Abstract
Avian parents initially provide their offspring with resources such as food and protection from predators but the offspring then undergo a period of transition from dependency to independence. That transition represents a crucial period of an individual’s life as their inexperience in simultaneously searching for food and avoiding predators’ means that the risks of mortality are higher than during other periods of their life. Unfortunately, our understanding of the transition to independence has lagged behind other life history stages due to the logistical challenges associated with tracking individuals after they have left the relatively safe confines of the nest. However, recent technological advances that have enabled individuals to be tracked remotely have dramatically increased our understanding of the process by which offspring acquire independence. First, interspecific studies of birds have demonstrated that the offspring of species that suffer both high levels of nest predation and ectoparasite-induced nestling mortality leave the nest comparatively sooner than the offspring of species that suffer lower levels of nest predation and ectoparasite-induced nestling mortality. Second, intraspecific studies have become much more prevalent in recent years as new technologies have been used to show that between-brood or litter variation in the timing of independence is influenced by predation risk, ectoparasite-induced nestling mortality and parent-offspring conflict over the provision of care. Further studies have shown that within-brood variation in the timing of independence also occurs as a result of both genetic effects such as parentage and offspring sex, and parental effects such as hatching asynchrony, that result in inequalities between siblings. In summary, there is considerable variation in the process by which avian offspring acquire independence at the interspecific and intraspecific levels. However, whilst we have a reasonable understanding of the causes of such variation, our understanding of the consequences of such variation is less developed and deserves further research.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Parents provide their developing offspring with resources such as food and protection from predators in species across many taxa, but at some stage, the offspring must undergo a period of transition from dependency to independence. That transition is one of the most important stages of an individual’s life as their inexperience in simultaneously searching for food and avoiding predators’ means that the risks of mortality are higher than at any other stage of their life (Clutton-Brock 1991; Stearns 1992). The process by which the offspring of vertebrates acquire independence differs between taxa with, for example, many studies examining the weaning process in mammals (e.g. Lee et al. 1991; Rehling and Trillmich 2007; reviewed by Hudson and Trillmich 2008; González-Mariscal et al. 2016). Unfortunately, our understanding of the transition from dependence to independence has lagged behind other life history stages, both in birds and other taxa, at least partly due to the logistical challenges associated with tracking individuals after they have left the relatively safe confines of the nest or burrow (Bennett and Owens 2002; Schlicht et al. 2012). However, recent technological advances that have enabled individual birds to be tracked remotely have dramatically increased our understanding of the process by which avian offspring acquire independence (e.g. Arroyo et al. 2002; Samuel et al. 2012; Bowers et al. 2013; Gow and Wiebe 2013; Johnson et al. 2013; Cox et al. 2014).
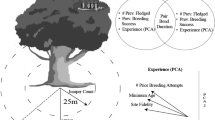
Here, I review the causes and consequences of both interspecific and intraspecific patterns of variation in the process by which avian offspring acquire independence from their parents. I begin by examining the relative influence of nest predation rates, food availability and ectoparasite-induced nestling mortality rates in determining interspecific patterns of the timing of nest departure in birds. I then examine the influence of predation risk, ectoparasite-induced nestling mortality and parent-offspring conflict over the provision of care in determining intraspecific patterns in the acquisition of independence. Next, I examine the influence of genetic effects such as parentage and sex, and parental effects such as hatching asynchrony, in determining within-brood variation in the timing of nest leaving and the duration of the post-fledging care period (summarised in Fig. 1). I then finish by synthesising our current knowledge of the process by which individuals acquire independence and by suggesting areas where further research should be focused.
Interspecific variation in the transition to independence
When compared to other taxa, birds make amenable study organisms as they are generally diurnal, they live above ground and in close proximity to humans, the adults are easy to identify to species level, their nest sites are usually easy to locate, their reproductive biology is easy to quantify and they are generally tolerant of monitoring activities. Consequently, a huge amount of research attention has focused on birds (Bennett and Owens 2002). This means that there are sufficient studies to perform comparative analyses examining the evolutionary causes of interspecific variation in the age at which birds leave the nest but that there are insufficient studies of other taxa.
Birds exhibit a broad range of interspecific variation in their life histories and, pertinently, they also exhibit a broad range of variation both in the timing and in the process by which offspring acquire independence (Roff 1992; Gebhardt-Henrich and Richner 1998). Such variation largely exists along the altricial-precocial continuum with groups such as gamebirds and rails having precocial chicks that are relatively mobile, have feathers and can feed themselves within a few hours of hatching. Whilst they undoubtedly have a degree of independence shortly after hatching, they nevertheless remain dependent on their parents for the acquisition of foraging skills and predator avoidance behaviours. Meanwhile, passerines are at the other end of the spectrum and their altricial nestlings are born virtually naked, blind and are fully dependent upon their parents for both food and warmth for a period of several weeks. Nevertheless, they gain a degree of independence daily as they acquire the ability to thermoregulate and also become increasingly able to detect predators independently of their parents as their senses develop. There are other groups of species between these two extremes which are defined as being semi-precocial or semi-altricial, depending on their level of dependence after hatching (Harrison 1975; O’Connor 1984). Considerable variation in the acquisition of independence exists at the between-species level depending on the species’ position on the altricial-precocial spectrum with the offspring of precocial species generally gaining a higher degree of independence much earlier than the offspring of altricial species. In this section, I will examine the relative influence of the risk of predation, the availability of food and ectoparasite-induced nestling mortality in determining interspecific variation in the process by which offspring acquire independence.
The risk of predation
With the exception of a few apex predatory species, the risk of predation is ubiquitous for birds both as nestlings and then during adulthood and thus exerts a strong influence on avian life histories (Caro 2005). The risk of predation for parent birds whilst visiting the nest site has been reviewed elsewhere (Lima 2009), and so here I concentrate on the risk of nest predation. Natural selection favours those life history traits that minimise the risk of predation, with the duration of nestling periods being such a trait. The importance of the duration of nestling periods was recognised by Lack (1968) when he suggested that those species with relatively short nestling periods tended to be open cup nesting species that suffered high levels of nestling predation rates whilst those species with relatively long nestling periods tended to be hole nesting species that suffered low levels of nestling predation rates. However, the earliest comparative analyses (Ricklefs 1969; Ricklefs et al. 1998) suggested that neither the risk of predation nor any other environmental factors influenced nestling periods and growth rates, which was interpreted as nestlings being able to develop at maximum rates within physiological constraints (Arendt 1997; Dmitriew 2011).
More recent studies have suggested that those previous estimates of nestling growth rates were confounded by variation in the length of nestling periods. So, after the growth rates of 115 species of North American passerine birds were re-estimated by taking the length of their nestling periods into account (Remeš and Martin 2002), the growth rates of altricial nestlings was found to be strongly positively correlated to daily nest predation rates, even after controlling for phylogenetic non-independence and adult body mass. Further, the nestlings of those species that suffer from high daily nest predation rates had shorter nestling periods, meaning that they fledged at lower body masses relative to adult body masses than species that suffer from lower daily nest predation rates. Thus, those species that suffer from high levels of nest predation have evolved shorter nestling periods and higher growth rates than species that suffer from lower levels of nest predation in order to escape the nest sooner (Remeš and Martin 2002). Further evidence that nestling periods and nestling growth rates are determined by the risk of predation comes from subsequent comparative analyses. Using data from 64 species of passerine birds breeding in North America, Venezuela and South Africa, a comparative analysis showed that the risk of predation was the main determinant of growth rate variation between species (Martin et al. 2011).
