Abstract
Hatching, the life history switch point between embryonic and larval or subadult stages, has traditionally been regarded as a fixed event in an organism’s development. This notion has been challenged by reports of environmentally cued hatching in recent years, which show embryos improve fitness by hatching in response to mortality risks. Here, we present evidence of accelerated hatching due to predation at two points during embryonic development in Chiromantis hansenae. Young embryos (0 day old) exposed to simulated predation hatched earlier compared to undisturbed clutches. Old embryos (4 days old) subjected to direct katydid predation had more immediate responses, hatching <1 h after predation on average. Hatching time was not correlated with female frog size, egg attendance time, or other predator cues. Results confirm predator-cued hatching in a new family of amphibians and support hatching plasticity being a widespread and potentially ancestral condition. We suggest mechanisms and ecological basis of cue transmission and response in C. hansenae and point out potential further research.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
For organisms with complex life cycles, transitions between life stages represent key events in development and often a significant niche change. Consequently, timing of transitions between life stages should be altered to maximize survivorship (Werner and Gilliam 1984; Werner 1986). The first transition for many organisms is hatching. The acceleration or delay of hatching time has been reported across diverse taxa (reviewed in Warkentin 2011a; Armstrong et al. 2013). Embryos can respond to many types of cues, including physical and chemical cues. Many studies suggest that hatching may be plastic more often than previously known or assumed (Warkentin 2011a). If hatching is plastic, individuals may improve their survival by altering hatching time (Warkentin 2011a). For example, hatching can be a way for embryos, which are relatively immobile, to escape predation (Warkentin 1995, 2000; Saenz et al. 2003; Strathmann et al. 2010).
Of the various cues embryos can respond to, those associated with predation may signify some of the most direct risks of mortality. For mature embryos in a clutch that is being consumed by a predator, costs of hatching prematurely are clearly outweighed by imminent mortality. Changes in hatching time due to predation have been observed in several taxa, with different cues and response mechanisms. For example, gastropods and crustaceans can delay hatching in response to cues of predators of larvae (Blaustein 1997; Miner et al. 2010); arachnids and reptiles can accelerate hatching in response to cues from predators of eggs (Li 2002; Doody 2011); and amphibians are able to accelerate or delay hatching depending on whether cues are from predators of eggs or larvae (Ireland et al. 2007).
Cues can be differentiated by how they are transmitted and by duration of exposure. For example, embryos can respond to predators by detecting physical cues (Warkentin 1995, 2000) or chemical signals (Blaustein 1997; Chivers et al. 2001; Li 2002; Capellán and Nicieza 2007; Ireland et al. 2007; Lehman and Campbell 2007). Moreover, embryos could be responding to acute (Warkentin 1995, 2000; Strathmann et al. 2010) or chronic (Sih and Moore 1993; Blaustein 1997; Chivers et al. 2001; Li 2002; Capellán and Nicieza 2007; Ireland et al. 2007; Lehman and Campbell 2007) exposure to predator cues. In some cases, embryos may have a specific window of time when they can respond to predator cues (Lehman and Campbell 2007). Mechanisms of cue transmission and detection vary among organisms and are dependent on the egg environment. In amphibians, for example, different cues are available to aquatic compared to terrestrial or arboreal eggs. Ability of embryos to respond to different cues suggests complexity of plastic hatching responses, requiring different neurological and physiological mechanisms for both risk assessment and subsequent behavioral responses. Currently, predator-cued hatching has only been reported in 7 (Warkentin 2011b) of 73 families of amphibians (IUCN 2013). As the study of environmentally cued hatching is still a relatively new field, discovery of its existence in new taxa and geographic regions, assessment of cues, and examination of directional responses by embryos are necessary to form a basis for further investigations on its adaptive values and evolutionary trajectories.
Focusing on Chiromantis hansenae, a Southeast Asian Rhacophorid tree frog, our current study extends both the geographic and taxonomic evaluation of this phenomenon (but see Brown and Iskandar 2000). The primary cause of embryonic mortality in C. hansenae is predation (Poo and Bickford 2013) and one of the most common egg predators is katydids (Hexacentrus cf. unicolor, Fig. 1). Both young and old embryos can experience katydid predation. However, while older embryos have the potential to respond immediately to predator cues by hatching, younger embryos continue to develop in partially predated clutches for a number of days (SP, personal observation). Therefore, C. hansenae offers the chance to examine the effects of both chronic and acute predator cues on hatching time by looking at predation on young and old embryos. Adding another dimension to factors that influence embryonic development, C. hansenae is a species with parental care. Egg attendance is exclusively by female parents and is essential for offspring survival (Poo and Bickford 2013).
