Abstract
Sexual conflict is magnified during the post-fledging period of birds when the sexes face different trade-offs between continuing parental care or investing in self maintenance or other mating opportunities. Species with reversed sex roles provide a unique opportunity to study the relationship between mating systems and investment in parental care. Here, we provide the first detailed study of the length of care by males versus females (n = 24 pairs) during the post-fledging period, assessing factors that may promote care within and between the sexes. In the northern flicker Colaptes auratus, a species with partly reversed sex roles, males cared longer than females (average 16 versus 12 days, respectively). Overall, 36 % of females but no males deserted the brood prior to fledgling independence. Parents that provisioned nestlings at a high rate also spent more days feeding fledglings. Among males, age and nestling feeding rates were positively associated with the length of care. Among females, a low level of feather corticosterone (CORTf) was associated with a longer length of care. About 45 % of fledglings died within the first week, but fledglings with intermediate body mass had the highest survival suggesting stabilizing selection on mass. Fledgling survival was also higher in individuals with larger broods and lower levels of CORTf. We demonstrate that because females can be polyandrous they often desert the brood before males, and that the sexes respond to different cues relating to their energy balance when deciding the length of care given to their offspring.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Parental care promotes the survival and fitness of offspring but males and females often differ in their evolutionary interests surrounding reproduction which leads to sexual conflict (Chapman et al. 2003). Life history theory predicts that when biparental care is required (e.g. birds with altricial young) sexual conflict over brood desertion will be low (Olson et al. 2008). However, brood desertion may occur if an individual benefits more from additional reproductive opportunities than any loss of offspring from the current brood (Kokko and Jennions 2008; Olson et al. 2008). Among the key factors promoting desertion by one partner are low offspring quality (Erikstad et al. 1997), small brood size (Beissinger and Snyder 1987), low perceived paternity (reviewed in Olson et al. 2008), high probability of re-mating (Székely et al. 1999), and good body condition or intrinsic quality of the parent (Hõrak 2003). Furthermore, if an individual perceives that its mate has a good ability to care for the offspring, it may desert, leading to an evolutionary game of mutual assessment (Griggio et al. 2005). In some cases, mainly in species with precocial young, brood desertion is normal and one sex always wins (Székely et al. 2007). In other species, the sex that has the possibility of attracting additional mates may cease caring for offspring in order to pursue new partnerships.
In birds, offspring desertion can occur during the incubation stage (Székely et al. 2007), nestling rearing (Beissinger and Snyder 1987; Griggio et al. 2005) or immediately after fledging (Morton et al. 2010). Usually it is the male that deserts but in some species it may be either sex, or rarely the female (e.g. Eldegard and Sonerud 2009; Morton et al. 2010). Brood desertion may be more frequent than previously thought because most studies do not quantify care after the young leave the nest, due to the logistical difficulties in following families on the landscape. Factors that contribute to the length of care are important because the post-fledging period may be a time of high energy demands for the parents when each may face different trade-offs involving parental care, self maintenance, or other mating opportunities. Although parents may increase the survival of their young by continuing to provide care after they leave the nest (Grüebler and Naef-Daenzer 2010), extended parental care may negatively affect parental survival or delay moult or migration (Stutchbury et al. 2011). The later in the season the offspring fledge, the more severe the potential time and energy conflict for parents.
One way parents may reduce the amount of investment needed in the post-fledging period is to invest strongly at the nestling stage to fledge large and healthy offspring that may become independent sooner. If the body condition of nestlings at the time of fledging determines the length of subsequent investment, we predict that the amount of care given in the nestling period and the fledging mass of nestlings will be inversely correlated with care during the post-fledging period. Conversely, if parents differ in quality or the amount of resources, parents in good body condition may invest more both at the nestling and post-fledging stages (van Noordwijk and de Jong 1986). Other studies show that a parent may reduce parental care if it has a poor body condition (Angelier and Chastel 2009) so we predict that parents with high corticosterone (CORT) levels or relatively low mass will stop caring sooner. Environmental factors are unlikely to explain sex differences in care when both parents use the same habitat on the same home range and are equally susceptible to predation risk, weather events and food shortages.
Here, we study sexual conflict and brood desertion in a woodpecker, the northern flicker Colaptes auratus, a species with partly reversed sex roles in which males contribute more care than females (Wiebe 2005, 2008; Gow et al. 2013) and females may be polyandrous (Wiebe and Kempenaers 2009). This allows for a unique opportunity to study how mating systems and sex roles influence the amount of care. Furthermore, male flickers (Wiebe and Kempenaers 2009) and woodpeckers generally (Michalek and Winkler 2001) have a high assurance of paternity. These traits suggest that brood desertion by females would be favoured in woodpeckers and this was confirmed in mate-removal experiments in the northern flicker (Wiebe 2005). In the wild, 11 % of female lesser spotted woodpeckers Dendrocopos minor apparently stopped feeding nestlings but were later observed feeding fledglings (Wiktander et al. 2000) so it is uncertain whether brood desertion occurs in woodpeckers as a general pattern.