Geographical variation in the risk of predation for both adult and nestling birds should exist, however, if there is geographical variation in the relative value of offspring to caring parents. Having proven that birds living in the Southern Hemisphere have higher adult survival rates and smaller clutches than birds living in the Northern Hemisphere, Ghalambor and Martin (2001) examined parental responsiveness to the risk of mortality by manipulating predation risk to adults and offspring in ten species of birds that were paired between North and South America on the basis of their phylogeny and ecology. As predicted, parent birds in South America responded more strongly to reduced levels of mortality risk to themselves even though it came at a cost to their own offspring, whereas species in North America responded more strongly to reduced levels of risk to their offspring even when it came at a greater risk to themselves (Ghalambor and Martin 2001). Another study showed that the age of fledging was determined by the risk of predation and that songbirds fledge at the optimal time that predicts their survival to maturity (Roff et al. 2005). The optimal fledging time represents a trade-off between the risk of mortality within and outside of nests, and because the mortality rate of individuals within nests is significantly higher than for the same individuals after they have fledged, then selection favours a fledging age where post-fledging mortality is approximately 0.3–0.4 of that experienced in the nest (Roff et al. 2005).
Meanwhile, Remeš (2007) tested the theory that annual adult mortality rates may indirectly influence nestling periods by testing the hypothesis that those species with high levels of adult mortality would invest a greater amount of effort into caring for the offspring from a single reproductive event. Data from 84 species of North American passerine birds showed that the growth rates of altricial nestlings were strongly positively correlated with annual adult mortality rates, even after controlling for potentially confounding variables such as phylogenetic non-independence and nest predation rates. Thus, parents with high levels of annual mortality rates did invest more care in their offspring within a single reproductive event, which resulted in their offspring receiving more food and subsequently growing faster and having shorter developmental periods than species with low levels of annual adult mortality rates (Remeš 2007). However, an alternative explanation, especially as the author did not measure feeding rates, is that faster developing species died relatively sooner due, for instance, to a faster accumulation of metabolic damage in line with the pace of life syndrome hypothesis. Nevertheless, these studies strongly suggest that predation rates influence nestling growth rates, nestling periods and thus the time at which offspring fledge which is a major transition in the process by which they acquire independence from their parents (Bennett and Owens 2002; Gebhardt-Henrich and Richner 1998; Martin and Briskie 2009).
High levels of predation risk are generally associated with shorter nestling periods and higher nestling growth rates in birds (Remeš 2007; Mainwaring et al. 2015), yet in other taxa, high levels of predation risk are associated with longer developmental periods and slower growth rates as slower rates of development are associated with reduced food requirements and reduced parental feeding activity (Dmitriew 2011). Moreover, this pattern is not solely confined to non-avian taxa as a study of East African stonechats (Saxicola torquata axillaris) showed that the risk of predation was associated with lower rates of growth (Scheuerlein and Gwinner 2006). However, regardless of whether developmental periods and growth rates are increased or decreased, there can be little doubt that the risk of predation is an important agent of selection on developmental periods and growth rates (Remeš and Martin 2002; Remeš 2007; Martin et al. 2011). Nevertheless, these studies suggest that the risk of predation is not the only determinant of developmental periods and that factors such as the availability of food are also important.
The availability of food
Despite initially arguing that nestling growth rates were determined by the risk of predation, Lack (1968) later suggested that growth rates were determined by the availability of food. Thus, an interspecific study of 64 species of passerine birds from three continents sought to examine the relative influence of the risk of predation and the availability of food in determining nestling growth rates and hence indirectly the timing of fledging (Martin et al. 2011). Nestling growth rates were positively correlated with nest predation rates which in turn were negatively correlated with provisioning rates, meaning that parents feeding their nestlings in riskier environments did so at a lower rate than parents in safer environments. So, even if parents fully compensated for the fewer provisioning trips by increasing the quantity of food provisioned at each trip, then this suggests that the risk of predation was more important than the availability of food in determining growth rates and fledging times in birds (Martin et al. 2011).
Moreover, the timing of fledging did not differ between cooperatively and non-cooperatively breeding species, despite cooperative breeders having additional adults to provide care for the nestlings (Langen 2000). However, the duration of post-fledging care periods was significantly longer in cooperatively breeding species because the non-breeding adults provided food for the dependent fledglings. This increased the net amount of care that the fledglings received and particularly so in multi-brooded species where the breeding adults initiate the second clutch shortly after the nestlings from the first clutch fledge (Langen 2000). So, the availability of food does not influence nestling growth periods or the timing of fledging in birds but does influence the duration of the post-fledging care period in cooperative societies.
Ectoparasite-induced nestling mortality
The risk of predation has traditionally been viewed as the main determinant of nestling periods and the timing of fledging in birds (Remeš and Martin 2002; Remeš 2007; Martin et al. 2011), yet the role of ectoparasite-induced nestling mortality may have been under-estimated. Using data from 43 species of passerine birds, an interspecific study examined the relative influence of daily ectoparasite-induced nestling mortality rates and of daily nest predation rates in determining the duration of nestling periods and of the timing of fledging in birds (Møller 2005). Daily ectoparasite-induced nestling mortality rates were strongly negatively correlated with the length of nestling periods, and hence, the nestlings of those species that suffered high daily ectoparasite-induced nestling mortality rates fledged sooner than nestlings of those species that suffered lower levels of daily ectoparasite-induced nestling mortality rates (Møller 2005). This interspecific trend has also received empirical support from intraspecific studies, whereby ectoparasites induced shorter nestling periods and earlier fledging (eg Badyaev et al. 2006). These studies suggest that ectoparasite-induced nestling mortality is an important determinant of nestling periods and fledging times in birds. Consequently, further studies are required to elucidate the relative importance of the risk of predation, the availability of food and ectoparasite-induced nestling mortality in determining the transition to independence in birds.
Intraspecific variation in the transition to independence
Our understanding of intraspecific variation in the acquisition of independence has lagged behind other life history stages due to the logistical challenges associated with tracking individuals. In passerine birds, for example, it has been tough to track individuals as they leave the nest and then during the post-fledging period when they remain at least partially dependent on their parents for food and protection from predators. Illustratively, the earliest studies to quantify the process of fledging in birds saw researchers sitting in hides close to nests (Lemel 1989) whilst later studies used continuous videotaping to capture the process (Johnson et al. 2004). However, advances in remote technologies mean that recent studies have used passive integrated transponder (PIT) tags to study small passerine birds leaving their nests (Schlicht et al. 2012; Johnson et al. 2013; Radersma et al. 2015), radio transmitters to track the movements of passerines, parrots, raptors and game birds during the post-fledging period on land (Alonso et al. 1987; Naef-Daenzer et al. 2001; Salinas-Melgoza and Renton 2007; Gow and Wiebe 2013, 2014; Blomberg et al. 2015) and satellite transmitters to track the movements of seabirds during the post-fledging period at sea (Bentzen and Powell 2015). Such technological advances have increased our understanding of the transition to independence and in this section, I will examine both between- and within-brood variation in the process by which offspring acquire independence. First, I will begin by examining the relative influence of the risk of predation, ectoparasite abundance and parent-offspring conflict over the provision of care in determining between-brood variation in the acquisition of independence.
Between broods: the risk of predation
The risk of predation varies between nests and hence, varies between broods raised in different nests (Mainwaring et al. 2015). In seasonal environments in the northern hemisphere, the timing of reproduction is important because predatory birds often time their reproductive attempts to coincide with the peak availability of naïve fledglings with limited flight abilities and anti-predator defences, as exemplified by sparrowhawks (Accipiter nisus) preying upon naïve blue tit (Cyanistes caeruleus) and great tit (Parus major) fledglings. Those tit broods that fledged early and hence, before the peak of sparrowhawk reproduction, suffered lower mortality rates than later fledged broods as they were able to independently forage in more concealed locations in the woodland canopy (Geer 1982; Götmark 2002).