Herein we examine C. hansenae hatching in response to predation cues at two different developmental stages. Predator disturbance experiments were conducted on young and old embryos. We exposed young embryos to cues from simulated predation and a nonconsumptive predator. We exposed old embryos to direct katydid predation. Experiments allowed us to examine responses to chronic and acute predator cues in relation to hatching plasticity. We predict that embryos exposed to predator cues will hatch early in both experiments. However, young and old embryos may have different mechanisms of cue detection and response or the mechanism may have an ontogeny (and hence be different at various times in development).
Methods
Study species and experimental setup
This study was conducted from July to October 2012 at the Sakaerat Environmental Research Station in Thailand (14° 30′ N, 101° 55′ E, elevation 250–762 m asl, mean annual rainfall 980 mm). C. hansenae is a tree frog that breeds in temporary ponds in Thailand and parts of Cambodia during the rainy season. Egg clutches are hemispherical gelatinous masses attached to vegetation or other substrates directly overhanging water. Immediately preceding hatching, the entire clutch detaches from the substrate and falls into the water below, which is immediately followed by the emergence of hatchlings from the gelatinous mass. Adults are nocturnal, with females exhibiting egg attendance both day and night throughout the entire embryonic period (Poo and Bickford 2013). The katydid, H. cf. unicolor, is found in the same habitat as C. hansenae and is known to eat both frogs and their eggs.
C. hansenae males and gravid females were collected from ponds and brought to an open air laboratory. Frogs were placed in glass aquariums (40 × 20 × 25 cm) with a plastic mesh cover and central mesh divider (Fig. 2). Aquariums contained rocks, plants, and aged tap water (2 cm) to simulate pond habitats. A pair of frogs was placed together on one side of the mesh divider (“clutch side”), while the other side was initially left empty (“nonclutch side”). Frogs mate readily in captivity and the majority of clutches were laid between 2100 and 0300 hours. Aquariums were checked between 0300 and 1000 hours each morning for clutches. Clutches were monitored twice daily and sprayed with rainwater to provide hydration. A time lapse camera (Brinno GardenWatchCam) was set up to take photos at 10 s interval, starting when experiments were initiated. Videos from time lapse camera were used to obtain hatching time for embryos. H. cf. unicolor adults were collected from ponds 24 to 48 h prior to experiments and starved to standardize and maximize predatory behaviors. All animals were returned to their original location immediately after clutches hatched and experiments concluded. Methods followed the Institutional Animal Care and Use Committee-approved protocol (B11/12) at the National University of Singapore.
Experimental design for predator-cued hatching in Chiromantis hansenae. a Young embryo experiments with clutches with and without simulated predation and nonconsumptive predator treatments. Clutches with simulated predation are illustrated with half-spheres while undisturbed control clutches are whole spheres. Dotted line indicates mesh divider in tank, with a predator (katydid, Hexacentrus cf. unicolor) on the nonclutch side of the aquarium. From top left to bottom right, N = 10, 12, 13, and 12, respectively. b Old embryo experiments with and without direct katydid predation treatments. In control clutches, C. hansenae adults are allowed to remain with clutches to avoid interruption of parental care, while in experimental clutches, adults are removed to avoid frog mortality from katydids and ensure katydid predation efforts are focused on clutches. From left to right, N = 13 and 10, respectively
Young embryo experiments
Experiments started 3–10 h postoviposition (“HPO” hereafter, developmental stage = Gosner stage 14 or less) (Gosner 1960). Clutches were assigned to one of four treatments under a two-way factorial design, with or without simulated predation and nonconsumptive predator cues (Fig. 2a). For simulated predation cue treatment, a pocket knife was used to carefully bisect and remove half of the clutch in disturbed clutches, while control clutches were touched by the side of the blade without altering clutch structure or integrity of embryos. Removal of eggs in treatments is similar to katydid predation in natural settings. Field observations indicate katydid can consume part of a clutch, leaving some broken embryos along with remaining embryos that continue to develop under the care of adult frogs. For nonconsumptive predator cue treatment, an adult katydid was placed on the nonclutch side of the aquarium, while the nonclutch side of controls was left empty. Mesh dividers prevented physical contact between predator and egg clutches, but allowed for visual, chemical, or other cues of predator to be transmitted.