Woodpeckers may provide a model system to understand how brood desertion evolves in species with biparental care and we tested hypotheses relating the length of post-fledging care to (1) prior investment in the brood (nestling feeding rates), (2) adult body condition, (3) seasonal time of breeding, (4) parent age, and (5) sex. Because female flickers are usually single brooded (but see Gow and Wiebe 2012) they would be unlikely to re-pair and begin a second breeding attempt in the current year. However, females which abandon care early may gain a fitness advantage by scouting out future males for polyandry, or searching for additional nest cavities in which to lay parasitic eggs (Wiebe and Kempenaers 2009). The rate of juvenile survival may indicate for how long biparental care is beneficial for offspring and so we also modelled juvenile survival based on the covariates of fledgling mass, fledgling physiological condition (feather CORT; CORTf), brood size, and nestling sex.
Materials and methods
Study site and species
We studied northern flickers at Riske Creek, British Columbia, Canada (51°52′N, 122°21′W), from 2010 to 2012. Female flickers are facultatively polyandrous, where up to 5 % of females annually have two nests with two different males. In addition, intraspecific brood parasitism occurs in 17 % of broods and <1 % of young are extra-pair (Wiebe and Kempenaers 2009). Northern populations are migratory, imposing constraints on individuals to breed within a fixed time window. Incubation of clutches ranging from three to 13 eggs lasts about 12 days and the nestling period lasts from 25 to 28 days (Wiebe and Moore 2008). In this study, mean brood size at fledging was 5.9 ± 1.3 SD (range 3–8, n = 28 nests).
Radio-tag deployment
We accessed flickers by cutting small, replaceable doors in the tree trunk near the base of the nest cavity (Wiebe 2008). Adults were captured at the cavity by plugging the entrance and then flushing the bird into a net over the entrance. Captured adults were weighed, measured (length of wing, bill, tail, tarsus, 9th primary, and bill depth), aged up to 4 years based on moult (Pyle et al. 1997) and banded with a unique colour combination. We monitored parental movements during the post-fledgling period using radio-telemetry and 10-week, 1.5 g radio-tags (Holohil, Carp, ON) attached with cyanoacrylate glue to the central rectrices, of the male and female of a pair. We walked in on birds to sight them directly using TRX-2000S receivers (Wildlife Materials, Murphysboro, IL) with Yagi 3-element antennae. Signals could be detected up to 5 km from high points on the study site. In 2010, we radio-tracked 11 pairs during the post-fledging period and in 2011 and 2012 we followed eight and ten pairs, respectively. However, two females (one in 2012 and one in 2010) dropped their tail feathers and tag prior to their young fledging. We tracked only those pairs in which both the male and female were alive at the time their nestlings fledged (n = 27 females and 28 males). Because some adults moved off of the study site to private property or disappeared (i.e. may have died) we could confirm the number of days of care for 24 females and for 24 males.
Measurements on fledglings
Nestlings were weighed when 20 days old and colour banded with unique combinations and the wing chord was measured. The mass of flicker nestlings plateaus after 18 days old (Gow et al. 2013), and so we assumed mass at day 20 was a reliable indicator of condition at fledging, which occurred about 5 days later. To maintain statistical independence we chose randomly one fledgling per nest to receive a 0.5–0.6 g radio-tag which represented about 0.004 % of its body mass. Sample sizes of radio-tagged nestlings were 13 in 2011 and 25 in 2012. From these tagged nestlings we measured the amount of CORTf by cutting the tip of a secondary feather (S2 or S3). CORT is a glucocorticoid hormone that is secreted in high levels during periods of food limitation and CORTf is an integrative measure of hormone levels deposited in the feather over the period of its growth (Bortolotti et al. 2008). Therefore, the collected feather reflected CORT secreted during days 6–20 of the nestling period. We calculated nestling condition on day 20 by using the residual body mass versus wing chord and averaged the condition of nestlings in a brood to obtain brood condition, which was used in the analysis of length of care by the parents. Finally, we determined date of fledging by visually checking nests or radio signals every day after nestlings were 20 days old.
Previous studies have found that several factors increase fledgling survival including early date of fledging, high body mass (Naef-Daenzer et al. 2001), higher values of the ‘stress hormone’ CORT (Rivers et al. 2012), and having few siblings (Styrsky et al. 2005). The relationship between some of these variables and fledgling survival may not be linear but instead an intermediate value (e.g. of body mass or CORT level) may be optimal and so we entered quadratic terms into the statistical models.