Offspring raised in nests that are at a greater risk of predation are expected to leave the nest comparatively earlier than conspecifics raised in nests that are at a lower risk of predation (Caro 2005). Empirical studies support this expectation and, for example, when experimental great tit broods were exposed to higher perceived levels of predation risk, nestlings in those broods grew their feathers faster and fledged earlier than nestlings in control broods where the perceived risk of predation remained unchanged (Coslovsky and Richner 2011). Meanwhile, colonially breeding pied babblers (Turdoides bicolor) breed in colonies of varying sizes, with larger colonies having more adults to both spot approaching predators and defend the nestlings from those predators. Accordingly, nestlings raised by solitary pairs in isolation from other conspecifics fledged earlier than nestlings raised in larger colonies where the higher number of adults meant that the risk of predation was lower (Raihani and Ridley 2007). More broadly, the nestlings of altricial species usually fledge within six hours of sunrise and a study of 17 species showed that nestlings in nests with a greater risk of predation fledged earlier in the day than nestlings in comparatively safer nests, presumably to decrease their chances of being predated whilst in the nest (Chiavacci et al. 2015). This has also received empirical support from an intraspecific study in which it was shown that those individuals that fledge early in the day subsequently have a higher probability of recruitment into the breeding population (Radersma et al. 2015).
The conditions in which nestlings are raised can also affect the risk of predation for the nestlings during the subsequent post-fledging period. In seasonally breeding passerine birds in temperate environments, earlier-breeding pairs generally match their timing of reproduction with their insectivorous food supply much more closely than later breeding pairs, meaning that those nestlings born earlier in the season generally receive more food and are usually heavier than conspecifics born later in the season (Naef-Daenzer et al. 2001). In a study of seasonally breeding great tits and coal tits (Parus ater), 47% of fledglings died during the post-fledging period, with predation being the main cause of mortality. However, the survival of individual fledglings was not random and instead, was positively correlated with their mass at fledging. Thus, as the mass of fledglings declined as the breeding season progressed because later breeding pairs poorly matched the timing of reproduction with their insectivorous food supply, then the mortality rate of fledglings was five times higher for those individuals fledgling later in the season than for those individuals fledging earlier in the season (Naef-Daenzer et al. 2001). Together, these studies demonstrate that offspring raised in nests with higher levels of predation risk do adaptively leave the sooner than conspecifics raised in nests with lower levels of predation risk.
The avoidance of predators is a ubiquitous challenge for birds, yet that challenge is particularly acute during the transition from dependence to independence when their anti-predatory responses are not fully developed. During this time, birds are often reliant on the alarm calls of either conspecifics or heterospecifics and a series of studies (Magrath et al. 2006; Haff and Magrath 2012, 2013) involving white-browed scrubwrens (Sericornis frontalis) have shown just how finely tuned individuals are to such alarm calls. Whilst in the nest, nestling scrubwrens ceased calling when the alarm calls of the adults of three heterospecifics were transmitted close to nests (Haff and Magrath 2012) but they also quickly adapted to respond to new challenges once they had left the nest. A playback experiment showed that whilst nestling scrubwrens continued to call after a parents’ aerial alarm call, recently fledged scrubwrens outside of nest immediately ceased calling and fell silent. This is important as whilst aerial predators such as hawks pose no threat to nestlings which remain concealed within nests, they pose a big threat once the nestlings fledge and are exploring the environment outside of the nest (Magrath et al. 2006). Further, fledgling scrubwrens also learn to respond to the alarm calls of heterospecifics who have similar predators to them. Shortly after fledging, the young scrubwrens responded strongly to conspecific alarm calls but weakly to the alarm calls of superb fairy-wrens (Malarus cyaneus) and new holland honeyeaters (Phylidonyris novaehollandiae) but only two weeks later, most fledglings responded to the alarm calls of the fairy-wrens and the honeyeaters. Fascinatingly, fledgling scrubwrens in territories without honeyeaters ignored honeyeater alarm calls and yet three weeks later, they did respond to their calls (Haff and Magrath 2013) and so together, these studies demonstrate that birds rapidly acquire sophisticated anti-predatory behaviours and that such behaviours can vary over remarkably short temporal and spatial scales.
Theory suggests that the exact time at which passerine birds leave the nest represents a trade-off between the risk of mortality within and outside of the nest (Roff et al. 2005). This concept has received empirical support from a study of great tits in which parents produce acoustically distinctive alarm calls for the two main predators of their offspring: jungle crows (Corvus macrorhynchos) which snatch nestlings from the entrance of nest holes and Japanese rat snakes (Elaphe climacophora) which invade nest holes. Great tit nestlings crouched down inside their nest holes when they heard alarm calls given for crows, whilst they fled from the nest holes in response to alarm calls given for snakes (Suzuki 2011). This elegant experiment demonstrates that parents can help nestlings selectively evade ecologically different predators and more broadly, shows that prey species develop sophisticated anti-predator behaviours around the timing of fledging.
Between broods: ectoparasite-induced nestling mortality
Ectoparasites often have detrimental effects on the development of nestling birds (e.g. Møller 1990; Oppliger et al. 1994), and consequently, nestlings raised in nests containing high numbers of ectoparasites are expected to grow faster and fledge quicker than conspecifics raised in nests containing comparatively fewer ectoparasites. In a study of house finches (Haemorhous mexicanus), preliminary observations suggested that male nestlings were affected much more severely by ectoparasites than their female siblings (Badyaev et al. 2006). Thus, when the abundance of ectoparasites was increased at experimental nests, females reduced their sons’ exposure to the detrimental ectoparasites by laying male nestlings in earlier eggs and female nestlings in later eggs so that the male nestlings in experimental nests were able to fledge earlier than males in control nests (Badyaev et al. 2006). Meanwhile, when the abundance of ectoparasites was increased in experimental barn swallow nests, the nestlings in those nests grew their wing feathers faster so that they could fledge sooner than nestlings in control nests where the abundance of ectoparasites remained unchanged (Saino et al. 1998).
Intriguingly, the study by Saino et al. (1998) suggested a possible trade-off between body mass and immunity, as nestlings in experimental nests with increased numbers of ectoparasites developed stronger immune systems and yet were lighter than nestlings in control nests. A trade-off between body mass and immunity has been experimentally demonstrated in Eurasian magpies (Pica pica) (Soler et al. 2003) which suggested that whilst nestlings may preferentially allocate resources towards those aspects of growth that facilitate adaptive fledging times in the short term (Mainwaring and Hartley 2012), they may suffer in the longer term. This is because their reduced body mass may lower their chances of surviving the subsequent winter months when body mass is often positively correlated with the survival rates of individual birds (Naef-Daenzer et al. 2001). Further studies that examine the fitness consequences of such temporal shifts in the selection pressures determining growth patterns could prove fruitful. In summary, the limited number of empirical studies’ performed to date have supported the expectation that nestlings raised in nests containing high numbers of ectoparasites develop their wings faster and leave the nest sooner than conspecifics raised in nests containing comparatively fewer ectoparasites.
Between broods: parent-offspring conflict over the provision of care
In those species where parents provide their offspring with food after hatching, the offspring must at some point during their development undergo a transition from nutritional dependency to independence. Parent-offspring conflict theory predicts that the optimal timing of that transition differs between the two generations, with offspring preferring it to occur at a later stage of their development than the parents would prefer (Trivers 1974). The actual timing of the transition reflects the outcome of the ways in which both generations attempt to resolve this evolutionary conflict of interest. Offspring attempt to maximise the period of dependency through a range of vocal and colourful begging behaviours, whilst parents can minimise the period of dependency by either reducing their responsiveness to offspring begging behaviours or by ceasing to provision their offspring (Trivers 1974; Hussell 1988; Nilsson and Svensson 1993).