Hatching time was defined as the time when a clutch detached from its substrate and fell into the water below, which is immediately prior to the emergence of hatchlings. In addition to experimental treatments, snout vent length (SVL) of female frogs and egg attendance time were measured to account for potential maternal effects on embryonic development. Attendance time was obtained by watching video from time lapse cameras and calculating the percentage of time female frogs spent in direct physical contact with egg clutches.
Old embryo experiments
Clutches for all treatments were allowed to develop normally until experiments were started (89–91 HPO, Gosner stages 22–23; Fig. 2b). Clutches were then assigned to one of two treatments. For direct katydid predation treatments, an adult katydid was placed on the clutch side of the aquarium and adult frogs were removed. Removal of frogs was done to ensure katydids focused on the eggs instead of the frogs and to prevent defensive parental frog behavior from interfering with katydid predation of eggs. These measures were taken based on field and laboratory observations of predatory behavior of katydids and antipredator defense behaviors of egg attending frogs (SP, unpublished data). For controls, an adult katydid was placed in the nonclutch side, preventing physical contact between katydid and clutches. Frogs in the control group were allowed to remain with clutches, in order to minimize disturbance to the normal course of development and hatching of embryos. Since embryos can hatch individually in response to predation, hatching time was defined as the time when the first hatchling dropped out of the clutch. Female SVL was measured to account for potential maternal effects.
Statistical analyses
Hatching time among treatment groups was analyzed with generalized linear models (GLMs) with an underlying gamma distribution and inverse link function. For young embryo experiments, effects of simulated predation, nonconsumptive predator cues, interactions between treatments, female frog SVL, and egg attendance were examined in the initial model. For old embryo experiments, effects of katydid predation and female frog SVL were examined in the initial model. Stepwise model simplification was done for all GLMs based on the Akaike information criterion (AIC). All statistical analyses were performed in R (R Core Team 2013). Means are presented with ±1 SE.
Results
Effects of predator cue on young embryos
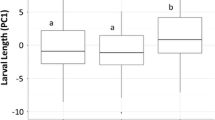
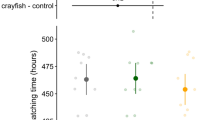
A total of 47 clutches were observed (Fig. 2a). Simulated predation was the only significant factor correlated with hatching time (p = 0.01, clutch size reduced = 40.9 ± 1.7 %, Table 1). Clutches with eggs removed hatched 7 % earlier on average compared to control clutches (N = 24 and 23, hatching time = 106.3 ± 2.2 and 114.1 ± 2.0 HPO, respectively, Fig. 3a). No effects were found for nonconsumptive predator cues, interaction with simulated predation treatment, female frog SVL, and egg attendance in the final model (Table 1). Female frog SVL was 24.4 ± 0.1 mm (range = 23.4–25.8 mm) and egg attendance time was 83.7 ± 1.4 % of the total observation (range = 61.8–95.0 %; 56.1–110.2 h).
Effects of predator cue on old embryos
A total of 23 clutches were observed (Fig. 3a). Katydid predation of clutches and induced hatching of embryos were observed for all experimental clutches. Predation of clutches occurred 0.8 ± 0.4 h after the experiments began and clutches hatched 0.9 ± 0.5 h after the start of predation (range = 0–4.6 and 0–6.2 h, respectively). Hatching time for predated clutches was 22 % earlier than controls (91.7 ± 0.5 and 117.0 ± 3.0 HPO, respectively, p < 0.01, Fig. 3b, Table 2). Successful escape from predation by hatching was observed in all cases after clutches were partially consumed. Hatchlings emerged within 1 h of initial predator contact in 77 % of the experimental clutches (N = 10). Multiple predation events were observed in 46.2 % of treatments (N = 6). In these cases, katydids left clutches after consuming part of the clutch and returning for up to six times to continue predation until the last embryo hatched (interval between predation events = 1.7 ± 0.3 h). For all clutches with only a single predation event (N = 7), hatching response was immediate and all embryos dropped into the water within 4 min of initial predator contact. Since embryos were submerged in water after they dropped, subsequent predation events were not possible. No effects were found for female SVL (24.7 ± 0.2 mm, range 23.4–26 mm, Table 2).
