Abstract
Social relationships are composed of both positive (affiliative) and negative (agonistic) interactions, representing opposing effects. Social network theory predicts that positive relationships should be transitive; thus, the friend of a friend is more likely to be a friend. Further, when considering both positive and negative relationships jointly, structural balance theory predicts that certain configurations of positive and negative relationships in a triad are inherently less stable (unbalanced) and should tend to be eliminated. However, structural balance has been rarely examined in nonhuman social systems. We tested for transitivity and structural balance in social networks of socially flexible yellow-bellied marmots (Marmota flaviventer) and asked if group size, network density, or group composition affected the degree of structural balance. We found a consistent pattern of significant transitivity in positive interactions, some transitivity in negative interactions, and some evidence of structural balance. In particular, a “weak” definition of structural balance is probably more common than “strong” structural balance, which used a stricter definition of balance. Network size limited the ability to detect these social processes, and smaller networks were less likely to show significant transitivity or structural balance. The proportion of adult females in a group affected the level of transitivity but did not affect the degree of structural balance. Our study suggests that there are intriguing similarities in social processes across diverse animal societies and that studying triads and network motifs may help identify basic social mechanisms linking local to global structure.
Significance statement
Social network theory predicts that basic social mechanisms should lead to similar structural properties across different societies. For example, positive relationships should be transitive (a friend of a friend is a friend), and certain combinations of positive and negative relationships represent conflict and should be unstable over time (e.g., a friend of a friend being an enemy is an unstable state). This latter theory, called structural balance, has rarely been examined in nonhuman societies; hence, we tested for transitivity and structural balance in groups of free-living yellow-bellied marmots. Positive interactions were generally transitive, but evidence for structural balance was inconsistent. Furthermore, group composition could affect network transitivity, and small network size (associated with few interactions) limits ability to detect significant patterns. Our results suggest that transitivity is fundamental in structuring positive relationships, while some forms of structural balance are present but not widespread.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Understanding the form and function of social relationships is of fundamental interest in behavioral ecology (Whitehead 2008). In cooperative societies, developing and maintaining social relationships can be a means to increase fitness in both sexes. Female-female bonds in primates (Silk 2007) and ungulates (Cameron et al. 2009) can be important for increasing lifetime reproductive fitness. In other cases, female social bonds form the core of matrilineal societies, which are critical for maintaining social knowledge and group success (McComb et al. 2001; Brent et al. 2015). Some male primates (Harcourt 1992) and cetaceans (Connor et al. 2001) form alliances to obtain and guard mates, and male primates (Schülke et al. 2010) and manakins (McDonald 2007; Edelman and McDonald 2014) build social capital to access future reproductive opportunities. Another important role of social relationships can be to establish social roles and reduce costly social conflict. Dominance hierarchies are common in many animal societies (Dewsbury 1982; Drews 1993; Ellis 1995; Shizuka and McDonald 2015), and the establishment of stable, predictable rank relationships is thought to reduce escalation of aggression, which can be energetically costly and more likely to result in injury (Drews 1993). Active conflict resolution is also important in primate societies (de Waal 2000), with some individuals even playing policing roles to maintain social stability (Flack et al. 2006). Even in less structured societies, there is evidence that social instability might negatively impact fitness components (Barocas et al. 2011; Wey et al. 2013).
Recent interest in animal social networks, where the patterning of social connections among individuals is represented and analyzed as a network of nodes (vertices) and ties (edges, links), has emphasized the utility of studying both global and local structural properties (Wey et al. 2008; Farine and Whitehead 2015; Krause et al. 2015; Croft et al. 2016). Many studies have examined how affiliative networks are shaped (Croft et al. 2006, 2009; Wey and Blumstein 2010; Brent et al. 2011; Ilany et al. 2015) and their impacts on individual outcomes, such as reproductive success (McDonald 2007; Wey and Blumstein 2012; Vander Wal et al. 2015) and survival (Stanton and Mann 2012; Blumstein et al. 2018), in natural populations. Other studies have considered how conflict and conflict resolution shape social networks (Flack et al. 2006), particularly in dominance interactions (Dey et al. 2013; Shizuka and McDonald 2015). The structure and function of network motifs, i.e., repeated subgraphs or small-scale patterns, have long been of interest in the study of social processes because these local interactions bridge individual behaviors and emergent network patterns (Wasserman and Faust 1994; Milo et al. 2002). Sets of three nodes (triads or triangles) have been of particular interest as they represent a key transition from dyadic interactions, involving only two individuals, to more complex patterning of interactions among multiple individuals (Faust 2010). Triads have more possible configurations than dyads (which largely vary between having a connection or not), while still being simple enough to infer general local rules affecting larger-scale structure. The structure of interactions among triads has thus played an important role in a number of important sociological theories (Heider 1946; Cartwright and Harary 1956; Granovetter 1973; Holland and Leinhardt 1976). Among the most prominent is the concept of transitivity, which in the context of positive relationships can be expressed as “the friend of my friend is my friend”. Thus, if A is friends with B and C, B and C should also have (or be more likely to develop) a friendly relationship, resulting in a fully connected triangle (Fig. 1, upper left). Tendency towards this kind of transitivity in positive relationships is commonly seen in social networks across species and contexts (Newman and Park 2003; Faust 2010; Ilany et al. 2013, 2015; Edelman and McDonald 2014).
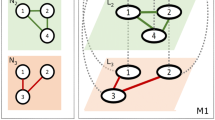
Possible configurations of positive and negative relationships in undirected triads. Under the original “strong” structural balance definition, the configurations on the left are considered balanced and the configurations on the right unbalanced, while under the “weak” structural balance definition, the - - - (bottom right) triad is also considered balanced. Unbalanced triads are expected to be unstable due to conflicts for the actors inherent to the configurations
An early triad-based hypothesis focused on the idea of “structural balance” in configurations of positive (+) and negative (-) ties among individuals or entities (Heider 1946; Cartwright and Harary 1956; Hummon and Doreian 2003; Zheng et al. 2015) (Fig. 1). This theory is based on the principle that, in social networks where both + and - relationships exist, certain configurations of triads are inherently more stable or balanced, whereas others are inherently unstable or unbalanced. In an undirected network, the four possible configurations are as follows: +++, ++-, +--, and ---. The +++ and +-- triads represent situations in which all three nodes are friendly or two nodes are friends with each other and both enemies of the third, respectively and are considered stable (Fig. 1, left side). On the other hand, a ++- triad would represent a situation in which one node is friends with the other two, who are enemies of each other, resulting in social tension (Fig. 1, right side). A --- triad also represents conflict and is considered unbalanced under the original theory of “strong” structural balance because a positive relationship is expected to eventually form between one dyad (turning the situation into an “alliance” between two individuals against the third). A relaxed definition of “weak” structural balance (Davis 1967) only considers the ++- configuration (Fig. 1, top right) unbalanced, i.e., the --- triad (Fig. 1, bottom right) is considered balanced under this definition. This definition is likely more relevant to situations where there are more than two subgroups in a network and --- triads might be common without representing strong social dissonance. Broadly, social networks, where nodes are individuals or entities, are generally expected to show many more balanced than unbalanced triads.