Avian offspring grow rapidly and in fact offspring mass usually exceeds that of their parents towards the end of the growth period but sometimes declines just before fledging (Ricklefs 1968a, b). In passerine birds, parents reduce their provisioning rates close to fledging in order to induce the offspring to leave the nest (Hiraldo et al. 1989; Ceballos and Donazar 1990; Bustamante and Negro 1994). However, they continue to provision their offspring for the first few days after they have left the nest at the same rate as they fed them in the nest (Kopachena and Falls 1993). Later, nestlings gradually cease begging for food and actively begin searching for food themselves as their hunting skills improve and the profitability of the later exceeds the profitability of the former (Davies 1976, 1978). Similarly to passerines, raptor parents reduce their provisioning rates close to fledging to encourage the offspring to leave the nest. Then, immediately after fledging, the offspring are unable to hunt for themselves and so the parents catch all of their food for them but the parents gradually reduce their provisioning rates as the ability of the offspring to hunt for themselves increases (Newton and Moss 1984; Johnson 1986; Konrad and Gilmer 1986; Alonso et al. 1987; Hiraldo et al. 1989; Bustamante and Hiraldo 1989a, 1989b, 1990a, 1990b; Ceballos and Donazar 1990; Bustamante 1993, 1994; Bustamante and Negro 1994; Weston et al. 2013).
Seabirds differ from both passerines and raptors in that their offspring undergo a period of significant mass reduction just before they leave the nest (Gaston 1997; Morbey et al. 1999; Hipfner and Gaston 1999; Salihoglu et al. 2001; Quillfeldt et al. 2004; Catry et al. 2006; Corbel et al. 2009), with some nestlings even becoming anorexic during this time (Harris 1978; Kitaysky 1999). The cause of this phenomenon has been ascribed to parental desertion (Hipfner and Gaston 1999; Catry et al. 2006) although it could equally result from the voluntary limitation of feeding by the offspring (Harris 1978; Kitaysky 1999). Either way, the timing of fledging is particularly important for seabirds as the parents often travel huge distances from the nest site to forage and so can be away for several days at a time (Gaston 1997; Corbel et al. 2009). Consequently, the parents are not present to defend their offspring from predators for the majority of the developmental period and yet the offspring face a dramatic life history transition as they shift from a terrestrial nest site where they are fed by their parents to a highly pelagic environment where they must forage for themselves (Gaston 2004).
So, which generation exerts control over the timing of fledging? The answer to this question is an important one as fledging is the major step by which birds acquire independence from their parents. In order to decipher the relative contributions of the parents and the offspring in determining the timing of fledging, empirical studies have either directly or indirectly manipulated the availability of food. This has been achieved either by directly providing supplementary food to offspring or indirectly by manipulating brood sizes to alter the broods’ demand for food or cross-fostering offspring of different ages to examine the flexibility of the duration of the parental care period.
The provision of supplementary food should increase the period of time that offspring remain dependent on their parents, thereby delaying the timing of fledging and increasing the duration of the post-fledging period. In raptors, an observational study showed that the length of the post-fledging period was negatively related to the availability of food (Arroyo et al. 2002), and whilst one experimental study showed that supplementary food increased the duration of the post-fledging period (Kenward et al. 1993), another study found no effect (Bustamante 1994). Studies of seabirds also report mixed findings with studies showing that supplementary food delayed the time to fledging (Gjerdrum 2004), reduced the time to fledging (Harfenist 1995) and had no effect (Corbel et al. 2009). These contrasting results suggest that the availability of food is not the main determinant of fledging times in birds.
Studies that have indirectly manipulated the availability of food by manipulating brood sizes often report that offspring in larger broods that receive less food per offspring have lower body masses but longer wings (Nilsson and Gårdmark 2001; Miller 2010; but see Martins 1997). This pattern of differential growth is adaptive as it enables offspring in larger broods to leave the nest as soon as their rapidly growing wings allow them to do so and then forage for food themselves. In mourning doves (Zenaida macroura), for example, the allocation of resources towards wing growth amongst nestlings in larger broods was adaptive as although they fledged at later ages than nestlings from smaller broods owing to their slower overall growth, the preferential allocation of resources towards wing growth reduced this effect by an estimated 11 % (Miller 2010). The slower growth exhibited by nestlings in experimentally enlarged broods’ means that parents control the supply of food as they never responded to increased levels of brood demand. However, a study of cooperatively breeding pied babblers showed that fledglings could influence carer provisioning rates by positioning themselves in risky locations where they were vulnerable to predators when they were hungry. This induced higher carer provisioning rates until the fledglings were satiated, when they moved to safer locations (Thompson et al. 2013).
Studies have also examined the relative contribution of parents and offspring in determining the length of the parental care period by cross-fostering offspring of different ages to manipulate the period of offspring dependency. Studies show that whilst seabirds do not alter the parental care period (Gray and Hamer 2001; Catry et al. 2006; Riou et al. 2012), passerines show more flexibility and extend the period of care as required by the demands of their offspring (Ridley and Raihani 2007; Rehling et al. 2012; Soler et al. 2013). The inflexibility of seabirds and the flexibility of passerines in the period of parental care probably reflect the relative value of each breeding attempt to the parents. Seabirds have very long lifespans and so one reproductive event is of little value to parents, which means that they act as ‘prudent parents’ and ignore excessive offspring solicitation behaviours (Catry et al. 2006). Meanwhile, passerine birds have relatively short lifespans and so the offspring in one brood represent a much larger proportion of the parents’ lifetime reproductive output which means that parents should be more responsive to the needs of their offspring (Thorogood et al. 2011; Rehling et al. 2012). Together, the studies that have directly or indirectly manipulated the supply of food to offspring report contrasting findings, thereby suggesting that the availability of food is not the main determinant of the duration of the period of dependence in birds. Rather, it suggests that parents are able to control the provision of food to their offspring quite effectively and are only responsive to the needs of their offspring when it is advantageous for them to be so.
Many studies report that in those species with bi-parental care, the two parents care for a different subset of offspring during the post-fledging period (Moreno 1984; Edwards 1985; Price and Gibbs 1987; Byle 1990; Middleton et al. 2007; Vega et al. 2007; Gow and Wiebe 2013). It is presently unclear why this occurs but it has been suggested that by dividing the brood, parents care for fewer offspring and are therefore able to keep track of how much food they have provided for those offspring more precisely than would be the case if both parents were caring for the whole brood together (Edwards 1985; Vega et al. 2007). Whilst this further suggests that it is parents that control how much food the offspring receive, it is also clear that offspring are unable to leave the nest until they are physically capable of doing so (Carrier and Auriemma 1992; Michaud and Leonard 2000) and when their endocrine physiology permits (Schwabl 1999; González-Solís 2004; Groothuis et al. 2005).
The fledging process is often synonymous with, or immediately proceeded by, an increase in hormones such as corticosterone in hole nesting birds (Heath 1997; Schwabl 1999; Sims and Holberton 2000; Kern et al. 2001; Love et al. 2003; but see Romero et al. 2006) and in seabirds (Catry et al. 2006; Quillfeldt et al. 2007; Corbel and Groscolas 2008; Corbel et al. 2008). This suggests that offspring are only able to fledge when their endocrine physiology allows them to although such changes are synonymous with reductions in parentally provided food. One study disentangled these two factors by providing supplementary food to experimental laysan albatross (Phoebastria immutabilis) chicks for the month before fledging and found that experimental chicks had less pronounced pre-fledging mass recessions, a slower increase in corticosterone levels and remained at the colony longer after reaching a morphological threshold than unfed control chicks (Sprague and Breuner 2010). These studies suggest that although offspring are only able to leave the nest when they are physically capable of doing so and when their endocrine physiology permits, it is the parents who control the supply of food and ultimately determine when offspring fledge.