Monitoring parental care
For a measure of parental effort prior to the post-fledging period, we quantified parental feeding rates (trips/h) at each nest during the nestling period at two stages: middle (nestlings 10–15 days old), and late (days 19–21). The feeding trips were videotaped with analog Sony Handycams placed about 5 m from the nest tree for 3- to 4-h sessions on days it was not raining. Preliminary analysis showed that estimates of feeding rates remained the same after 3 h of observation and did not vary throughout the day or with temperature (unpublished data). To see how long parents fed their young, we began radio-tracking each pair within two days of fledging and every second day thereafter. The tracking sessions lasted 1 h and occurred between 0700–1900 hours. Parental care was assumed to have ended after two consecutive sessions in which parents never fed fledglings. We defined a parent as abandoning the brood when it was seen alive for two sequential tracking sessions and never fed nestlings although its partner was feeding them. Our accuracy in determining when parents stopped feeding was strong because parents were observed not feeding fledglings in 3 % of 162 tracking sessions (males) and 6 % of 126 tracking sessions (females) but then feeding during the next tracking session. The radio-tracking and vocalizations given by young meant that we almost always detected feeding visits if they occurred during the session, but we were sometimes unable to see the duration of the food transfer or specific identify of the fledging being fed when tree branches blocked our view. Thus, assessing how much food each fledgling received was impossible.
The mobility of fledglings also made it difficult to record precisely the instantaneous locations of both fledglings and parents, and so we calculated the distance between parents and offspring based on four categories: <50 m, where parents were in visual contact with fledglings and could quickly react to predators by alarm calling or mobbing; 50–300 m, where parents could return to fledglings relatively quickly; 300–700 m, a typical foraging distance from the nest during the nestling period; and >700 m. We eliminated tracking sessions and times during a tracking session when the locations of the parents or fledglings were unknown (142 h) and thus report the proportion of time at each distance based on a total of 256 h of tracking (female 112 h, male 144 h). We assumed parents investing more in the protection of their fledglings stayed closer to them. We used a multivariate ANOVA to investigate factors that were associated with the proportion of time parents spent at the four distance categories from at least one of their fledglings as the dependent variable. For some analyses, we divided the tracking data into two time periods based on the age (and hence mobility) of the fledglings: early (1–7 days post-fledging) when fledglings were less mobile and typically did not follow parents and late (from 8 days to independence) when fledglings flew >100 m during tracking sessions, and often followed parents.
Body condition and CORTf analysis
We used two measures to assess physiological condition of adults trapped during the nestling period; CORTf and a body condition index calculated as the residual of body mass regressed against a multivariate measure of body size (see Wiebe 2008). We plucked a secondary (S2) feather when the adult was first captured during incubation. During recapture, 2–4 weeks later (nestlings were 14–18 days old), we re-weighed the adult and cut the regrown secondary.
For CORTf assays we followed Bortolotti et al. (2008) where CORT was extracted from feathers using a methanol-based technique. Samples were measured in two assays with an intra-assay coefficient of variation of 8.32 %, an inter-assay coefficient of variation of 14.1 %, and mean (±SD) limit of detection (ED80) of 10.99 ± 2.33 pg CORTf/assay tube. Data values are expressed as picograms CORT per millimetre of feather, which gives a valid estimate of CORTf per unit time of feather growth (Bortolotti et al. 2008). CORTf assays were performed at the University of Saskatchewan, Canada.
Statistical analysis
The tagged fledglings were tracked every other day, weather permitting, to determine whether they were alive or dead. If a fledgling’s signal disappeared from the study area and it was not re-sighted with the family group for two consecutive tracking sessions it was assumed dead. We estimated individual survival of 33 fledgling flickers using known-fate models in Program MARK (White and Burnham 1999). These models use the Kaplan–Meier product-limit estimator which is advantageous because it allows biological covariates to be evaluated and multiple models to be compared (White and Burnham 1999). We examined whether juvenile survival during the post-fledging dependency period varied over the first 16 days post-fledging by using the biologically meaningful covariates: year, fledging date, fledging mass, fledgling CORTf, brood size at fledging, and fledgling sex. We ranked models using Akaike’s information criterion corrected for small sample sizes (AICc). We considered all models with ΔAICc ≤2 to show substantial support, while ΔAICc ≤4 showed some support (Burnham and Anderson 2002). We considered parameter estimates with 95 % confidence intervals that overlapped zero to be insignificant. We used model averaged weights to identify the most important models and variables (Burnham and Anderson 2002). We increased the power of our analysis by using a base model with two time intervals. To assess model validity (all models with Δ < 2) we conducted a bootstrap test with 1,000 simulations (White et al. 2001).
We used linear mixed effects models (lme) to analyse influences on the length of parental care for males and females during the post-fledging period, with length of time each parent fed their fledglings as the dependent variable and year as a random factor. Because only a subset of birds were re-trapped, we ran one analysis with a reduced data set of the re-trapped parents which included the variables of CORTf and body condition during the nestling period and one analysis with the larger sample of parents without those variables. Because of a small sample size in the reduced dataset (n = 13 females, 12 males), we limited the number of independent variables to four: parent age, body condition, date of fledging and CORTf. To meet the assumptions of normality CORTf was log transformed. For the full data set the independent variables were: brood size at fledging, the condition of the brood at fledgling, parent age, feeding rate during the nestling period, and the date of fledging.