Despite this long-standing interest in structural balance theory, there have been relatively few explicit tests of its predictions for the structuring of positive and negative interactions. Some human networks display structural balance, while others do not (Doreian and Mrvar 1996; Hummon and Doreian 2003; Jordán 2009; Leskovec et al. 2010; Facchetti et al. 2011; Zheng et al. 2015) and, to our knowledge, this specific hypothesis has only been empirically tested in a single nonhuman species. Ilany et al. (2013) found evidence for structural balance in groups of wild rock hyraxes (Procavia capensis) and that immigrant animals create social instability, demonstrating the emergence of seemingly complex structural balance without advanced cognition. More generally, various studies have demonstrated how social complexity might emerge from simple local processes in diverse systems, including repeated basic network motifs (Milo et al. 2002; Ohtsuki et al. 2006), individual differences in behavioral rules affecting position in networks (Krause et al. 2010; Firth et al. 2017), or partner preferences affecting cooperation (Croft et al. 2015) or mating behavior (McDonald and Pizzari 2016). Thus, it seems likely that further tests for structural balance in diverse species will provide important insight into how social processes, including those involving more than one type of social interaction, generally emerge in real systems.
In this study, we used long-term data from a free-living population of yellow-bellied marmots (Marmota flaviventer) (previously M. flaviventris) to test several hypotheses about the structuring of animal social networks. We used social interactions observed over the course of several months as a proxy for social relationships. First, we hypothesized that social affiliation should be transitive (friends of friends are likely to be friends). In contrast, we did not expect to see this pattern in agonistic interactions. (Note that network “transitivity” in this study differs from the form discussed elsewhere (Shizuka and McDonald 2015).) Second, we hypothesized that networks of positive and negative interactions should tend towards structural balance; that is, there should be a high number of balanced triads. Third, given that adult females of this species settle permanently in one colony while adult males are more likely to move among colonies and juveniles often disperse, we hypothesized that maintaining social stability should be more important for adult females than for other demographic groups. Thus, we predicted that networks with a higher proportion of adult females would be more likely to show transitivity and structural balance.
Methods
Study system and data collection
Yellow-bellied marmots are large burrowing North American sciurid rodents, and a wild population under long-term study at the Rocky Mountain Biological Laboratory (RMBL), CO, provides a model system for many research questions (Blumstein 2013; Armitage 2014). Adult females are traditionally viewed as the social glue holding colonies together, as they settle in colonies permanently, often forming matrilineal groups and using a reproductive strategy of “recruiting” daughters and female relatives to settle in the same colony (Armitage 2014). Hence, colonies are often composed of one or more groups of related adult females. However, adult female marmots also compete with other females, including kin, for resources and reproductive success (Armitage 2014). Social interactions appear to reflect these competing interests. Adult females tend to direct affiliation towards close kin (Armitage 2014), but agonistic interactions are common among relatives and overlap with affiliation (Wey and Blumstein 2010). Moreover, adult females do not appear to gain direct reproductive benefits from stronger social relationships (Wey and Blumstein 2012).
Long-term trapping and observational methods for the RMBL study are the same as described elsewhere (Wey and Blumstein 2010, 2012; Blumstein 2013). Briefly, marmots are observed and live-trapped repeatedly and regularly from spring emergence (typically mid-April) until late summer (mid-September) at several permanent colonies. Data used in the current study come from 5 colonies (River, Bench, Town, Marmot Meadow, Picnic) that received similar levels of sampling in each year (average > 100 h per colony per year). We attempt to observe each colony daily (in the morning, 07:00–10:00 h, and/or the afternoon, 16:00–19:00 h) and trap colonies every 2 weeks between mid-May and mid-September, weather permitting. Animals receive uniquely numbered ear tags the first time they are trapped for permanent identification and a distinctive dye mark as needed on their dorsal pelage so that they can be identified during observations. Age, sex, reproductive status, mass, and other morphological indices are recorded every time animals are trapped. Behavioral observations are conducted at a distance through spotting scopes so as not to disturb the animals. It was not possible to record data blind as identifying individuals was key to observing animals in the field.
From 2003 on, observers conducted detailed continuous scan sampling at each colony and recorded all occurrences of social interaction. For each interaction, the type and identities of the initiator and recipient were recorded, where possible. Interaction types were defined from an ethogram previously developed for this system (Blumstein et al. 2009). Affiliative interactions—which we considered positive interactions—included greeting, grooming, sitting in close proximity, foraging together, and playful interactions. Agonistic interactions—which we considered as negative interactions—included aggression and displacements (where one individual moves away in response to the approach of another individual). Interaction rates are typically low in this species, and most interactions and participants could be clearly identified. We excluded any interactions whose nature (affiliative or agonistic) or participants were not clear from analysis. Only individuals that were observed or trapped at a colony more than 5 times in a year were included in analysis to avoid including transient animals as group members.
Network analysis and assignment of positive and negative interactions
We used behavioral observations from 2003 to 2011 to construct annual social networks for each colony. We chose this level of analysis rather than a higher population level because colonies are spatially distinct and all individuals within the colony are able to interact (although, of course, some choose not to). These marmots do not typically move outside their colony unless dispersing (either as yearlings or as breeding adult males); hence, there are no persistent interactions among colonies. Marmots rely on their burrows for protection and generally stay physically close to colonies, emerging and moving about temporarily during the day mainly to feed, so colony members are consistently within or directly around the colony site. We constructed affiliative (positive only) networks where binary undirected connections between every pair of individuals was assigned as being present if that pair was observed in any affiliative interaction that year, and absent otherwise. We created agonistic (negative only) networks in the same way with agonistic interactions. The concepts of transitivity and structural balance were developed and are better studies in the context of considering binary undirected ties, and we consider the definition of a signed binary tie between two individuals as inferring the quality of a social relationship from multiple interactions. Previous analysis in this system suggests that unweighted and weighted measures of centrality are highly correlated (Wey and Blumstein 2010, 2012).