Discussion
Our study demonstrates that both young and old C. hansenae embryos are able to hatch earlier when disturbed. Factors that affected hatching time were simulated and direct katydid predation in young and old embryos, respectively. The presence of a nonconsumptive predator did not have an effect on hatching time. Furthermore, hatching time was not correlated with clutch size, female frog SVL, or attendance time. These results provide the first experimental documentation of environmentally cued hatching in Southeast Asian amphibians and for the family Rhacophoridae.
Accelerated hatching by younger embryos
Simulated predation on embryos early in the egg stage resulted in accelerated hatching, while no response to nonconsumptive predator was found. Simulated predation treatment may have signified a change in embryonic environment through release of chemical cues by broken embryos or reduce in integrity of clutch structure. Chemical cues are known to affect hatching time in amphibians. Aquatic amphibian eggs can accelerate hatching in response to cues from predators, injured conspecifics, and a combination of both (Chivers et al. 2001; Touchon et al. 2006; Capellán and Nicieza 2010; Ferrari and Chivers 2010). The embryonic environment of arboreal eggs, however, can be vastly different from aquatic eggs. For instance, aquatic eggs can be continuously exposed to chemical cues in the surrounding environment. To our knowledge, response to chemical predation cues by terrestrial or arboreal eggs has not been documented in the past (see Warkentin 2011b). However, it is possible that C. hansenae embryos are responding to chemical cues through similar mechanisms as aquatic amphibian eggs. Chemicals released from broken embryos may remain on clutches and be detected by the remaining embryos over time. Given that embryos are at an early stage when treatments started, it is possible that cue detection happens at a later point, when embryos have developed the sensory systems required. As in the case of hatching plasticity in aquatic eggs, cues from injured conspecifics could signal a less desirable environment to remaining embryos. Given that predation of a clutch can be split into multiple events spread out over time, accelerating hatching time in response to perceived threats can be adaptive and reduce subsequent mortality. Response to chemical cues in nonaquatic eggs, however, requires more detailed studies. Specifically, these would be examining response to different types of chemical cues, onset and durations of exposure, and level of response elicited.
Another possible reason for accelerated hatching in simulated predation treatments is reduction in physical integrity of clutches when embryos were removed. In certain arboreal-breeding amphibian, some embryos can hatch while others remain in the overall clutch structure. The natural hatching process of C. hansenae, however, starts with the entire gelatinous clutch detaching from its substrate and falling into the water as a whole. Individuals then break out of their egg capsules after the clutch is submerged in water. It is possible that loss of embryos within the clutch affected its adhesive properties, causing the clutch to drop into the water at an earlier time. In this case, accelerated hatching may be an indirect result of predation, with embryos hatching in response to flooding once clutches drop into the water.
Hatching time was not correlated with female frog SVL or egg attendance time in our study. Studies in other taxa have shown that the level of parental care can affect hatching time (Buckley et al. 2005; Delia et al. 2014) and parents can facilitate or elicit hatching response (Brown et al. 2008; Brown et al. 2010; Li 2002; Ishimatsu and Graham 2011). However, attending parents in C. hansenae did not appear to play an active role in hatching and can be absent from clutches when it occurs.
Our study establishes the presence of accelerated hatching by young embryos due to simulated predator cues. However, we can only propose possible mechanisms of cue types and cue detection in embryos. Further studies targeting specific cues are required to determine the particular mechanism of this behavioral adaptation in C. hansenae. Predation in natural settings results in mortality of embryos accompanied by both chemical cues and structural damage similar to those simulated in the present study. As such, either factor, or a combination of both, can be responsible for the accelerated hatching observed. We do not know if it is both or just one.