For the lme models, we confirmed model validity by using likelihood ratio tests to compare the fixed effects models to null models with only the random effects using ANOVA (Zuur et al. 2010). We present P-values estimated from Markov chain Monte Carlo methods with statistical significance set at α = 0.05. All other statistical analyses were run in R 2.14.2 (R Core Development Team 2012), were two-tailed, and met the assumptions of normality.
Results
Fledgling behaviour and survival
Flicker fledglings could fly when leaving the nest and one first flight from the cavity was about 200 m across an open field. During the first week after their young fledged, parents left them at a certain location (often clinging to the side of a tree) and periodically returned to feed them. To initiate feeding, parents gave a soft series of ‘wicka’ calls that signalled fledglings to move towards the parent to receive the regurgitated meal. Between 7 and 10 days post-fledging, offspring followed their parents to foraging sites and either foraged themselves or watched their parents. A few fledglings attempted to forage as soon as 2 days post-fledging, by hopping and pecking at the ground. Parents did not usually split the brood, but in three of 25 cases certain young were only seen with one of the parents.
Fledgling mortality was high within the first 8 days after fledging with 45 % (17/38) of radio-tagged fledglings dying and the daily survival calculated by program MARK was 0.866 ± 0.032. In comparison, daily survival was 0.984 ± 0.015 between 9 to 16 days post-fledging (Fig. 1). The location and nature of the fledgling remains, either in raptor nests or as plucked piles of feather and bones, suggested avian predators were responsible for most of the mortality. We observed an American kestrel Falco sparverius catch and consume a recently fledged flicker. One tag was located in a red-tailed hawk Buteo jamaicensis nest, and on several occasions Cooper’s hawks Accipiter cooperii chased the flicker fledglings. A chewed radio-tag was also observed in black bear Ursus americanus scat and two others were cached in, or buried under logs suggesting mammalian predators.
The program MARK analysis resulted in three models with ΔAIC values ≤2; these contained four covariates (mass, mass2, CORT, and brood size at fledging) and accounted for 61.8 of the AICc weight (Table 1). Mass2 and mass occurred in all six of the top models with ΔAIC values ≤4, suggesting that these variables were important for predicting fledgling survival and cumulatively had an AICc weight of 0.87. The AICc weights for the variables of brood size and CORT were 0.70 and 0.45, respectively. Fledglings with high and low mass had lower survival probabilities than those with intermediate mass (Fig. 2), those fledglings from larger broods had higher survival than those from small broods and survival was negatively correlated with the amount of CORTf. The quadratic relationship with fledgling mass was not driven by the two extreme values because it remained in the top models with these two data points removed. The bootstrap goodness of fit test showed that the data fit the top three models (P = 0.24, 0.001, and 0.001).
Length of post-fledging parental care
After the first week, fledglings often acted aggressively towards their parents once the adults began to rebuff their begging attempts, by pecking and flying at the parent, sometimes knocking it off a branch. Likewise, parents sometimes attacked begging offspring aggressively, or flew away or ignored solicitation attempts from their young. After about 5–30 min of begging, the fledglings usually stopped and began to forage on their own. On average, fledglings received food from at least one parent for 16.6 ± 0.7 SE days. Males fed their fledglings on average for 16.6 ± 0.7 SE days (range 11–22 days), which was longer than the care period of females, which fed fledglings for 12.3 ± 1.1 SE days (range 0–21 days; paired t-test, t 23 = −2.93, P = 0.0076). The length of care was not correlated between members of a pair (r = 0.13, n = 25, P = 0.082; Fig. 3) suggesting that an adult did not compensate for the amount of care given by its mate.
Only one male stopped caring for his young before his partner, and this was in a pair that appeared to have split the brood. However, nine of 25 females (36 %) deserted the brood while the male was still feeding the fledglings. These deserting females typically spent several days either on the nesting home range or at another location, sometimes 1–2 km away. During this time, females were always at least several hundred metres from the family and were observed ‘wica dancing’ (displaying) with neighbouring males, prospecting for new cavities, or engaging in self maintenance such as preening or foraging. Five of the nine females that deserted the brood soon disappeared from radio range (i.e., >3 km away), and were not relocated again on our study site despite extensive searching. In two cases, females paired-up the following year with the neighbouring males they were observed displaying with after they abandoned their broods.
Factors influencing the length of post-fledging parental care
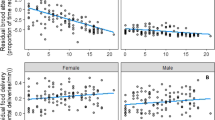
We re-trapped 17 female parents of which 15 re-grew their feather, and 19 males of which 15 re-grew their feather. Among these recaptured adults for which we obtained CORTf, older males (t = −2.76, P = 0.011) fed fledglings for significantly longer. The mean duration of care for yearling males was 14.5 ± 0.82 (SE) days and that for older males was 17.2 ± 0.67 (SE) days. Male body condition (t = 1.3, P = 0.23), the date of fledging (t = 0.25, P = 0.81), and CORTf (t = 0.87, P = 0.40) were not significant predictors of the length of care. For females, CORTf was negatively related to the length of post-fledging care (t = −2.71, P = 0.03; Fig. 4), but age (t = −1.49, P = 0.18), date of fledging (t = 0.94, P = 0.38), and body condition (t = 1.82, P = 0.11) were not significant.