For each network, we then calculated the transitivity (i.e., clustering coefficient), which measures the proportion of completely connected triads out of the total possible. Rather than directly analyzing transitivity scores, whose likely values will be affected by network size and density, we used network permutations to assess the significance of this structural measure for each network and looked for overall patterns of significantly transitive structure across networks. We created 1000 permuted versions of each observed network by rewiring the ties while maintaining the degree sequence, where the number of iterations in each rewiring was 10 × the number of nodes (to allow for more iterations in larger networks, which also had more ties). In permuting networks, we did not have a reliable way to track more detailed spatial overlap of individuals, so could not account for this factor that likely influences social patterns at a smaller scale. However, the size of marmot colonies is well within distances animals can move to forage, so all colony members have the potential to interact. We then recalculated transitivity for each of the permuted networks and calculated the proportion of permutations that resulted in a value as high or higher than the level of transitivity in the original network. Reported P values are these proportions and are 1-tailed as we were specifically interested in testing the hypothesis of higher transitivity in social networks.
For analysis of structural balance, we assigned each pair of individuals a + or - interaction. If only + interactions occurred within the pair, they were assigned a + interaction. Likewise, if only - interactions occurred within a pair, they were assigned a - interaction. For pairs that had both + and - interactions, we compared results from two different assignment rules. In the first method, pairs that had both + and - interactions were assigned a - relationship, which represents this type of relationship as tending towards conflict. This conservatively interprets conflict in the interaction and could be biologically relevant for social relationships that are not yet resolved or are in flux. In the second method, we omitted any ties between pairs that had both + and - interactions. This would remove interactions for which there was uncertainty about their nature. In the interest of space, we only present results from the first method in the main text but provide network and triad summaries for both in supplemental material and address potential differences in interpretation in the “Discussion” section.
For each network, we then counted the number of balanced and unbalanced triads, as defined first by “strong” and then by “weak” structural balance. We again created 1000 permutations of each network by rewiring the observed ties 10 × the number of nodes, recalculated the number of balanced and unbalanced triads in each network permutation, and calculated the proportion of balanced triads (again for both “strong” and “weak” definitions) out of the total triads. We assessed significance of structural balance two ways: first, as the probability of getting a number of balanced triads as large as or larger than the number in the observed network, and second, as the probability of getting a proportion of balanced triads out of total triads as large as or larger than the level in the observed network. The second method is more restrictive and will be more difficult to measure in networks with fewer triads. Reported P values again come from the probabilities of the observed statistic compared with permutations and are 1-tailed because the hypothesis was specifically that social networks should tend towards higher transitivity and structural balance. Because we used 1-tailed P values, we considered P < 0.025 as significant. We did not consider the opposite pattern (networks showing lower transitivity and structural balance than expected) here but consider the implications of this possibility in the Discussion.
We examined the potential effect of group size and composition on transitivity in affiliative and agonistic networks separately using linear mixed models (LMMs). We modeled network transitivity as a function of the fixed effects of number of nodes (centered to 0 and scaled to 1 standard deviation), network density, and proportion of adult females. We also modeled these same fixed effects on structural balance in a generalized linear model (GLMM) with binomial error distribution, where the dependent variable is specified as a 2-column variable with the number of balanced and unbalanced triads in the network. All models included random intercepts for year and colony, and we tested for significance of random effects with log-likelihood ratio tests with 1 degree of freedom.
We conducted analyses in R version 3.1.2 (R Development Core Team 2018), network-specific analyses with the package “igraph” (Csardi and Nepusz 2006), and LMMs with “lme4” (Bates et al. 2015). Code for calculating structural balance is provided in Supplementary Material.
Results
In total, we included 43 networks (colony-years) in triad analysis (Table 1). We excluded the 2009 River colony from this study due to an extremely low population and low rate of interactions in the colony that precluded triad analysis. We also excluded a few other networks from analysis of agonistic structure as there were too few interactions for triad analysis (see Supplementary Material, Table S1).
Most marmot social networks composed of positive interactions showed significant transitivity, while half or fewer of networks of negative interactions showed significant transitivity (Fig. 2, Table S1). In affiliative networks, only relatively small networks ever failed to show significant transitivity. Across all affiliative networks, 28 out of 43 (63.6%) showed significant transitivity, and if networks with 10 or fewer nodes are excluded (the lowest quartile of network size), 27 out of 32 (84.4%) showed significant transitivity. The extreme differentiation in P values in Fig. 2 (generally either highly significant or not at all) likely reflects the lack of variation in smaller networks with few interactions, i.e., there are fewer ways to permute links and differentiate between permuted and observed networks, even if there is a high level of transitivity. In contrast, bigger networks with more affiliative interactions always had very significant levels of transitivity. Agonistic networks showed a less consistent pattern of transitivity than affiliative networks (Fig. 2, Table S1). Across all agonistic networks, 13 out of 38 (34.2%) showed significant transitivity, and 13 out of 32 (40.6%) with more than 10 nodes had significant transitivity. In contrast with affiliative networks, the significance of transitivity in agonistic networks seemed to be less strongly tied to network size.
Transitivity and probabilities of obtaining transitivity scores plotted against number of nodes in the network, for affiliative and agonistic networks. Probability was calculated as the number of values from permuted networks that were as large as or larger than in the observed network. Affiliative networks were more likely to show significant transitivity overall, and smaller affiliative networks were less likely to show significant transitivity. The dashed vertical line indicates network size of 10, and the solid horizontal line indicates P = 0.025
The level of transitivity in affiliative networks (model intercept = 0.493, P < 0.001) was not affected by the number of nodes (β = 0.061, P = 0.123). However, denser affiliative networks tended to have higher transitivity (β = 0.750, P = 0.002), and networks with a greater proportion of adult females tended to be less transitive (β = − 0.391, P = 0.033). In contrast, the level of transitivity in agonistic networks (model intercept = 0.516, P = 0.001) was not affected by the number of nodes (β = − 0.052, P = 0.225), network density (β = − 0.049, P = 0.878), or proportion of adult females (β = − 0.402, P = 0.080), although there was a tendency for networks with greater proportion of adult females to be less transitive. Random effects of year and colony were never significant (all P > 0.499).
We found some evidence for structural balance, but the level differed between “strong” and “weak” structural balance and also depended on the statistic used. When we looked at the number of strongly balanced triads, 26 out of 43 networks (60.5%) had more balanced triads than comparable permuted networks, but when we looked at the proportion of strongly balanced triads out of all triads, only 2 out of 43 (4.6%) showed a significantly higher proportion than comparable permuted networks (Fig. 3, Table S1). Excluding the smallest networks (10 or fewer nodes) leads to 19 out of 32 (59.4%) and 2 out of 32 (6.3%) significant networks according to the first or second method, respectively. When we looked at the number of weakly balanced triads, 28 out of 43 networks (65.1%) had more balanced triads than comparable permuted networks, and when we looked at the proportion of weakly balanced triads out of all triads, 9 out of 43 (20.9%) showed a significantly higher proportion than comparable permuted networks (Fig. 4, Table S1). Excluding the smallest networks (10 or fewer nodes) leads to 26 out of 32 (81.3%) and 8 out of 32 (25.0%) significant networks according to the first or second method, respectively.