To summarise, the risk of predation and the abundance of ectoparasites within nests both influence fledging times with broods in nests at greater levels of predation and that contain more ectoparasites being more likely to fledge first. Also, the theory of parent-offspring conflict over the provision of care means that parents would prefer the offspring to acquire independence before the offspring would prefer to do so and empirical studies that have either directly or indirectly manipulated the supply of food to offspring have reported mixed results. This suggests that the availability of food is not the main determinant of fledging times or the duration of post-fledging care in birds. Rather, it suggests that parents are generally able to control the provision of food to their offspring quite effectively and that they are only responsive to the needs of their offspring when it is beneficial for them to be so.
Within-brood variation
Variation in the process by which individual offspring acquire independence can also occur within broods, as offspring raised in the same nest often differ substantially in size. Such variation exists because of differences in genotypes resulting from sexual size dimorphism (Kalmback and Benito 2007) or extra-pair parentage (Ferree et al. 2010) and/or because of differences in phenotypes resulting from parental effects such as hatching asynchrony (Glassey and Forbes 2002). Such within-brood variation in offspring sizes is generally exaggerated, but can also be mitigated, by offspring begging behaviours and parental provisioning rules (Draganoiu et al. 2005; Kilner and Hinde 2008; Mainwaring et al. 2011) and have important consequences for offspring throughout the nestling period and during fledging (Nilsson 1990).
Within broods, offspring may vary in size due to differences in genotypes resulting from extra-pair parentage or sexual size dimorphism, with the latter referring to sexual differences in body size (Kalmback and Benito 2007). Patterns of dimorphism range from both sexes being similar in passerines, males being larger than females in gamebirds and females being larger than males in raptors. Illustratively, female sparrowhawks are 60 % heavier than males and one study showed that males grew their wing feathers significantly faster than females (Moss 1979), probably to fledge simultaneously with their female siblings. However, that study did not quantify the fledging process due to the logistical problems of doing so, although a more recent study did by implanting transponders in 14-day-old great tit nestlings (Radersma et al. 2011). Male great tit nestlings are around 10 % heavier than females and although the sex ratios of broods were manipulated by swapping nestlings of both sexes when they were 6 days old, brood sex ratios had no effect on fledging times. Instead, nestlings with longer wings at day 14 fledged earlier than siblings with shorter wings (Radersma et al. 2011) and so there is currently no evidence that sexual size dimorphism influences variation in the fledging process in birds.
Meanwhile, molecular studies have revealed that far from having monogamous pair bonds (Lack 1968), birds routinely indulge in extra-pair matings (Griffith et al. 2002) which means that mixed paternity within broods is common. This is important as kin selection theory predicts that when offspring compete for parental care, individual offspring should compete more intensely for food against non-related brood members than with related brood members, as shown empirically in birds (Boncoraglio et al. 2009). Extra-pair offspring are usually larger than their within-pair siblings as a result of genetic influences via good genes or genetic compatibility effects (Griffith et al. 2002) and/or by hatching order effects that give them a head start (Magrath et al. 2009; Ferree et al. 2010). Either way, larger extra-pair offspring are expected to fledge before their within-pair siblings and a study of great tits showed that even after controlling for hatching order, male but not female extra-pair nestlings fledged earlier than their within-pair siblings (Schlicht et al. 2012). Thus, extra-pair paternity influences patterns of fledging, although more studies are required to fully understand how parentage influences the transition to independence in birds.
Phenotypic variation within broods arises primarily from nestling size asymmetries caused by hatching asynchrony (Glassey and Forbes 2002; Forbes and Wiebe 2010). Hatching asynchrony is common within birds although its evolution remains much debated as whilst adaptive hypotheses view the size hierarchies as a selected trait that allow for adaptive brood reduction when resources are limited (Lack 1947), non-adaptive hypotheses view the size hierarchies as a side effect of selection for other traits (Clark and Wilson 1981). Either way, hatching asynchrony is seemingly paradoxical as the size hierarchies result in asymmetric sibling competition and increased mortality rates amongst younger and smaller nestlings (Leonard and Horn 1996; Parker et al. 2002; reviewed by Stoleson and Beissinger 1995). The older and larger nestlings are expected to fledge before their younger and smaller siblings, and this idea has received empirical support (Lemel 1989; Nilsson 1990; Nilsson and Svensson 1993, 1996; Nilsson and Gårdmark 2001; Johnson et al. 2004, 2013; Radersma et al. 2011). However, a recent study of house wrens (Troglodytes aedon) showed that both within broods that hatched naturally asynchronously and in broods where the degree of hatching asynchrony was experimentally manipulated, that the older and larger nestlings delayed fledging until their younger and smaller siblings were ready to do so. This meant that the younger siblings continued to be fed in the nest and reached a suitable morphological threshold before fledging which benefited the younger siblings as it improved their survival and reproductive prospects but also benefitted the older siblings by increasing their inclusive fitness (Bowers et al. 2013). This demonstrates that hatching asynchrony has complex effects on the fledging process and that patterns of fledging within broods are far from well understood at present.
Conclusions and further work
Avian parents provide their developing offspring with resources such as food and protection from predators, but at some stage, the offspring must undergo a period of transition from dependence to independence. That period of transition is one of the most important stages of an individual’s life as their inexperience in simultaneously searching for food and avoiding predators’ means that the risks of mortality are higher than at any other stage of their life. Interspecific studies that have collated data from passerines have demonstrated that the offspring of species that suffer both high levels of nest predation and ectoparasite-induced nestling mortality leave the nest comparatively sooner than the offspring of species that suffer lower levels of nest predation and ectoparasite-induced nestling mortality.
Intraspecific studies, meanwhile, have become more prevalent in recent years as new technologies that allow individuals to be tracked as they leave the nest and during the post-fledging period have been used to show that between-brood variation in the timing of independence is influenced by predation risk, ectoparasite-induced nestling mortality and parent-offspring conflict over the provision of care. Further studies have shown that within-brood variation in the timing of fledging also occurs as a result of both genetic effects such as parentage and maternal effects such as hatching asynchrony that result in size inequalities between siblings.
Our understanding of the transition from dependence to independence in birds still remains poorly understood when compared to other life history stages and so I strongly urge further studies, and particularly experimental ones, to examine the causes and consequences of the fledging process and of the post-fledging period of care. However, there are six areas where further research may prove particularly fruitful. First, whilst we have a good understanding of the roles of nest predation and ectoparasite-induced nestling mortality in determining the duration of time which the offspring of passerines remain in the nest, we have only a poor understanding of such issues in non-passerine birds. Therefore, broader studies that incorporate data from non-passerines would be useful despite the logistical problems of collating data to complete such a task. Second, although we have a good understanding of the determinants of the duration of the nestling period across bird species, the consequences of such variation remains much less well understood and so studies that examine, for example, possible trade-offs between the length of time spent developing in nests and the accomplishment of tasks once having left the nest could be extremely informative.
Third, intraspecific studies have demonstrated that the presence of ectoparasites within nests reduces the duration of the nestling period but studies could also usefully examine how the risk of interspecific brood parasitism by, for example, cuckoos or cowbirds influences the duration of the nestling period in those species where the hosts’ offspring are not evicted by the parasitic offspring. Fourth, the fledging process is often accompanied by, or immediately proceeded by, an increase in hormones and although this suggests that offspring are only able to fledge when their endocrine physiology allows them to, such hormonal changes are also accompanied by reductions in parental provisioning rates. Thus, further studies (but see Sprague and Breuner 2010) are required to develop our understanding of the relative contributions of increases in hormones and reductions in parental provisioning in determining the timing of fledging in birds. Fifth, studies that examine the consequences of variation in the age of the acquisition of independence may well be useful as, for example, it would be interesting to examine the fitness costs and benefits of variable fledging times and post-fledging periods. Illustratively, it may well be that individuals with longer periods of parental care have greater reproductive success or cognitive abilities during adulthood and such issues could elegantly be examined by experimental studies that either increase or decrease the period of dependence upon adults for care. Sixth, our understanding of the determinants of within-brood variation in the acquisition of independence is poor and more studies that experimentally examine the influence of both genetic effects such as parentage and sex and maternal effects such as hatching asynchrony on the fledging process could be enlightening.