In the full data set containing all parents, males that had provisioned more frequently during the nestling period also fed their fledglings longer in the post-fledging period (t = 2.74, P = 0.012; Fig. 5a) and similar to the reduced dataset, older males also fed their fledglings longer (t = −2.2, P = 0.039). The brood size at fledging (t = 0.3, P = 0.76), the mean offspring body condition (t = −0.97, P = 0.34), and the date of fledging (t = 0.3, P = 0.76) did not significantly influence how long males cared for their offspring. Females that had high feeding rates during the nestling period also cared for their fledglings longer (t = 2.8, P = 0.012; Fig. 5b). Brood size at fledging (t = −1.41, P = 0.17), brood condition at fledging (t = 0.38, P = 0.71), the age of the female (t = −0.45, P = 0.66) and date of fledging (t = −0.78, P = 0.44) did not affect the duration of female parental care.
Distance between parents and fledglings
There were significant differences between males and females in the time they spent at various distances from fledglings (F 4,85 = 6.05, P = 0.0002), and a strong suggestion that these distances differed between the early versus late time periods (F 4,85 = 2.45, P = 0.052). Despite the effect of sex and time period on parental proximity to their young, there was no interaction between the two variables (F 4,85 = 0.032, P = 0.58), and older parents did not spend more time close to their fledglings (F 4,85 = 0.048, P = 0.38). A post hoc ANOVA revealed that males spent more time close to fledglings (<50 m) than females and they tended to remain closer to the fledglings as the offspring aged (Table 2; Fig. 6). In comparison, the distance between females and their offspring tended to increase as the fledglings aged and females spent more time far from juveniles, regardless of the fledglings’ ages.
Discussion
Sex-biased brood desertion
Overall, 36 % of females deserted their brood and 18 % did so within 10 days after their offspring left the nest. We did not observe any desertion by males. Our results suggest that a female’s propensity to desert depends on her physiological state in relation to how she responded to stressors as revealed by high CORTf levels, and that some females reach a point at which they are unwilling to trade-off increased offspring care with increased ‘stress’. A previous mate-removal experiment conducted during the nestling period showed that male flickers were about twice as effective as single parents than females (Wiebe 2005). Females do not naturally desert during the nestling period, apparently because a lack of unpaired males and an even population sex ratio (Wiebe 2005) would mean little chance of initiating a new brood with a second partner.
During the late part of the breeding season, the trade-off parents face between continuing to invest in parental care and initiating moult or migration is particularly strong because heavy investment can negatively affect post-breeding physiological condition (Done et al. 2011) or the scheduling of moult and migration (Stutchbury et al. 2011). Although both sexes of flickers presumably benefit by reducing the length of care to fledglings, male flickers may be more willing to invest than males of many other species because they have a high assurance of paternity (Wiebe and Kempenaers 2009). In addition, the potential for polyandry in flickers may cause higher intrasexual competition among females compared to males. High intrasexual competition may make females less willing than males to undergo moult when energetically stressed and more willing to trade-off parental care with the courting of potential future mates.
Brood desertion during the post-fledging period has not been recorded, to our knowledge, in other single-brooded altricial species, but records of the length of male and female contributions to parental care are lacking. Both sexes appear to care for fledglings among woodpeckers [e.g. middle spotted woodpeckers Dendrocopos medius, and great spotted woodpeckers Dendrocopos major (Michalek and Winkler 2001)], but the length of care was not documented. In another study, temporary female desertion was observed during the nestling period of middle-spotted woodpeckers, but all females returned to care for fledglings during the post-fledging period (Wiktander et al. 2000). Clearly, more studies of the post-fledging care period are needed not only among woodpeckers, but among all birds, to understand the length of investment by each sex.
Factors influencing the length of post-fledging care
A parent’s willingness to desert offspring is related to their value and the parent’s perception of the capability of their partner to raise the brood without help (Olson et al. 2008). Although larger broods are expected to be more valuable and to need more care, brood size did not influence the length of care for either parent. Brood size may still have influenced the amount of food delivered by parents but we could not measure it. In our study, 45 % of fledglings died within the first 8 days post-fledging, so presumably the energy demand on parents declined rapidly after the first week. In addition, the good flying ability of young flickers at fledging and the relative ease with which they can learn to collect their main prey (ants) from the ground may reduce the need for prolonged care compared to many other altricial species.
The prediction that parents which invested heavily during the nestling period would be those that also invested heavily during post-fledging was supported. This suggests that differences in quality between individuals masked a trade-off between high parental effort in the nestling period and high effort in the fledging period and that high-quality parents could provide substantial care throughout the reproductive cycle (Nakagawa et al. 2007). Females were more sensitive than males to their ‘stress level’ (i.e. high CORTf), which was perhaps linked to their physiological condition when deciding levels of investment, but care was not related to any measure of condition in males. Instead, care increased with age of the male. Terminal investment may explain high effort at older ages if the likelihood of future reproduction is low (Clutton-Brock 1984), as seems to be the case in flickers [annual mortality rate around 60 % (Fisher and Wiebe 2006)]. Another explanation is that males may invest more heavily in their first year to find, excavate, and defend a cavity tree, whereas in subsequent years, the energy saved by returning to the same nest may be invested in offspring care.