Number and proportion of balanced triads according to “strong” structural balance, and probabilities of obtaining these scores, plotted against number of nodes in the network. Probability was calculated as the number of values from permuted networks that were as large as or larger than in the observed network. The dashed vertical line indicates network size of 10, and the solid horizontal line indicates P = 0.025
Number and proportion of balanced triads according to “weak” structural balance, and probabilities of obtaining these scores, plotted against number of nodes in the network. Probability was calculated as the number of values from permuted networks that were as large as or larger than in the observed network. The dashed vertical line indicates network size of 10, and the solid horizontal line indicates P = 0.025
The proportion of strongly balanced triads in a network (model intercept = 0.327, OR (odds ratio) = 1.386, P = 0.411) was not affected by network size (β = − 0.113, OR = 0.893, P = 0.276), network density (β = − 0.391, OR = 0.676, P = 0.552), or the proportion of adult females (β = − 0.212, OR = 0.809, P = 0.686) in the network. Both colonies (χ2 = 20.552, P < 0.001) and years (χ2 = 5.901, P = 0.015) differed in this proportion. The proportion of weakly balanced triads in a network (model intercept = 0.968, OR = 2.634, P = 0.038) was not affected by network size (β = − 0.156, OR = 0.855, P = 0.154), network density (β = 0.649, OR = 1.913, P = 0.472), or the proportion of adult females (β = 0.038, OR = 1.039, P = 0.947) in the network. Colonies did not differ in this proportion (χ2 = 1.479, P = 0.224), but years did (χ2 = 23.121, P < 0.001).
Discussion
We provide one of the first tests of strong and weak structural balance in nonhuman social networks, which suggests that structural balance occurs to some degree but is not widespread, in groups of free-living marmots. Our results provide stronger evidence that significant transitivity is widespread in the structuring of positive interactions and that it is less prevalent in negative interactions. However, the ability to detect a significant pattern was limited in small networks, and network density increased affiliative transitivity. Contrary to our predictions, adult females were not drivers of social balance; a higher proportion of adult females did not increase levels transitivity or structural balance but instead appeared to decrease transitivity in affiliative networks. Overall, our study provides exciting insight into social processes occurring in nonhuman animals and highlights both similarities and diversity of social structure across very different systems.
Our results add to previous findings that transitivity in networks of positive interactions is common across species and contexts (Faust 2010), including among others in humans (Newman and Park 2003), bottlenose dolphins (Tursiops spp.) (Lusseau 2003), rock hyrax (Ilany et al. 2013, 2015), and long-tailed manakins (Chiroxiphia linearis) (Edelman and McDonald 2014). Interestingly, in contrast to other studied systems, yellow-bellied marmots are not highly cooperative, and the transitive structure might be expected to arise from simple mechanisms (Faust 2010). Marmot colonies frequently consist of multiple smaller subgroups of individuals that overlap more in space and interact more frequently (Blumstein 2013), and these subgroups could facilitate complete triads among neighbors. This spatial structure might explain the presence of transitivity in some agonistic networks, which was not predicted, but the higher level of significant transitivity in affiliative networks suggests that additional mechanisms are driving transitivity in positive interactions. Affiliative networks with a higher proportion of adult females had lower transitivity, suggesting that costs of competition might shape marmot sociality more strongly than benefits of cooperation in this system (Blumstein 2013). We had predicted that adult females might have the most motivation to maintain social stability, resulting in higher clustering and/or structural balance. Other work has also shown that, not only do individual females not appear to gain direct benefits from affiliative interactions, but there might actually be costs associated with more affiliation, although the causal mechanism is unclear (Wey et al. 2013; Blumstein et al. 2018). We also note that the negative effect in this study could be partly driven by the proportion of yearlings in a network being strongly inversely correlated with the proportion of adult females. Because of this strong negative correlation (r = − 0.87), we did not include the proportion of yearlings in the same models, but yearlings are highly affiliative and drive social connectedness (Wey and Blumstein 2010), so they are more likely to create complete triads.
Network size and density also play roles in transitivity of affiliative networks. Denser networks, with more links for a given network size, had higher levels of transitivity. On some level, this could be a byproduct of complete triads being more likely when there are more links. However, the lack of the equivalent effect of density in agonistic networks implies that there are additional social processes at work. While network size (number of nodes) did not have a direct effect on the level of transitivity in affiliative or agonistic networks, network size did seem to limit our ability to detect significant transitivity. The only affiliative networks that did not show significant transitivity were small (12 nodes or less). We note that small networks have fewer potential configurations, and thus the permutation method is unlikely to detect structure that is significantly different from permutations, even if the level of transitivity is high. Additionally, high densities in small networks will make them nearly completely connected, essentially saturating them and making it impossible to detect significant differences from permuted networks. Network size could also potentially affect the structure measured through observer ability to track activity in different colonies. The largest and most socially active networks will have the most potential for individual interactions to be missed. However, there is typically only a low level of social activity in marmot colonies, with the exception of bouts of play by yearlings, and we do not expect this to be a systematic issue with interactions observed over a season and usually with multiple interactions observed between pairs.
While at least some marmot social networks showed significant structural balance, the significance of the pattern depended on the measurement of structural balance used. Although many networks had more balanced triads (both strong and weak) than permuted networks, almost no networks (5%) showed strong structural balance when comparing proportion of balanced triads in method 1 (ambiguous +/- pairs assigned -). This percentage was higher if we considered weak structural balance (21% of networks) or if we omitted ambiguous +/- pairs (23% of networks). This percentage was also higher if we omitted the smallest networks, which were less likely to show a significant pattern, but overall this suggests that structural balance is not widespread in this system. Network size and density did not appear to have direct effects on the level of structural balance. Social networks are expected to tend towards structural balance over time (Harary 1961), so this process might be hard to detect in less well-established networks such as in this study. Marmot groups experience long hibernations and significant turnover in group membership each year, with adult females being the most stable members, which might limit establishment of long-term structural balance. The higher number of weakly balanced triads indicates that—triads are common and that there are multiple (more than 2) subgroups per network, and the increase in structural balance when ambiguous pairs were omitted suggests that selection of relevant relationships is important. It might be rare to find strong structural balance in most nonhuman social networks and more common to see weak structural balance, and this is a pattern to be tested more broadly.