References
Alonso JC, Gonzáleza LM, Heredia B, González JL (1987) Parental care and the transition to independence of Spanish Imperial Eagles (Aquila heliaca) in Doñana National Park, southwest Spain. Ibis 129:212–224
Arendt JD (1997) Adaptive intrinsic growth rates: an integration across taxa. Q Rev Biol 72:150–177
Arroyo BE, De Cornulier T, Bretagnolle V (2002) Parental investment and parent-offspring conflicts during the post-fledging period in Montagu’s harrier. Anim Behav 63:235–244
Badyaev AV, Hamstra T, Oh KP, Acevedo Seaman D (2006) Sex-biased maternal effects reduce ectoparasite-induced mortality in a passerine bird. Proc Natl Acad Sci U S A 103:14406–14411
Bennett PM, Owens IPF (2002) Evolutionary ecology of birds: life histories, mating systems and extinction. Oxford University Press, Oxford
Bentzen RL, Powell AN (2015) Dispersal, movements and site fidelity of post-fledging King Eiders Somateria spectabilis and their attendant females. Ibis 157:133–146
Blomberg EJ, Sedinger JS, Gibson D, Coates PS, Casazza ML (2015) Carryover effects and climatic conditions influence the postfledging survival of greater sage-grouse. Ecol Evol 4:4488–4499
Boncoraglio G, Caprioli M, Saino N (2009) Fine-tuned modulation of competitive ability according to kinship in barn swallow nestlings. Proc R Soc Lond B 276:2117–2123
Bowers EK, Sakaluk SK, Thompson CF (2013) Sibling cooperation influences the age of nest leaving in an altricial bird. Am Nat 181:775–786
Bustamante J (1993) Post-fledging dependence period and development of flight and hunting behaviour in the Red Kite Milvus milvus. Bird Study 40:181–188
Bustamante J (1994) Family break-up in black and red kites Milvus migrans and M. milvus: is time of independence an offspring decision? Ibis 136:176–184
Bustamante J, Hiraldo F (1989a) Post-fledging dependence period and maturation of flight skills in the Black Kite Milvus migrans. Bird Study 36:199–204
Bustamante J, Hiraldo F (1989b) The function of aggressive chases by breeding Black and Red Kites Milvus migrans and M. milvus during the post-fledging dependence period. Ibis 135:139–147
Bustamante J, Hiraldo F (1990a) Parental care and the transition to independence of Spanish Imperial Eagles Aquila heliaca in Doñana National Park. Ibis 132:58–67
Bustamante J, Hiraldo F (1990b) Factors influencing family rupture and parent-offspring conflict in the Black Kite Milvus migrans. Ibis 132:58–67
Bustamante J, Negro JJ (1994) The post-fledging-dependence period of the lesser kestrel (Falco neumanni) in southwestern Spain. J Raptor Res 28:158–163
Byle PAF (1990) Brood division and parental care in the period between fledging and independence in the dunnock (Prunella modularis). Behaviour 113:1–20
Caro T (2005) Antipredator defences in birds and mammals. Chicago University Press, Chicago
Carrier DR, Auriemma J (1992) A developmental constraint on the fledging time of birds. Biol J Linn Soc 47:61–77
Catry P, Phillips RA, Forcada J, Croxall JP (2006) Factors affecting the solution of parental dilemma in albatrosses; at what age should chicks be left unattended? Anim Behav 72:383–391
Ceballos O, Donazar JA (1990) Parent-offspring conflict during the post-fledging period in the Egyptian vulture Neophron percnopterus (Aves, Accipitridae). Ethology 85:225–235
Chiavacci SJ, Ward MP, Benson TJ (2015) Why fledge early in the day? Examining the role of predation risk in explaining fledging behaviour. Behav Ecol 26:593–600
Clark AB, Wilson DS (1981) Avian breeding adaptations: hatching asynchrony, brood reduction, and nest failure. Q Rev Biol 56:253–277
Clutton-Brock TH (1991) The evolution of parental care. Princeton University Press, Princeton
Corbel H, Groscolas R (2008) A role for cortocosterone and food restriction in the fledging of nestling white storks. Horm Behav 53:557–566
Corbel H, Morlon S, Groscolas R (2008) Is fledging in king penguin chicks related to changes in metabolic or endocrinal status? Gen Comp Endocrinol 155:804–813
Corbel H, Morlon S, Geiger S, Groscolas R (2009) State-dependent decisions during the fledging process of king penguin chicks. Anim Behav 78:829–838
Coslovsky M, Richner H (2011) Predation risk affects offspring growth via maternal effects. Funct Ecol 25:878–888
Cox WA, Thompson FR, Cox AS, Faaborg J (2014) Post-fledging survival in passerine birds and the value of post-fledging studies to conservation. J Wildl Manag 78:183–193
Davies NB (1976) Parental care and transition to independent feeding in the young spotted flycatcher (Muscicapa striata). Behaviour 59:280–295
Davies NB (1978) Parental meanness and offspring independence: an experiment with hand-reared great tits, Parus major. Ibis 120:509–514
Dmitriew CM (2011) The evolution of growth trajectories: what limits growth rate? Biol Rev 86:97–116
Draganoiu T, Nagle L, Musseau R, Kreutzerm M (2005) Parental care and brood division in a songbird, the black redstart. Behaviour 142:1495–1514
Edwards PJ (1985) Brood division and transition to independence in Blackbirds Turdus merula. Ibis 127:42–59
Ferree ED, Dickinson J, Rendell W, Stern W, Portere S (2010) Hatching order explains an extrapair chick advantage in western bluebirds. Behav Ecol 21:802–807
Forbes S, Wiebe M (2010) Egg size and asymmetric sibling rivalry in red-winged blackbirds. Oecologia 163:361–372
Gaston AJ (1997) Mass and date at departure affect the survival of ancient murrelet Synthliboramphus antiquus chicks after leaving the colony. Ibis 139:673–678
Gaston AJ (2004) Seabirds: a natural history. T & AD Poyser, London
Gebhardt-Henrich S, Richner H (1998) Causes of growth variation and its consequences for fitness. In: Starck JM, Ricklefs RE (eds) Avian growth and development: evolution within the altricial-precocial spectrum. Oxford University Press, Oxford, pp 324–339
Geer TA (1982) The selection of tits Parus spp. by sparrowhawks Accipiter nisus. Ibis 124:159–167
Ghalambor CK, Martin TE (2001) Fecundity-survival trade-offs and parental risk-taking in birds. Science 292:494–497
Gjerdrum C (2004) Parental provisioning and nestling departure decisions: a supplementary feeding experiment in tufted puffins (Fratercula cirrhata) on Triangle Island, British Columbia. Auk 121:463–472
Glassey B, Forbes S (2002) Begging and asymmetric nestling competition. In: Wright J, Leonard ML (eds) The evolution of begging: competition, cooperation and communication. Kluwer Academic Publishers, Dordrecht, pp 269–281
González-Mariscal G, Caba M, Martínez-Gómez M, Bautista A, Hudson R (2016) Mothers and offspring: the rabbit as a model system in the study of mammalian maternal behaviour and sibling interactions. Horm Behav 77:30–41
González-Solís J (2004) The regulation of incubation shifts near hatching: a timed mechanism, embryonic signalling or food availability? Anim Behav 67:663–671
Götmark F (2002) Predation by sparrowhawks favours early breeding and small broods in great tits. Oecologia 130:25–32
Gow EA, Wiebe KL (2013) Survival and habitat selection by fledgling Northern Flickers in a fragmented forest landscape. J Wildl Manag 78:273–281
Gow EA, Wiebe KL (2014) Determinants of parental care and offspring survival during the post-fledging period: males care more in a species with partially reversed sex roles. Oecologia 175:95–104