CORT may mediate the survival-reproduction trade-off in birds (Ricklefs and Wikelski 2002). Increases in CORT in adult birds have been linked to increased foraging behaviour (apparently to improve the individual’s energy balance) at the cost of parental behaviour, and in other studies there is a negative relationship between the levels of prolactin, ‘the parental care hormone’, and CORT (reviewed in Angelier and Chastel 2009). In contrast to plasma CORT, which is a single snapshot, CORTf is a summation of CORT levels over the time of feather growth and hence is an integrated measure of the response to stressors over a longer time (Bortolotti et al. 2008; Fairhurst et al. 2013). Therefore, flicker females with high CORTf likely experienced more chronic activation of the hypothalamic–pituitary–adrenal axis from response to various stressors (e.g. food deprivation, predators, etc.) during the nestling period. In a proximate sense, flicker females apparently reacted to a certain CORTf threshold after which they were not willing or capable of continuing parental care. It is intriguing that average CORTf levels did not differ for males and females (unpublished data); thus, males seem to be more tolerant of high CORTf than females. In contrast to our finding that the more ‘stressed’ female flickers abandoned broods it was the females with more fat reserves which abandoned offspring in Tengmalm’s owls Aegolius funereus. Apparently female owls which abandoned their offspring often initiated a second brood with a new mate and needed high energy reserves to do so (Eldegard and Sonerud 2009).
Males and females differed not only in their length of parental care but also in their proximity to fledglings, suggesting that the sexes valued the brood differently. Males spent more time within 50 m of their young at all fledgling ages, perhaps as a result of two (not mutually exclusive) mechanisms. First, fledglings may opt to follow the male rather than the female if he provides food more frequently. Second, males may be more attentive to the needs of fledglings and more willing to invest in vigilance and offspring defence compared to females. Females seemed to have more interest in prospecting for future mates or nest sites than in spending time near family. If females select future mates (the following season), in part based on the male’s ability to provide parental care, the post-fledging period may be a good time to assess future males because females can use social information to judge his production and care of offspring near the end of the breeding season (Betts et al. 2008).
Fledgling survival and parental care
Fledglings with higher mass have higher survival in some species such as great tits Parus major and coal tits Periparus ater (Naef-Daenzer et al. 2001), but stabilizing selection on fledgling mass occurs in juvenile and adult blue tits Cyanistes caeruleus in the presence of sparrowhawks Accipiter nisus (Adriaensen et al. 1998). We found stabilizing selection on mass of fledgling flickers during the first 2 weeks after they left the nest. Heavier birds may experience higher predation risk because body mass reduces flight manoeuverability (reviewed by Lind et al. 2010). Relatively lightweight fledglings may benefit if predation risk is high (Adriaensen et al. 1998) but face a higher risk of starvation if foraging success is unpredictable (Lima 1986). We did not find any fledglings that died of starvation as a proximate cause, but our results suggest that lightweight fledglings were also susceptible to predators, perhaps because they were less energetic fliers. Low body mass may also be reflective of other conditions that may affect survival, such as disease, high parasite loads or a reduced immune response (Møller et al. 1998).
Although sibling competition and food shortages can explain lightweight fledglings in a brood, the presence of heavy fledglings with apparently low survival is more difficult to explain. Perhaps an unpredictable food supply leads the more competitive fledglings in a brood to continue begging and gaining weight past the optimal level as a buffer against starvation? A second explanation is that we measured survival in the first 2 weeks post-fledging when predation risk was the main proximate cause of mortality. Over the longer term, as the young become independent from parental feeding, starvation as a mortality factor may have increased in importance such that a relatively heavy body mass may have become more advantageous (e.g. Witter and Cuthill 1993). In sociable weavers Philetairus socius, there was stabilizing selection on juvenile mass that carried over into future years, such that birds with an average fledgling mass had the highest survival over the 7-year study period (Covas et al. 2002). Unfortunately, flicker juvenile recruitment is <1 % per year at our study site and so the longer-term effects of fledging mass on survival could not be determined. A final explanation is that predation risk on our study area was unusually high, and a heavy fledgling mass may be advantageous in other populations with fewer predators.
Our results that the survival of flicker fledglings was negatively related to their levels of CORTf is in contrast to Rivers et al. (2012) who found a positive relationship between baseline plasma CORT and juvenile survival in Swainson’s thrushes Catharus ustulatus; but agree with two other studies relating handling induced plasma CORT (Blas et al. 2007) and experimentally elevated CORT levels (Goutte et al. 2010) to reduced juvenile and adult survival, respectively.