We currently have few other animal studies with which to directly compare our results for structural balance specifically, but the theory should have broad relevance to any system where individuals experience repeated overlap in positive and negative relationships and might benefit from maintaining social balance. Our results differ somewhat from those of Ilany et al. (2013), who found significant structural balance across wild rock hyrax groups. The disparity could arise from a difference in how positive and negative relationships were defined in the two studies. We considered pairs exhibiting both positive and negative interactions as having a negative (unstable) relationship (or we deleted the relationship), which tends to increase the frequency of negative relationships. Ilany et al. (2013) defined pairs that exhibited any affiliation as having positive relationships, which would tend to increase the frequency of positive relationships and might be more similar to our analysis of networks of only positive interactions. Moreover, as previously noted, yellow-bellied marmots are not highly cooperative, and maintaining structural balance might have fewer benefits than in humans or other highly cooperative species. It will be interesting to see if degree of sociality or context are predictors of tendency towards structural balance. For example, would more cooperative species typically show both transitivity in affiliative interactions and structural balance, while less cooperative species show less structural balance? Or is contextual motivation for social balance more important than degree of sociality per se? Even human social networks show some variation in whether or not structural balance is present (Hummon and Doreian 2003; Leskovec et al. 2010; Zheng et al. 2015), indicating that context is important. Importantly though, the question of social balance applies to the joint patterns of positive and negative interactions, not on level of cooperation. A number of other animal studies have examined clustering or multiple types of social interactions simultaneously, without explicitly testing for structural balance, and these could provide fruitful avenues for testing for balance between positive and negative relationships. Various studies have demonstrated how clustering and other local processes affect the emergence of cooperation, notably in a series of studies in guppies (Poecilia reticulata), which conduct cooperative predator inspection (Croft et al. 2015). Diverse factors ranging from the distribution of individuals in space and time to partner preference and reciprocity affect the patterns of this cooperative behavior, which is risky and relies on stable partnerships. Studies that have already examined both positive and negative network interactions include pig-tailed macaques (Macaca nemestrina) (Flack et al. 2005, 2006), rhesus macaques (Macaca mulatta) (Brent et al. 2013), chacma baboons (Papio hamadryas ursinus) (Barrett et al. 2012), white-faced capuchins (Cebus capunicus) (Crofoot et al. 2011), meerkats (Suricata suricatta) (Madden et al. 2009), and ring-tailed coatis (Nasua nasua) (Hirsch et al. 2012), among others (Faust 2010). Testing structural balance theory would be a natural further question in these systems but would likely provide insight into any system with triadic structure in positive and negative interactions.
The lack of consistent signatures of structural balance in our study might also suggest that agonistic interactions, and thus negative relationships, in this system are less clearly structured than affiliative interactions. For example, while affiliative partner choice was significantly influenced by similarity in age and relatedness, agonistic partner interactions were not well predicted by these factors (Wey and Blumstein 2010). It is also not uncommon for the same individuals to show both affiliation and agonism (17% of ties in this study; also see Wey and Blumstein 2010), and reproductive female marmots are motivated by both cooperation and conflict with their own kin (Armitage 2014). In this study, we focused on testing tendency towards higher transitivity or structural balance, but examining the opposite tendency (if there are colonies that show lower transitivity or less balance) could be interesting as well. Animals might actively avoid certain interactions, and we could hypothesize for instance that agonistic networks show lower clustering if some individuals are dominant and aggression is structured more linearly. It is also important to consider how well our definitions of positive and negative relationships capture how animals experience relationships. We included low-level agonistic encounters, such as displacements, in the same category as the much less common higher-level aggression (chases, fights), and it is possible that these types of interactions are qualitatively different. We also used a definition of negative relationships that considered mixed affiliative and agonistic interactions as a negative relationship, but it might be important to consider the frequency of each type of interaction or other weighting rules in determining the overall relationship quality. Finally, membership in marmot colonies typically changes significantly between years and to some extent even within years, so the social networks used here might represent a snapshot that represents multiple phases of integration or a system in flux. If flux is expected, perhaps it is not ultimately beneficial for marmots to invest in the cognitive capacity required to keep track of these relationships. Addressing temporal change in networks and studying network structure and consequences on the right timescale is an ongoing challenge (Gross and Blasius 2008; Blonder et al. 2012; Holme and Saramäki 2012; Sih and Wey 2014).
Overall, our study adds to the empirical understanding of how animal social networks are structured, and it also illustrates the potential utility of considering network motifs to study local network structures. Considering its long history in sociological studies and the intuitive applications to animal societies, it is somewhat surprising that there has not been more application of structural balance theory and analysis of network motifs more generally to nonhuman groups (but see Hobson and DeDeo 2015; Shizuka and McDonald 2012, 2015). More recent developments have also proposed variants to structural balance theory, such as status theory (Leskovec et al. 2010), which might better explain certain human social networks, such as directed relationships in online social networks. A recent review and analysis by Yap and Harrigan (2015) found that both balance and status (along with a third major social network paradigm—homophily; McPherson et al. 2001) explained a large majority of student social networks. Status theory makes some predictions similar to expectations for the kind of transitive relationships commonly studied in animal dominance hierarchies (Shizuka and McDonald 2015), and it will be intriguing to see if findings in the two fields converge. Even most studies of human social networks only analyze positive ties without considering negative ties (Leskovec et al. 2010).
Given the complexity and common presence of multiple types of relationships in real social networks, further tests of structural balance theory will be highly informative in studying the broader form and function of social networks. A number of intriguing directions for further empirical work readily come to mind. Most simply, further studies in diverse systems would help establish how general these social processes are and if there is variation across different social systems. Methodologically, experimental manipulations could greatly enhance our understanding of the role that environment or individuals play in triadic structure. For example, network structure responds to different environmental factors in diverse species: resource availability in New Caledonian crows (Corvus moneduloides) (St Clair et al. 2015), food quality in bees (Apis spp.) (Naug 2008), habitat structure in sleepy lizards (Tiliqua rugosa) (Leu et al. 2016), and water levels and sex ratio in guppies (Wilson et al. 2015), so we could hypothesize that environmental factors could generally increase or decrease social stability. We could also test predictions about how network structure, either at a local or global scale, might respond to removal of different nodes depending on their role in triads. For example, based on clustering and balance theory, we might predict that removing a node that was part of an unbalanced triad might reduce tension in the remaining dyad and in the network, whereas removing a node that was part of a balanced triad might weaken the bond between the remaining dyad and increase tension in the network. Interestingly, Facchetti et al. (2011) suggest that certain individuals in online communities that are involved in the most negative interactions (general “enemies” of many others) are not creating social tension in the sense of the original structural balance theory but instead are creating disorder.