Rapid response hatching by older embryos
Katydid predation on embryos late in the egg stage resulted in early hatching in all experimental clutches. Old embryos were able to respond relatively quickly to predator cues by hatching and escaping into an aquatic environment. Difference in hatching time between control and experimental treatments is within the range of previous reports of predator-cued hatching for terrestrial amphibian eggs (Warkentin 2011b). However, since all experimental clutches were able to hatch in response to predation in our study, it is reasonable to assume that embryos had reached hatching competency at an earlier point. Therefore, the start of the plastic hatching period may be earlier than what we observed.
Predation in one section of the clutch may signal mortality risks to remaining embryos through chemical or physical (vibrational) cues. Of these, physical cues are likely to have a faster transmission speed than chemical cues and may be responsible for the acute hatching responses observed. In Agalychnis callidryas, a Neotropical tree frog that also lays arboreal gelatinous clutches, embryos use vibrational cues to detect predator attacks and are able to hatch almost immediately in response (Warkentin 2005). Hatching response in A. callidryas is signal specific and based on vibrational characteristics of each type of predator (Warkentin et al. 2006; Caldwell et al. 2010). Similarly, C. hansenae embryos may be relying on vibration cues from predators to decide on an optimal hatching time. However, hatching in C. hansenae in response can occur more than 30 min after predators have left. In these cases, turning and hatching movements from neighboring embryos may play a significant role in transmitting hatching signals to those remaining. Vibrations from neighboring embryos in the turtle Carettochelys insculpta induce early and synchronized hatching, potentially reducing mortality due to hatching latency when clutches are flooded (Doody et al. 2012). These hatching cues and conditions are similar to that of C. hansenae, since normal hatching occurs after clutches are submerged in water. It is therefore possible that C. hansenae embryos are using a similar strategy of hatching in response to sibling vibration. Due to rapid pond level rises during the rainy season, flooding is the second major source of mortality for embryos (Poo and Bickford 2013). Although flood-induced hatching has yet to be examined in this species, it is possible that sibling vibration is one of the hatching cues embryos rely on. Again, further studies are needed to elucidate the mechanisms of immediate hatching responses in C. hansenae. However, response of embryos to katydid predation is a clear indication that hatching time is indeed a plastic event for C. hansenae and embryos can respond to acute signals by escaping into the next life stage.
Potential effects of early hatching
Plasticity in hatching time can have carryover effects on an individual’s survival in the next life stage. Regardless of whether accelerated hatching is in response to cues from an early or late egg stage predation, C. hansenae tadpoles that hatched prematurely may have different developmental and behavior characteristics compared to those of spontaneous hatchlings. Exposure to predators during egg stage affects larvae in various taxa (Mathis et al. 2008; Ferrari and Chivers 2010; Jozet-Alves and Hebert 2013; Nelson et al. 2013). These effects are highly variable even within amphibians (e.g., Warkentin 1995, 1999; Buckley et al. 2005; Vonesh and Bolker 2005; Capellán and Nicieza 2007). Early hatching can have effects on size (Sih and Moore 1993; Johnson et al. 2003), morphology (Capellán and Nicieza 2007), and behavior of tadpoles (Vonesh and Bolker 2005). These effects depend on the posthatching environment and can carry over into subsequent life stages (Touchon et al. 2013). There are trade-offs, however, of hatching early (i.e., avoiding egg mortality) with longer vulnerable larval stage development (Gibbons and George 2013) and larval mortality (Willink et al. 2013). Consequently, studies have increasingly shown the importance of placing comparisons of plasticity in hatching in a larger environmental context once the existence of such plasticity has been established. Plasticity for accelerated hatching in C. hansenae suggests an evolutionary advantage of maintaining a longer egg stage and having a later spontaneous hatching time. However, given its potential complexity and context-dependent nature, the effects of early hatching in C. hansenae require further studies before assumptions can be made on its significance to survivorship in subsequent life stages.
Conclusion
Our study confirms accelerated hatching in response to predation cues at two distinct developmental stages in an arboreal-breeding Rhacophorid frog. We present experimental evidence of environmentally cued hatching in a new family of amphibians, which supports previous suggestions of prevalence of hatching plasticity. Discoveries and confirmations of hatching in response to predator cues suggest that further studies on C. hansenae may lead to a novel understanding of the mechanisms of cue detection and decisions involved in behavioral responses leading to the time of hatching.