In conclusion, the shorter duration of parental care by female compared to male flickers may be explained by a high assurance of paternity and by partly reversed sex roles where females appear to have more to gain than males by prospecting for future breeding sites and partners. At the proximate level, for females length of care is mediated by CORTf levels, whereas males are not as sensitive to their own condition when deciding levels of care. However, further tests on the effects of CORT on the length of parental care using larger samples are needed to substantiate this result. Our study provides an example of how reversed sex roles can contribute to a reduction in care by females, and we encourage others to study parental contributions at the post-fledging stage for a more complete understanding of investment by the sexes.
References
Adriaensen F, Dhondt AA, van Dongen S, Lens L, Matthysen E (1998) Stabilizing selection in blue tit fledgling mass in the presence of sparrowhawks. Proc R Soc Lond B 265:1011–1016
Angelier F, Chastel O (2009) Stress, prolactin and parental investment in birds: a review. Gen Comp Endocrinol 163:142–148
Beissinger SR, Snyder NFR (1987) Mate desertion in the snail kite. Anim Behav 35:477–487
Betts MB, Hadley AS, Rodenhouse N, Nocera JJ (2008) Social information trumps vegetation structure in breeding-site selection by a migrant songbird. Proc R Soc Lond B 275:2257–2263
Blas J, Bortolotti GR, Tella JL, Baos R, Marchant TA (2007) Stress response during development predict fitness in a wild, long lived vertebrate. Proc Natl Acad Sci USA 104:8880–8884
Bortolotti GR, Marchant TA, German T (2008) Corticosterone in feathers is a long-term, integrated measure of avian stress physiology. Funct Ecol 22:494–500
Burnham KP, Anderson DR (2002) Model selection and multi-model inference: a practical information-theoretic approach, 2nd edn. Springer, Berlin
Chapman T, Arnqvist G, Bangham J, Rowe L (2003) Sexual conflict. Trends Ecol Evol 18:41–47
Clutton-Brock TH (1984) Reproductive effort and terminal investment in iteroparous animals. Am Nat 123:212–229
Covas R, Brown CR, Anderson MD, Bomberger Brown M (2002) Stablizing selection on body mass in the sociable weaver Philetairus socius. Proc R Soc Lond B 269:1905–1909
Done T, Gow EA, Stutchbury BJM (2011) Corticosterone stress response and plasma metabolite levels during breeding and molt in a free-living migratory songbird, the wood thrush (Hylocichla mustelina). Gen Comp Endocrinol 171:176–182
Eldegard K, Sonerud GA (2009) Female offspring desertion and male-only care increase with natural and experimental increase in food abundance. Proc R Soc Lond B 276:1713–1721
Erikstad KE, Asheim M, Fauchald P, Dahlhaug L, Tveraa T (1997) Adjustment of parental effort in the puffin: the roles of adult body condition and chick size. Behav Ecol Sociobiol 40:95–100
Fairhurst GD, Marchant TA, Soos C, Machin KL, Clark RG (2013) Experimental relationships between levels of corticosterone in plasma and feathers in a free-living brid. J Exp Biol 216:4071–4081. doi:10.1242/jeb.091280
Fisher RJ, Wiebe KL (2006) Effects of sex and age on survival of northern flickers: a six-year field study. Condor 108:193–200
Goutte A, Angelier F, Welcker J, Moe B, Clement-Chastel C, Gabrielsen GW, Bech C, Chastel O (2010) Long-term survival effect of corticosterone manipulation in black-legged kittiwakes. Gen Comp Endocrinol 167:246–251. doi:10.1016/j.ygcen.2010.03.018
Gow EA, Wiebe KL (2012) An unusually synchronous double brooding attempt by a northern flicker pair. Wilson J Ornithol 124:389–392
Gow EA, Musgrove AB, Wiebe KL (2013) Brood age and size influence sex-specific parental provisioning patterns in a sex-role reversed species. J Ornithol 154:525–535
Griggio M, Matessi G, Pilastro A (2005) Should I stay or should I go? Female brood desertion and male counterstrategy in rock sparrows. Behav Ecol 16:435–441
Grüebler MU, Naef-Daenzer B (2010) Survival benefits of post-fledging care: experimental approach to a critical part of avian reproductive strategies. J Anim Ecol 79:334–341
Hõrak P (2003) When to pay the cost of reproduction? A brood size manipulation experiment in great tits (Paus major). Behav Ecol Sociobiol 54:105–112
Kokko H, Jennions MD (2008) Parental investment, sexual selection and sex ratios. J Evol Biol 21:919–948
Lima SL (1986) Predation risk and unpredictable feeding conditions: determinants of body mass in birds. Ecology 67:377–385
Lind J, Jakobsson S, Kullberg C (2010) Impaired predator evasion in the life history of brids: behavioral and physiological adaptations to reduced flight ability. In: Thompson CF (ed) Current ornithology. Springer Science, New York