While we are not aware of animal studies that have directly manipulated group composition to study structural balance specifically, previous works show how manipulation can elucidate network dynamics. Targeted short-term removals in captive pig-tailed macaques, used in combination with simulated removals, showed that individuals with different conflict management roles differentially affected affiliative and agonistic network structure (Flack et al. 2005, 2006). A natural extension to this would be to ask if there were corresponding changes to structural balance. In general, captive animals that are subject to group changes due to management might provide fruitful opportunities for examining resulting effects on network structure, such as in a study of patterns of aggression and pair bonding in American flamingos (Phoenicopterus ruber) after introductions and removals of individuals (Frumkin et al. 2016). Invertebrates also generally represent systems suitable group manipulations. For instance, Bhadra and Jordán (2013) took advantage of previously collected data on queen removals in two species of paper wasps (Gadagkar 2001), also in conjunction with simulated removals, to examine species differences in queen succession. Many natural systems, such as in the marmots, will not be amenable to experimental manipulation due to research focus on long-term natural processes with the goal of avoiding consequential manipulations. However, even when direct manipulation is not desirable, researchers could take advantage of natural events, such as changes in group composition due to immigration and emigration, to test if group-level structure or local structure changes in ways predicted by social network theory. This approach has already been applied to study structural balance in rock hyrax (Ilany et al. 2013) and response of social networks in multiple dimensions to perturbation in chacma baboons (Barrett et al. 2012). Going forward, experimental manipulations and natural changes in group composition, used in conjunction with simulations and other technologies, continue to offer exciting opportunities to gain insight into animal social networks in general (Croft et al. 2016), and applications to structural balance theory represent a promising avenue to test established theory in a new range of social systems.
Data availability
The datasets generated during and/or analyzed during the current study are not publicly available as they are part of ongoing research on a long-term dataset but are available from the corresponding author upon reasonable request. R code for calculating strong and weak structural balance are provided in supplementary material.
References
Armitage KB (2014) Marmot biology: sociality, ind ividual fitness, and population dynamics. Cambridge University Press, Cambridge
Barocas A, Ilany A, Koren L, Kam M, Geffen E (2011) Variance in centrality within rock hyrax social networks predicts adult longevity. PLoS One 6:e22375. https://doi.org/10.1371/journal.pone.0022375
Barrett L, Henzi SP, Lusseau D (2012) Taking sociality seriously: the structure of multi-dimensional social networks as a source of information for individuals. Phil Trans R Soc B 367:2108–2118. https://doi.org/10.1098/rstb.2012.0113
Bates D, Mächler M, Bolker B, Walker S (2015) Fitting linear mixed-effects models using lme4. J Stat Softw 67:1–48. https://doi.org/10.18637/jss.v067.i01
Bhadra A, Jordán F (2013) Cryptic successors unrevealed even by network analysis: a comparative study of two paper wasp species. Netw Biol 3:54–66
Blonder B, Wey TW, Dornhaus A, James R, Sih A (2012) Temporal dynamics and network analysis. Methods Ecol Evol 3:958–972. https://doi.org/10.1111/j.2041-210X.2012.00236.x
Blumstein DT (2013) Yellow-bellied marmots: insights from an emergent view of sociality. Phil Trans R Soc B 368:20120349. https://doi.org/10.1098/rstb.2012.0349
Blumstein DT, Wey TW, Tang K (2009) A test of the social cohesion hypothesis: interactive female marmots remain at home. Proc R Soc Lond B 276:3007–3012. https://doi.org/10.1098/rspb.2009.0703
Blumstein DT, Williams DM, Lim AN, Kroeger S, Martin JGA (2018) Strong social relationships are associated with decreased longevity in a facultatively social mammal. Proc R Soc B 285:20171934. https://doi.org/10.1098/rspb.2017.1934
Brent LJN, Semple S, Dubuc C, Heistermann M, MacLarnon A (2011) Social capital and physiological stress levels in free-ranging adult female rhesus macaques. Physiol Behav 102:76–83. https://doi.org/10.1016/j.physbeh.2010.09.022
Brent LJN, MacLarnon A, Platt ML, Semple S (2013) Seasonal changes in the structure of rhesus macaque social networks. Behav Ecol Sociobiol 67:349–359. https://doi.org/10.1007/s00265-012-1455-8
Brent LJN, Franks DW, Foster EA, Balcomb KC, Cant MA, Croft DP (2015) Ecological knowledge, leadership, and the evolution of menopause in killer whales. Curr Biol 25:746–750. https://doi.org/10.1016/j.cub.2015.01.037
Cameron EZ, Setsaas TH, Linklater WL (2009) Social bonds between unrelated females increase reproductive success in feral horses. P Natl Acad Sci USA 106:13850–13853. https://doi.org/10.1073/pnas.0900639106
Cartwright D, Harary F (1956) Structural balance: a generalization of Heider’s theory. Psychol Rev 63:277–293. https://doi.org/10.1037/h0046049
Connor RC, Heithaus MR, Barre LM (2001) Complex social structure, alliance stability and mating access in a bottlenose dolphin ‘super-alliance’. Proc R Soc Lond B 268:263–267. https://doi.org/10.1098/rspb.2000.1357
Crofoot MC, Rubenstein DI, Maiya AS, Berger-Wolf TY (2011) Aggression, grooming and group-level cooperation in white-faced capuchins (Cebus capucinus): insights from social networks. Am J Primatol 73:821–833. https://doi.org/10.1002/ajp.20959
Croft DP, James R, Thomas POR, Hathaway C, Mawdsley D, Laland KN, Krause J (2006) Social structure and co-operative interactions in a wild population of guppies (Poecilia reticulata). Behav Ecol Sociobiol 59:644–650. https://doi.org/10.1007/s00265-005-0091-y
Croft DP, Krause J, Darden SK, Ramnarine IW, Faria JJ, James R (2009) Behavioural trait assortment in a social network: patterns and implications. Behav Ecol Sociobiol 63:1495–1503. https://doi.org/10.1007/s00265-009-0802-x
Croft DP, Edenbrow M, Darden SK (2015) Assortment in social networks and the evolution of cooperation. In: Krause J, James R, Franks DW, Croft DP (eds) Animal social networks. Oxford University Press, New York, NY, pp 13–23
Croft DP, Darden SK, Wey TW (2016) Current directions in animal social networks. Curr Opin Behav Sci 12:52–58. https://doi.org/10.1016/j.cobeha.2016.09.001
Csardi G, Nepusz T (2006) The igraph software package for complex network research. Int J Commun Syst 1695:1695
Davis JA (1967) Clustering and structural balance in graphs. Hum Relat 20:181–187. https://doi.org/10.1177/001872676702000206
