi.org/10.1016/S0065-3454(08)60380-0
Smith JE, Memenis SK, Holekamp KE (2007) Rank-related partner choice in the fission–fusion society of the spotted hyena (Crocuta crocuta). Behav Ecol Sociobiol 61(5):753–765. https://doi.org/10.1007/s00265-006-0305-y
Smith JE, Kolowski JM, Graham KE, Dawes SE, Holekamp KE (2008) Social and ecological determinants of fission–fusion dynamics in the spotted hyaena. Anim Behav 76(3):619–636. https://doi.org/10.1016/j.anbehav.2008.05.001
Smith JE, Powning KS, Dawes SE, Estrada JR, Hopper AL, Piotrowski SL, Holekamp KE (2011) Greetings promote cooperation and reinforce social bonds among spotted hyaenas. Anim Behav 81(2):401–415. https://doi.org/10.1016/j.anbehav.2010.11.007
Stanton MA, Mann J (2012) Early social networks predict survival in wild bottlenose dolphins. PLoS One 7(10):e47508. https://doi.org/10.1371/journal.pone.0047508
Stanton MA, Gibson QA, Mann J (2011) When mum’s away: a study of mother and calf ego networks during separations in wild bottlenose dolphins (Tursiops sp.) Anim Behav 82(2):405–412. https://doi.org/10.1016/j.anbehav.2011.05.026
Suomi SJ (2005) Mother-infant attachment, peer relationships, and the development of social networks in rhesus monkeys. Hum Dev 48(1-2):67–79. https://doi.org/10.1159/000083216
Swanson EM, Dworkin I, Holekamp KE (2011) Lifetime selection on a hypoallometric size trait in the spotted hyena. Proc R Soc Lond B 278(1722):3277–3285. https://doi.org/10.1098/rspb.2010.2512
Swanson EM, McElhinny TL, Dworkin I, Weldele ML, Glickman SE, Holekamp KE (2013) Ontogeny of sexual size dimorphism in the spotted hyena (Crocuta crocuta). J Mammal 94(6):1298–1310. https://doi.org/10.1644/12-MAMM-A-277.1
Szykman M, Engh AL, Van Horn RC, Funk S, Scribner K, Holekamp KE (2001) Association patterns among male and female spotted hyenas (Crocuta crocuta) reflect male mate choice. Behav Ecol Sociobiol 50(3):231–238. https://doi.org/10.1007/s002650100356
Tanner JB, Zelditch ML, Lundrigan BL, Holekamp KE (2009) Ontogenetic change in skull morphology and mechanical advantage in the spotted hyena (Crocuta crocuta). J Morphol 271:353–365
Van Horn RC, McElhinny TL, Holekamp KE (2003) Age estimation and dispersal in the spotted hyena (Crocuta crocuta). J Mammal 84(3):1019–1030. https://doi.org/10.1644/BBa-023
Vandeleest JJ, Beisner BA, Hannibal DL, Nathman AC, Capitanio JP, Hsieh F, Atwill ER, McCowan B (2016) Decoupling social status and status certainty effects on health in macaques: a network approach. PeerJ 4:e2394. https://doi.org/10.7717/peerj.2394
Walker R, Burger O, Wagner J, Von Rueden CR (2006) Evolution of brain size and juvenile periods in primates. J Hum Evol 51(5):480–489. https://doi.org/10.1016/j.jhevol.2006.06.002
Watts HE, Tanner JB, Lundrigan BL, Holekamp KE (2009) Post-weaning maternal effects and the evolution of female dominance in the spotted hyena. Proc R Soc Lond B 276(1665):2291–2298. https://doi.org/10.1098/rspb.2009.0268
Wilson A, Krause S, Dingemanse NJ, Krause J (2013) Network position: a key component in the characterization of social personality types. Behav Ecol Sociobiol 67(1):163–173. https://doi.org/10.1007/s00265-012-1428-y
Acknowledgments
We thank the Kenyan National Commission for Science, Technology, and Innovation for permission to conduct this research and the Kenya Wildlife Service, Narok County Government and the Senior Warden of the Masai Mara National Reserve for assistance. We are indebted to all those who have contributed to long-term data collection on the Mara Hyena Project. Special thanks to T. Getty, L. Smale, J. Neal, and A. Wolfe for helpful comments along the way. We would also like to thank three very thoughtful anonymous reviewers for their comments. This research was supported by National Science Foundation Grants DEB1353110 and OISE1556407 to KEH, a Student Research Grant from the Animal Behavior Society to JWT, a Grant-in-Aid from the American Society of Mammalogists to JWT, and a Dissertation Continuation Fellowship to JWT from the College of Natural Science at Michigan State University.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving animals were in accordance with the ethical standards of Michigan State University and following all applicable guidelines in Kenya. Ethical approval for use of animals in this study was issued by Michigan State University under IACUC approval no. 05/11-110-00 on 22 August 2013.
Additional information
Communicated by D. P. Croft
Electronic supplementary material
ESM 1
(DOCX 617 kb)
Rights and permissions
About this article
Cite this article
Turner, J.W., Bills, P.S. & Holekamp, K.E. Ontogenetic change in determinants of social network position in the spotted hyena. Behav Ecol Sociobiol 72, 10 (2018). https://doi.org/10.1007/s00265-017-2426-x
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00265-017-2426-x