Gray CM, Hamer KC (2001) Prefledging mass recession in Manx shearwaters: parental desertion or nestling anorexia? Anim Behav 62:705–709
Griffith SC, Owens IPF, Thuman KA (2002) Extra pair paternity in birds: a review of interspecific variation and adaptive function. Mol Ecol 11:2195–2212
Groothuis TGG, Müller W, von Engelhardt N, Carare C, Eising C (2005) Maternal hormones as a tool to adjust offspring phenotype in avian species. Neurosci Biobehav Rev 29:329–352
Haff TM, Magrath RD (2012) Learning to listen? Nestling response to heterospecific alarm calls. Anim Behav 84:1401–1410
Haff TM, Magrath RD (2013) Eavesdropping on the neighbours: fledglings learn to respond to heterospecific alarm. Anim Behav 85:411–418
Harfenist A (1995) Effects of growth-rate variation on fledging of Rhinoceros Auklets Cerorhinca monocerata. Auk 112:60–66
Harris MP (1978) Supplementary feeding of young puffins, Fratercula arctica. J Anim Ecol 47:15–23
Harrison C (1975) A field guide to the nests, eggs and nestlings of British and European birds. Collins, London
Heath J (1997) Corticosterone levels during nest departure of juvenile American kestrels. Condor 99:806–811
Hipfner JM, Gaston AJ (1999) Timing of nest departure in the thick-billed murre and razorbill: tests of Ydenberg’s model. Ecology 80:587–596
Hiraldo F, Delibes M, Estrella RR (1989) Observations of a Zone-tailed Hawk family during the post fledging period. J Raptor Res 23:103–106
Hudson R, Trillmich F (2008) Sibling competition and cooperation in mammals: challenges, developments and prospects. Behav Ecol Sociobiol 62:299–307
Hussell DJT (1988) Supply and demand in tree swallow broods: a model of parent-offspring food provisioning interactions in birds. Am Nat 131:103–106
Johnson SJ (1986) Development of hunting and self-sufficiency in juvenile Red-tailed Hawks (Buteo jamaicensis). Raptor Res 20:29–34
Johnson LS, Rauch RL, Dellone SN (2004) The process and causes of fledging in a cavity-nesting bird, the house wren (Troglodytes aedon). Ethology 110:693–705
Johnson LS, Hebert RM, Napolillo FM, Allen A (2013) The process of fledging in the Mountain Bluebird. J Field Ornithol 84:367–376
Kalmback E, Benito MM (2007) Sexual size dimorphism and offspring vulnerability in birds. In: Fairburn DJ, Blanckenborn WU, Székely T (eds) Sex, size and gender roles: evolutionary studies of sexual size dimorphism. Oxford University Press, Oxford, pp 133–142
Kenward RE, Marcström V, Karlbom M (1993) Post-nestling behaviour in goshawks, Accipiter gentilis: 1. The causes of dispersal. Anim Behav 46:365–370
Kern M, Bacon D, Long D, Cowie RJ (2001) Possible roles for corticosterone and critical size in the fledging of nestling pied flycatchers. Physiol Biochem Zool 74:651–659
Kilner RM, Hinde CA (2008) Information warfare and parent-offspring conflict. Adv Study Behav 38:283–336
Kitaysky AS (1999) Metabolic and developmental responses of alcid chicks to experimental variation in food intake: functional significance of juvenile traits in varying environments. Physiol Biochem Zool 72:462–473
Konrad PM, Gilmer DS (1986) Post-fledging behaviour of Ferruginous Hawks in North Dakota. Raptor Res 20:35–39
Kopachena JG, Falls JB (1993) Postfledging parental care in the white-throated sparrow (Zonotrichia albicollis). Can J Zool 71:227–232
Lack D (1947) The significance of clutch size. Ibis 89:302–352
Lack D (1968) Ecological adaptations for breeding in birds. Methuen and Co, London
Langen TA (2000) Prolonged offspring independence and cooperative breeding in birds. Behav Ecol 11:367–377
Lee PC, Majluf P, Gordon IJ (1991) Growth, weaning and maternal investment from a comparative perspective. J Zool 225:99–114
Lemel J (1989) Body-mass dependent fledging order in the great tit. Auk 106:490–492
Leonard M, Horn A (1996) Provisioning rules in tree swallows. Behav Ecol Sociobiol 38:341–347
Lima SL (2009) Predators and the breeding bird: behavioural and reproductive flexibility under the risk of predation. Biol Rev 84:485–513
Love OP, Bird DM, Shutt LJ (2003) Plasma corticosterone in American kestrel siblings: effects of age, hatching order, and hatching asynchrony. Horm Behav 43:480–488
Magrath RD, Platzen D, Kondo J (2006) From nestling calls to fledgling silence: adaptive timing of change in response to aerial alarm calls. Proc R Soc Lond B 273:2335–2341
Magrath MJL, Vedder O, van der Velde M, Komdeur J (2009) Maternal effects contribute to the superior performance of extra-pair offspring. Curr Biol 19:792–797
Mainwaring MC, Hartley IR (2012) Causes and consequences of differential growth in birds: a behavioral perspective. Adv Study Behav 44:225–277
Mainwaring MC, Lucy D, Hartley IR (2011) Parentally biased favouritism in relation to offspring sex in zebra finches. Behav Ecol Sociobiol 65:2261–2268
Mainwaring MC, Reynolds SJ, Weidinger K (2015) The influence of predation on the location and design of nests. In: Deeming DC, Reynolds SJ (eds) Nests, eggs, and incubation: new ideas about avian reproduction. Oxford University Press, Oxford, pp 50–64
Martin TE, Briskie JV (2009) Predation on dependent offspring: a review of the consequences for mean expression and phenotypic plasticity in avian life history traits. Ann NY Acad Sci 1168:201–217
Martin TE, Lloyd P, Bosque C, Barton DC, Biancucci AL, Cheng Y-R, Ton R (2011) Growth rate variation among passerine species in tropical and temperate sites: an antagonistic interaction between parental food provisioning and nest predation risk. Evolution 65:1607–1622
Martins TLF (1997) Fledging in the common swift, Apus apus: weight watching with a difference. Anim Behav 54:99–108
Michaud T, Leonard M (2000) The role of development, parental behaviour, and nestmate competition in fledging of nestling tree swallows. Auk 117:996–1002
Middleton HA, Green DJ, Krebs EA (2007) Fledgling begging and parental responsiveness in American dippers (Cinclus mexicanus). Behaviour 144:485–501
Miller DA (2010) Morphological plasticity reduces the effect of poor developmental conditions on fledging age in mourning doves. Proc R Soc Lond B 277:1659–1665
Møller AP (1990) Effects of parasitism by a haematophagous mite on reproduction in the barn swallow. Ecology 71:2345–2357
Møller AP (2005) Parasites, predators and the duration of developmental periods. Oikos 111:291–301
Morbey YE, Ydenberg RC, Knechtel HA, Harfenist A (1999) Parental provisioning, nestling departure decisions and prefledging mass recession in Cassin’s auklets. Anim Behav 57:873–881
Moreno J (1984) Parental care of fledged young, division of labour, and the development of foraging techniques in the Northern Wheatear (Oenanthe oenanthe L). Auk 101:741–752
Moss D (1979) Growth of nestling sparrowhawks (Accipiter nisus). J Zool 187:297–314
Naef-Daenzer B, Widmer F, Nuber M (2001) Differential post-fledging survival of great and coal tits in relation to their condition and fledging date. J Anim Ecol 70:730–738
Newton I, Moss D (1984) Post-fledging survival of Sparrowhawks (Accipiter nisus) in relation to mass, brood size and brood composition at fledging. Ibis 128:73–80