de Waal FBM (2000) Primates--a natural heritage of conflict resolution. Science 289:586–590. https://doi.org/10.1126/science.289.5479.586
Dewsbury DA (1982) Dominance rank, copulatory behavior, and differential reproduction. Q Rev Biol 57:135–159
Dey CJ, Reddon AR, O’Connor CM, Balshine S (2013) Network structure is related to social conflict in a cooperatively breeding fish. Anim Behav 85:395–402. https://doi.org/10.1016/j.anbehav.2012.11.012
Doreian P, Mrvar A (1996) A partitioning approach to structural balance. Soc Netw 18:149–168. https://doi.org/10.1016/0378-8733(95)00259-6
Drews C (1993) The concept and definition of dominance in animal behaviour. Behaviour 125:283–313. https://doi.org/10.1163/156853993X00290
Edelman AJ, McDonald DB (2014) Structure of male cooperation networks at long-tailed manakin leks. Anim Behav 97:125–133. https://doi.org/10.1016/j.anbehav.2014.09.004
Ellis L (1995) Dominance and reproductive success among nonhuman animals: a cross-species comparison. Ethol Sociobiol 16:257–333. https://doi.org/10.1016/0162-3095(95)00050-U
Facchetti G, Iacono G, Altafini C (2011) Computing global structural balance in large-scale signed social networks. P Natl Acad Sci USA 108:20953–20958. https://doi.org/10.1073/pnas.1109521108
Farine DR, Whitehead H (2015) Constructing, conducting and interpreting animal social network analysis. J Anim Ecol 84:1144–1163. https://doi.org/10.1111/1365-2656.12418
Faust K (2010) A puzzle concerning triads in social networks: graph constraints and the triad census. Soc Netw 32:221–233. https://doi.org/10.1016/j.socnet.2010.03.004
Firth JA, Sheldon BC, Brent LJN (2017) Indirectly connected: simple social differences can explain the causes and apparent consequences of complex social network positions. Proc R Soc B 284:20171939. https://doi.org/10.1098/rspb.2017.1939
Flack JC, Krakauer DC, de Waal FBM (2005) Robustness mechanisms in primate societies: a perturbation study. Proc R Soc Lond B 272:1091–1099. https://doi.org/10.1098/rspb.2004.3019
Flack JC, Girvan M, de Waal FBM, Krakauer DC (2006) Policing stabilizes construction of social niches in primates. Nature 439:426–429. https://doi.org/10.1038/nature04326
Frumkin NB, Wey TW, Exnicios M, Benham C, Hinton MG, Lantz S, Atherton C, Forde D, Karubian J (2016) Inter-annual patterns of aggression and pair bonding in captive American flamingos (Phoenicopterus ruber). Zoo Biol 35:111–119. https://doi.org/10.1002/zoo.21274
Gadagkar R (2001) The social biology of Ropalidia marginata: toward understanding the evolution of eusociality. Harvard University Press, Cambridge, MA
Granovetter MS (1973) The strength of weak ties. Am J Sociol 78:1360–1380. https://doi.org/10.1086/225469
Gross T, Blasius B (2008) Adaptive coevolutionary networks: a review. J R Soc Interface 5:259–271. https://doi.org/10.1098/rsif.2007.1229
Harary F (1961) A structural analysis of the situation in the Middle East in 1956. J Confl Resolut 5:167–178. https://doi.org/10.1177/002200276100500204
Harcourt AH (1992) Coalitions and alliances: are primates more complex than nonprimates. In: Harcourt AH, de Waal FBM (eds) Coalitions and alliances in humans and other animals. Oxford University Press, Oxford, pp 445–471
Heider F (1946) Attitudes and cognitive organization. J Psychol 21:107–112. https://doi.org/10.1080/00223980.1946.9917275
Hirsch BT, Stanton MA, Maldonado JE (2012) Kinship shapes affiliative social networks but not aggression in ring-tailed coatis. PLoS One 7:e37301. https://doi.org/10.1371/journal.pone.0037301
Hobson EA, DeDeo S (2015) Social feedback and the emergence of rank in animal society. PLoS Comput Biol 11:e1004411. https://doi.org/10.1371/journal.pcbi.1004411
Holland PW, Leinhardt S (1976) Local structure in social networks. Sociol Methodol 7:1–45. https://doi.org/10.2307/270703
Holme P, Saramäki J (2012) Temporal networks. Phys Rep 519:97–125. https://doi.org/10.1016/j.physrep.2012.03.001
Hummon NP, Doreian P (2003) Some dynamics of social balance processes: bringing Heider back into balance theory. Soc Netw 25:17–49. https://doi.org/10.1016/S0378-8733(02)00019-9
Ilany A, Barocas A, Koren L, Kam M, Geffen E (2013) Structural balance in the social networks of a wild mammal. Anim Behav 85:1397–1405. https://doi.org/10.1016/j.anbehav.2013.03.032
Ilany A, Booms AS, Holekamp KE (2015) Topological effects of network structure on long-term social network dynamics in a wild mammal. Ecol Lett 18:687–695. https://doi.org/10.1111/ele.12447
Jordán F (2009) Children in time: community organization in social and ecological systems. Curr Sci 97:1579–1585
Krause J, James R, Croft DP (2010) Personality in the context of social networks. Phil Trans R Soc B 365:4099–4106. https://doi.org/10.1098/rstb.2010.0216
Krause J, James R, Franks DW, Croft DP (eds) (2015) Animal social networks. Oxford University Press, New York, NY
Leskovec J, Huttenlocher D, Kleinberg J (2010) Signed networks in social media. In: Proceedings of the SIGCHI Conference on Human Factors in Computing Systems. ACM, New York, NY, pp 1361–1370
Leu ST, Farine DR, Wey TW, Sih A, Bull CM (2016) Environment modulates population social structure: experimental evidence from replicated social networks of wild lizards. Anim Behav 111:23–31. https://doi.org/10.1016/j.anbehav.2015.10.001
Lusseau D (2003) The emergent properties of a dolphin social network. Proc R Soc Lond B 270:S186–S188. https://doi.org/10.1098/rsbl.2003.0057
Madden JR, Drewe JA, Pearce GP, Clutton-Brock TH (2009) The social network structure of a wild meerkat population: 2. Intragroup interactions. Behav Ecol Sociobiol 64:81–95. https://doi.org/10.1007/s00265-009-0820-8
McComb K, Moss C, Durant SM, Baker L, Sayialel S (2001) Matriarchs as repositories of social knowledge in African elephants. Science 292:491–494. https://doi.org/10.1126/science.1057895
McDonald DB (2007) Predicting fate from early connectivity in a social network. P Natl Acad Sci USA 104:10910–10914. https://doi.org/10.1073/pnas.0701159104
McDonald GC, Pizzari T (2016) Why patterns of assortative mating are key to study sexual selection and how to measure them. Behav Ecol Sociobiol 70:209–220. https://doi.org/10.1007/s00265-015-2041-7
McPherson M, Smith-Lovin L, Cook JM (2001) Birds of a feather: homophily in social networks. Annu Rev Sociol 27:415–444. https://doi.org/10.1146/annurev.soc.27.1.415
Milo R, Shen-Orr S, Itzkovitz S, Kashtan N, Chklovskii D, Alon U (2002) Network motifs: simple building blocks of complex networks. Science 298:824–827. https://doi.org/10.1126/science.298.5594.824
Naug D (2008) Structure of the social network and its influence on transmission dynamics in a honeybee colony. Behav Ecol Sociobiol 62:1719–1725. https://doi.org/10.1007/s00265-008-0600-x