References
Armstrong AF, Blackburn HN, Allen JD (2013) A novel report of hatching plasticity in the phylum Echinodermata. Am Nat 181:264–272
Blaustein L (1997) Non-consumptive effects of larval Salamandra on crustacean prey: can eggs detect predators? Oecologia 110:212–217
Brown RM, Iskandar DT (2000) Nest site selection, larval hatching, and advertisement calls of Rana arathooni from southwestern Sulawesi (Celebes) Island, Indonesia. J Herpetol 34:404–413
Brown JL, Twomey E, Morales V, Summers K (2008) Phytotelm size in relation to parental care and mating strategies in two species of Peruvian poison frogs. Behaviour 145:1139–1165
Brown JL, Morales V, Summers K (2010) A key ecological trait drove the evolution of biparental care and monogamy in an amphibian. Am Nat 175 (4):436–446
Buckley CR, Michael SF, Irschick DJ (2005) Early hatching decreases jumping performance in a direct-developing frog, Eleutherodactylus coqui. Funct Ecol 19:67–72
Caldwell MS, McDaniel JG, Warkentin KM (2010) Is it safe? Red-eyed treefrog embryos assessing predation risk use two features of rain vibrations to avoid false alarms. Anim Behav 79:255–260
Capellán E, Nicieza AG (2007) Trade-offs across life stages: does predator-induced hatching plasticity reduce anuran post-metamorphic performance? Evol Ecol 21:445–458
Capellán E, Nicieza A (2010) Constrained plasticity in switching across life stages: pre- and post-switch predators elicit early hatching. Evol Ecol 24:49–57
Chivers DP, Kiesecker JM, Marco A, DeVito J, Anderson MT, Blaustein AR (2001) Predator-induced life history changes in amphibians: egg predation induces hatching. Oikos 92:135–142
Delia JRJ, Ramírez-Bautista A, Summers K (2014) Glassfrog embryos hatch early after parental desertion. Proc R Soc B 281:20133237
Doody JS (2011) Environmentally cued hatching in reptiles. Integr Comp Biol 51(1):49–61
Doody JS, Stewart B, Camacho C, Christian K (2012) Good vibrations? Sibling embryos expedite hatching in a turtle. Anim Behav 83:645–651
Ferrari MCO, Chivers DP (2010) The ghost of predation future: threat-sensitive and temporal assessment of risk by embryonic woodfrogs. Behav Ecol Sociobiol 64:549–555
Gibbons ME, George MP (2013) Clutch identity and predator-induced hatching affect behavior and development in a leaf-breeding treefrog. Oecologia 171:831–843
Gosner KL (1960) A simplified table for staging anuran embryos and larvae with notes on identification. Herpetologica 16:183–190
Ireland DH, Wirsing AJ, Murray DL (2007) Phenotypically plastic responses of green frog embryos to conflicting predation risk. Oecologia 152:162–168
Ishimatsu A, Graham JB (2011) Roles of environmental cues for embryonic incubation and hatching in mudskippers. Integr Comp Biol 51(1):38–48
IUCN Red List of Threatened Species. Version 2013.2 (2013) www.iucnredlist.org. Accessed 12 Feb 2014
Johnson JB, Saenz D, Adams CK, Conner RN (2003) The influence of predator threat on the timing of a life-history switch point: predator-induced hatching in the southern leopard frog (Rana sphenocephala). Can J Zool 81:1608–1613
Jozet-Alves C, Hebert M (2013) Embryonic exposure to predator odour modulates visual lateralization in cuttlefish. Proc R Soc Lond B 280:20122575
Lehman EM, Campbell CD (2007) Developmental window of response to predator chemical cues in rough-skinned newt embryos. Funct Ecol 21:880–885
Li D (2002) Hatching responses of subsocial spitting spiders to predation risk. Proc R Soc Lond B 269:2155–2161
Mathis A, Ferrari MCO, Windel N, Messier F, Chivers DP (2008) Learning by embryos and the ghost of predation future. Proc R Soc B 275:2603–2607
Miner BG, Donovan DA, Andrews KE (2010) Should I stay or should I go: predator- and conspecific-induced hatching in a marine snail. Oecologia 163:69–78
Nelson AB, Alemadi SD, Wisenden BD (2013) Learned recognition of novel predator odour by convict cichlid embryos. Behav Ecol Sociobiol 67:1269–1273
Poo S, Bickford DP (2013) The adaptive significance of egg attendance in a South-east Asian tree frog. Ethology 119:671–679