Nilsson J-Å (1990) What determines the timing and order of nest-leaving in the marsh tit (Parus palustris)? In: Blondel J, Gosler A, Lebreton J-D, McCleery R (eds) Population biology of passerine birds. Springer, Berlin, pp 369–380
Nilsson J-Å, Gårdmark A (2001) Sibling competition affects individual growth strategies in marsh tit, Parus palustris, nestlings. Anim Behav 61:357–365
Nilsson J-Å, Svensson M (1993) Fledging in altricial birds; parental manipulation or sibling competition? Anim Behav 46:379–386
Nilsson J-Å, Svensson M (1996) Sibling competition affects nestling growth strategies in marsh tits. J Anim Ecol 65:825–836
O’Connor RJ (1984) The growth and development of birds. John Wiley and Sons, New York
Oppliger A, Richner H, Christe P (1994) Effect of an ectoparasite on lay date, nest-site choice, desertion, and hatching success in the Great Tit (Parus major). Behav Ecol 5:130–134
Parker GA, Royle NJ, Hartley IR (2002) Intra-familial conflict and parental investment: a synthesis. Phil Trans R Soc B 375:295–307
Price TD, Gibbs HL (1987) Brood division in Darwin’s ground finches. Anim Behav 35:299–301
Quillfeldt P, Masello JF, Hamer KC (2004) Sex differences in provisioning rules and honest signalling of need in Manx shearwaters Puffinus puffinus. Anim Behav 68:613–620
Quillfeldt P, Poisbleau M, Chastel O, Masello JF (2007) Corticosterone in thin-billed prion Pachyptila belcheri chicks: diel rhythm, timing of fledging and nutritional stress. Naturwissenschaften 94:919–925
Radersma R, Tinbergen JM, Komdeur J (2011) Do brood sex ratio, nestling development and sex affect fledging timing and order? An experimental study on great tits. Anim Behav 81:69–75
Radersma R, Komdeur J, Tinbergen JM (2015) Early morning fledging improves recruitment in Great Tits Parus major. Ibis 157:351–355
Raihani NJ, Ridley AR (2007) Variable fledging age according to group size: trade-offs in a cooperatively breeding bird. Biol Lett 3:624–627
Rehling A, Trillmich F (2007) Weaning in the guinea pig (Cavia aperea f. porcellus): who decides and by what measure? Behav Ecol Sociobiol 62:149–157
Rehling A, Spiller I, Krause ET, Nager RG, Monaghan P, Trillmich F (2012) Flexibility in the duration of parental care: zebra finch parents respond to offspring needs. Anim Behav 83:35–39
Remeš V (2007) Avian growth and development rates and age-specific mortality: the roles of nest predation and adult mortality. J Evol Biol 20:320–325
Remeš V, Martin TE (2002) Environmental influences on the evolution of growth and developmental rates in passerines. Evolution 56:2505–2518
Ricklefs RE (1968a) Patterns of growth in birds. Ibis 110:320–325
Ricklefs RE (1968b) Weight recession in nestling birds. Auk 85:30–35
Ricklefs RE (1969) Preliminary models for growth rates in altricial birds. Ecology 50:1031–1039
Ricklefs RE, Starck JM, Konarzewski M (1998) Internal constraints on growth rates in birds. In: Starck JM, Ricklefs RE (eds) Avian growth and development: evolution within the altricial-precocial spectrum. Oxford University Press, Oxford, pp 266–287
Ridley AR, Raihani NJ (2007) Variable post-fledging care in a cooperative bird: causes and consequences. Behav Ecol 18:994–1000
Riou S, Chastel O, Hamer KC (2012) Parent-offspring conflict during the transition to independence in a pelagic seabird. Behav Ecol 23:1102–1107
Roff DA (1992) The evolution of life histories. Chapman and Hall, London
Roff DA, Remeš V, Martin TE (2005) The evolution of fledging age in songbirds. J Evol Biol 18:1425–1433
Romero LM, Holt DW, Maples M, Wingfield JC (2006) Corticosterone is not correlated with nest departure in snowy owl chicks (Nyctea scandiaca). Gen Comp Endocrinol 149:119–123
Saino N, Calza S, Møller AP (1998) Effects of a dipteran ectoparasite on immune response and growth trade-offs in barn swallow (Hirundo rustica) nestlings. Oikos 81:217–228
Salihoglu B, Fraser WR, Hofmann EE (2001) Factors affecting fledging weight of Adélie penguin (Pygoscelis adeliae) chicks: a modelling study. Polar Biol 18:1425–1433
Salinas-Melgoza A, Renton K (2007) Post-fledging survival and development of juvenile lilac-crowned parrots. J Wildl Manag 71:43–50
Samuel R, Olivier C, Hamer KC (2012) Parent-offspring conflict during the transition to independence in a pelagic seabird. Behav Ecol 23:1102–1107
Scheuerlein A, Gwinner E (2006) Reduced nestling growth of East African Stonechats (Saxicola torquata axillaris) in the presence of a predator. Ibis 148:468–476
Schlicht L, Girg A, Loës P, Valcu M, Kempenaers B (2012) Male extrapair nestlings fledge first. Anim Behav 83:1335–1343
Schwabl H (1999) Developmental changes and among-sibling variation of corticosterone levels in an altricial avian species. Gen Comp Endocrinol 116:403–408
Sims CG, Holberton RL (2000) Development of the corticosterone stress response in young Northern Mockingbirds (Mimus polyglottos) in the presence of a predator. Gen Comp Endocrinol 119:193–201
Soler JJ, de Neve L, Pérez-Contreras T, Soler M (2003) Trade-off between immunocompetence and growth in magpies: an experimental study. Proc R Soc Lond B 270:241–248
Soler M, Pérez-Contreras T, de Neve L (2013) Magpies do not desert after prolonging the parental care period: an experimental study. Behav Ecol 24:1292–1298
Sprague RS, Breuner CW (2010) Timing of fledging is influenced by glucocorticoid physiology in Laysan albatross chicks. Horm Behav 58:297–305
Stearns SC (1992) The evolution of life histories. Oxford University Press, Oxford
Stoleson SH, Beissinger SR (1995) Hatching asynchrony and the onset of incubation in birds, revisited: when is the critical period? Curr Ornithol 12:191–270
Suzuki TN (2011) Parental alarm calls warn nestlings about different predatory threats. Curr Biol 21:R15–R16
Thompson AM, Raihani NJ, Hockey PAR, Britton A, Finch FM, Ridley AR (2013) The influence of fledging location on adult provisioning: a test of the blackmail hypothesis. Proc R Soc B 280:20130558
Thorogood R, Ewen JG, Kilner RM (2011) Sense and sensitivity: responsiveness to offspring signals with the parents’ potential to breed again. Proc R Soc Lond B 2780:2638–2645
Trivers RL (1974) Parent-offspring conflict. Am Zool 14:249–264
Vega LB, Holloway GJ, Millet JE, Richardson DS (2007) Extreme gender-based post-fledging brood division in the toc-toc. Behav Ecol 18:730–735
Weston ED, Whitfield D, Travis JMJ, Lambin X (2013) When do young birds disperse? Tests from studies of golden eagles in Scotland. BMC Ecol 13:42
Acknowledgments
I thank Ian Hartley, Jenni Taylor and Lydia Atkinson for useful discussions and both Peter Kappeler and two anonymous reviewers for providing useful comments that improved this manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
No animals were used in this study and so no ethical approval was necessary and no informed consent was required.
Conflict of interest
The author declares that they have no conflicts of interest.
Funding
This study received no outside funding.
Additional information
Communicated by P. M. Kappeler
Rights and permissions
About this article
Cite this article
Mainwaring, M.C. The transition from dependence to independence in birds. Behav Ecol Sociobiol 70, 1419–1431 (2016). https://doi.org/10.1007/s00265-016-2186-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00265-016-2186-z