Newman MEJ, Park J (2003) Why social networks are different from other types of networks. Phys Rev E 68:036122. https://doi.org/10.1103/PhysRevE.68.036122
Ohtsuki H, Hauert C, Lieberman E, Nowak MA (2006) A simple rule for the evolution of cooperation on graphs and social networks. Nature 441:502–505. https://doi.org/10.1038/nature04605
R Development Core Team (2018) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria, http://www.R-project.org. Accessed 3 April 2019
Schülke O, Bhagavatula J, Vigilant L, Ostner J (2010) Social bonds enhance reproductive success in male macaques. Curr Biol 20:2207–2210. https://doi.org/10.1016/j.cub.2010.10.058
Shizuka D, McDonald DB (2012) A social network perspective on measurements of dominance hierarchies. Anim Behav 83:925–934. https://doi.org/10.1016/j.anbehav.2012.01.011
Shizuka D, McDonald DB (2015) The network motif architecture of dominance hierarchies. J R Soc Interface 12:20150080. https://doi.org/10.1098/rsif.2015.0080
Sih A, Wey TW (2014) Dynamic feedbacks on dynamic networks: on the importance of considering real-time rewiring—comment on Pinter-Wollman et al. Behav Ecol 25:258–259. https://doi.org/10.1093/beheco/art081
Silk JB (2007) The adaptive value of sociality in mammalian groups. Phil Trans R Soc B 362:539–559. https://doi.org/10.1098/rstb.2006.1994
St Clair JJH, Burns ZT, Bettaney EM, Morissey MB, Otis B, Ryder TB, Fleischer RC, James R, Rutz C (2015) Experimental resource pulses influence social-network dynamics and the potential for information flow in tool-using crows. Nat Commun 6:7197. https://doi.org/10.1038/ncomms8197
Stanton MA, Mann J (2012) Early social networks predict survival in wild bottlenose dolphins. PLoS One 7:e47508. https://doi.org/10.1371/journal.pone.0047508
Vander Wal E, Festa-Bianchet M, Réale D, Coltman DW, Pelletier F (2015) Sex-based differences in the adaptive value of social behavior contrasted against morphology and environment. Ecology 96:631–641. https://doi.org/10.1890/14-1320.1
Wasserman S, Faust K (1994) Social network analysis: methods and applications. Cambridge University Press, Cambridge
Wey TW, Blumstein DT (2010) Social cohesion in yellow-bellied marmots is established through age and kin structuring. Anim Behav 79:1343–1352. https://doi.org/10.1016/j.anbehav.2010.03.008
Wey TW, Blumstein DT (2012) Social attributes and associated performance measures in marmots: bigger male bullies and weakly affiliating females have higher annual reproductive success. Behav Ecol Sociobiol 66:1075–1085. https://doi.org/10.1007/s00265-012-1358-8
Wey T, Blumstein DT, Shen W, Jordán F (2008) Social network analysis of animal behaviour: a promising tool for the study of sociality. Anim Behav 75:333–344. https://doi.org/10.1016/j.anbehav.2007.06.020
Wey TW, Burger JR, Ebensperger LA, Hayes LD (2013) Reproductive correlates of social network variation in plurally breeding degus (Octodon degus). Anim Behav 85:1407–1414. https://doi.org/10.1016/j.anbehav.2013.03.035
Whitehead H (2008) Analyzing animal societies. The University of Chicago Press, Chicago, IL
Wilson ADM, Krause S, Ramnarine IW, Borner KK, Clément RJG, Kurvers RHJM, Krause J (2015) Social networks in changing environments. Behav Ecol Sociobiol 69:1617–1629. https://doi.org/10.1007/s00265-015-1973-2
Yap J, Harrigan N (2015) Why does everybody hate me? Balance, status, and homophily: the triumvirate of signed tie formation. Soc Netw 40:103–122. https://doi.org/10.1016/j.socnet.2014.08.002
Zheng X, Zeng D, Wang F-Y (2015) Social balance in signed networks. Inf Syst Front 17:1077–1095. https://doi.org/10.1007/s10796-014-9483-8
Acknowledgments
We thank Adriana Maldonado-Chaparro and Julien Martin for database curation and all the marmoteers over the years for data collection. This work was also assisted through participation in the Animal Social Networks Investigative Workshop at the National Institute for Mathematical and Biological Synthesis, sponsored by the National Science Foundation (award no. DBI-1300426), with additional support from the University of Tennessee, Knoxville. We thank Josh Firth and an anonymous reviewer for extremely constructive feedback on an earlier version of the manuscript.
Funding
This work was supported by a U.S. Department of Education Graduate Assistance in Areas of National Need Fellowship, a National Science Foundation GK-12 Fellowship, a UCLA Chancellor’s Prize, and a Rocky Mountain Biological Laboratory (RMBL) Snyder Graduate Research Fellowship (to TWW); by the National Geographic Society (grant no. 8140-06), the UCLA Faculty Senate and Division of Life Sciences, and a RMBL research fellowship (to DTB); by the National Science Foundation (IDBR-0754247, DEB-1119660, and 1557130 to DTB; DBI 0242960 and 0731346 to the RMBL); and by the National Research, Development, and Innovation Office (NKFIH grant OTKA K 11607 to FJ).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed. All procedures performed in studies involving animals were in accordance with the ethical standards of the institution or practice at which the studies were conducted. Marmots are studied under protocols approved by UCLA (2001-191, renewed annually) and the Colorado Division of Wildlife (TR914, renewed annually).
Additional information
Communicated by D. P. Croft
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
Table S1 Network properties and triad summaries in marmot social networks. “AF” = adult females. “Structural Balance (Method 1)” assigns links with both + and - interactions as - (tending towards conflict). “Structural Balance (Method 2)” omits links with both + and – interactions (ambiguous relationships not included). The “P-value” is the probability of obtaining a transitivity score from 1000 permutations of the observed network (i.e., maintaining network size and density) that is as large as or larger than the value from the observed network. “P-value1” and “P-value 2” are the probabilities of obtaining a level of structural balance as large as or larger than the observed level, again from 1000 permutations, where structural balance is defined as either the number of balanced triads or the proportion of balanced triads, respectively. “Link Diff.” is the difference in number of links from Method 1 and Method 2 (i.e., the number of node pairs that had both + and - interactions). “Prop. Diff.” is the Link Diff. / Links from Method 1. (XLSX 23 kb)
ESM 2
R functions to calculate strong and weak structural balance (R 2 kb)
Rights and permissions
About this article
Cite this article
Wey, T.W., Jordán, F. & Blumstein, D.T. Transitivity and structural balance in marmot social networks. Behav Ecol Sociobiol 73, 88 (2019). https://doi.org/10.1007/s00265-019-2699-3
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00265-019-2699-3