Michalek KG, Winkler H (2001) Parental care and parentage in monogamous great spotted woodpeckers (Picoides major) and middle spotted woodpeckers (Picoides medius). Behaviour 138:1259–1285
Møller AP, Christe P, Erritzoe J, Mavarez J (1998) Condition, disease and immune defence. Oikos 83:301–306
Morton ES, Stutchbury BJM, Chiver I (2010) Parental conflict and brood desertion by females in blue-headed vireos. Behav Ecol Sociobiol 64:947–954
Naef-Daenzer B, Widmer F, Nuber M (2001) Differential post-fledging survival of great and coal tits in relation to their condition and fledging date. J Anim Ecol 70:730–738
Nakagawa S, Gillespie DOS, Hatchwell BJ, Burke T (2007) Predicatble males and unpredictable females: sex differences in repeatability of parental care in a wild bird population. J Evol Biol 20:1674–1681
Olson VA, Liker A, Freckleton RP, Székely T (2008) Parental conflict in birds: comparative analyses of offspring development, ecology and mating opportunities. Proc R Soc Lond B 275:301–307
Pyle P, Howell AB, DeSante DF, Yunick RP, Gustafson M (1997) Identification guide to North American birds. Slate Creek Press, New York
R Core Development Team (2012) R: a language and environment for statistical computing. Version 2.14.2. R Foundation for Statistical Computing, Vienna
Ricklefs RE, Wikelski M (2002) The physiology/life-history nexus. Trends Ecol Evol 17:462–468
Rivers JW, Liebl A, Owen JC, Martin LB, Betts MB (2012) Baseline corticosterone is positively related to juvenile survival in a migrant passerine bird. Funct Ecol 26:1127–1134
Stutchbury BJM, Gow EA, Done T, MacPherson M, Fox JW, Afanasyev V (2011) Effects of post-breeding moult and energetic condition on timing of songbird migration into the tropics. Proc R Soc Lond B 278:131–137
Styrsky JN, Brawn JD, Robinson SK (2005) Juvenile mortality increase with clutch size in a Neotropical bird. Ecology 86:3238–3244
Székely T, Cuthill IC, Kis J (1999) Brood desertion in Kentish plover: sex differences in remating opportunities. Behav Ecol 10:185–190
Székely T, Kosztolányi A, Küpper C, Thomas GH (2007) Sexual conflict over parental care: a case study of shorebirds. J Ornithol 148:S211–S217
van Noordwijk AJ, de Jong G (1986) Acquisition and allocation of resources: their influence on variation in life history tactics. Am Nat 128:137–142
White GC, Burnham KP (1999) Program MARK: survival estimation from populations of marked animals. Bird Study 46:120–138
White GC, Burnham KP, Andersen DR (2001) Advanced features of program MARK. In: Field R, Warren RJ, Okarma H, Sievert PR (eds) Wildlife, land, and people: priorities for the 21st century. The Wildlife Society, Bethesda
Wiebe KL (2005) Asymmetric costs favor female desertion in the facultatively polyandrous Northern Flicker (~Colaptes auratus~): a removal experiment. Behav Ecol Sociobiol 57:429–437
Wiebe KL (2008) Division of labour during incubation in a woodpecker Colaptes auratus with reversed sex roles and facultative polyandry. Ibis 150:115–124
Wiebe KL, Kempenaers B (2009) The social and genetic mating system in flickers linked to partially reversed sex roles. Behav Ecol 20:453–458
Wiebe KL, Moore WS (2008) Northern flicker (Colaptes auratus). In: Poole A (ed) The birds of North America online. Cornell Lab of Ornithology, Ithaca
Wiktander U, Olsson O, Nilsson SG (2000) Parental care and social mating system in the lesser spotted woodpecker Dendrocopos minor. J Avian Biol 31:447–456
Witter MS, Cuthill IC (1993) The ecological costs of avian fat storage. Philos Trans R Soc Lond B Biol Sci 340:73–92
Zuur AF, Ieno EN, Walker N, Saveliev AA, Smith GM (2010) Mixed effects models and extensions in ecology with R. Springer, New York
Acknowledgments
We thank J. Allsop, B. Griffiths, H. Hanbridge M. Mitsutani, A. Musgrove and M. Van der Pol for help in the field. T. Marchant, S. Cabezas, G. Treen and G. Fairhurst for laboratory analysis of CORTf. This research was funded by the Kenneth Molson Foundation, NSERC Discovery (K. L. W.), NSERC Canada Graduate Scholarship (E. A. G.), the Society of Canadian Ornithologists, and the Isabel Maria López Matínez Memorial Scholarship (E. A. G.). This study was conducted with Animal Care Permit number 20010113 from the University of Saskatchewan and corresponds with the current laws of Canada.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Indrikis Krams.
Rights and permissions
About this article
Cite this article
Gow, E.A., Wiebe, K.L. Determinants of parental care and offspring survival during the post-fledging period: males care more in a species with partially reversed sex roles. Oecologia 175, 95–104 (2014). https://doi.org/10.1007/s00442-014-2890-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00442-014-2890-1