R Core Team (2013) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria, www.r-project.org
Saenz D, Johnson JB, Adams CK, Dayton GH (2003) Accelerated hatching of southern leopard frog (Rana sphenocephala) eggs in response to the presence of a crayfish (Procambarus nigrocinctus) predator. Copeia 2003:646–649
Sih A, Moore RD (1993) Delayed hatching of salamander eggs in response to enhanced larval predation risk. Am Nat 142:947–960
Strathmann RR, Strathmann MF, Ruiz-Jones G, Hadfield MG (2010) Effect of plasticity in hatching on duration as a precompetent swimming larva in the nudibranch Phestilla sibogae. Invertebr Biol 129:309–318
Touchon JC, Gomez-Mestre I, Warkentin KM (2006) Hatching plasticity in two temperate anurans: responses to a pathogen and predation cues. Can J Zool 84:556–563
Touchon JC, McCoy MW, Vonesh JR, Warkentin KM (2013) Effects of plastic hatching timing carry over through metamorphosis in red-eyed treefrogs. Ecology 94:850–860
Vonesh JR, Bolker BM (2005) Compensatory larval responses shift trade-offs associated with predator-induced hatching plasticity. Ecology 86:1580–1591
Warkentin KM (1995) Adaptive plasticity in hatching age—a response to predation risk trade-offs. Proc Natl Acad Sci U S A 92:3507–3510
Warkentin KM (1999) The development of behavioral defenses: a mechanistic analysis of vulnerability in red-eyed tree frog hatchlings. Behav Ecol 10:251–262
Warkentin KM (2000) Wasp predation and wasp-induced hatching of red-eyed treefrog eggs. Anim Behav 60:503–510
Warkentin KM (2005) How do embryos assess risk? Vibrational cues in predator-induced hatching of red-eyed treefrogs. Anim Behav 70:59–71
Warkentin KM (2011a) Environmentally cued hatching across taxa: embryos respond to risk and opportunity. Integr Comp Biol 51:14–25
Warkentin KM (2011b) Plasticity of hatching in amphibians: evolution, trade-offs, cues and mechanisms. Integr Comp Biol 51:111–127
Warkentin KM, Caldwell MS, McDaniel JG (2006) Temporal pattern cues in vibrational risk assessment by embryos of the red-eyed treefrog, Agalychnis callidryas. J Exp Biol 209:1376–1384
Werner EE (1986) Amphibian metamorphosis—growth-rate, predation risk, and the optimal size at transformation. Am Nat 128:319–341
Werner EE, Gilliam JF (1984) The ontogenetic niche and species interactions in size structured populations. Annu Rev Ecol Syst 15:393–425
Willink B, Palmer MS, Landberg T, Vonesh JR, Warkentin KM (2013) Environmental context shapes immediate and cumulative costs of risk-induced early hatching. Evol Ecol 1–14
Acknowledgments
We thank Director T. Artchawakom and the entire staff of Sakaerat for their unwavering logistic support and hospitality. We thank K.M. Warkentin for early discussions, directions, and comments on the manuscript and L.R. Carrasco for advice on statistical analyses. We are grateful to S.D. Howard and three anonymous reviewers for their constructive suggestions. Collection of field data was made possible by the wonderful assistance of A.F. McNear, J.J. Reinig, and J.S. Sherrock. Katydid species was identified by M.K. Tan and illustrations were made by A.K.S. Wee. Funding support was provided by the Ministry of Education and the National University of Singapore (Grant # R-154-000-383-133) and the Singapore International Graduate Award.
Ethical standards
All experiments were approved by the Institutional Animal Care and Use Committee at National University of Singapore (Protocol B11/12). Frog handling was done with care and all animals were returned to their original locations as soon as the experiments concluded. In addition, work at the Sakaerat Environmental Research Station was approved by the National Research Council of Thailand (Project I.D.: 2010/063).
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by M. Gibbons
Rights and permissions
About this article
Cite this article
Poo, S., Bickford, D.P. Hatching plasticity in a Southeast Asian tree frog. Behav Ecol Sociobiol 68, 1733–1740 (2014). https://doi.org/10.1007/s00265-014-1781-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00265-014-1781-0