Abstract
Carry-over effects influence trait responses in later life stages as a result of early experience with environmental cues. Predation risk is an influential stressor and selection exists for early recognition of threats. In particular, invasive species may benefit from carry-over effects by preemptively recognizing and responding to novel predators via latent developmental changes and embryonic learning. In a factorial experiment, we conditioned invasive American bullfrog embryos (Lithobates catesbeianus) to the odor of a novel fish predator, largemouth bass (Micropterus salmoides) alone or in combination with injured conspecific cues. We quantified developmental carryover in the larval life stage and found that individuals conditioned to the highest risk (fish and injured conspecific cues) grew into longer bodied larvae relative to larvae from lower risk treatments. We also assessed embryonic learning, a behavioral carry-over effect, and found an interaction between embryonic conditioning and larval exposure. Behavioral responses were only found in scenarios when predation risk varied in intensity across life history stages, thus requiring a more flexible antipredator strategy. This indicates a potential trade-off between the two strategies in larval growth and development rates, and time until metamorphosis. Our results suggest that early predator exposure and carry-over effects have significant impacts on life history trajectories for American bullfrogs. This research contributes to our understanding of a potentially important invasion mechanism in an anuran species of conservation concern.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Due to the costly nature of predation risk, there are considerable advantages for prey to gain information about potential predators before they pose a significant threat (Lima and Dill 1990). As such, the ability of prey to evaluate future predation risk should be highly beneficial (Mathis et al. 2008). Embryonic learning represents a behavioral carry-over effect, whereby embryos exposed to environmental cues have the opportunity to learn about the identity of predators that will pose a threat for them in the next life stages (Marshall et al. 2003; Ferrari and Chivers 2010; Nelson et al. 2013; Polo-Cavia and Gomez-Mestre 2014). In some systems, this latent learning has been shown to be as sophisticated as that of post-embryonic prey (Ferrari and Chivers 2009c; Mitchell and McCormick 2013). For instance, wood frog tadpoles can modulate the intensity and timing of their antipredator responses to a predator threat based on information learned as embryos (Ferrari and Chivers 2009a). Prey that can use early information to display appropriate antipredator responses upon predator encounters later in life should benefit from similar survival benefits than those provided by non-latent learning (Mathis et al. 2008; Ferrari and Chivers 2013).
Embryonic learning requires exposure to novel predator cues coupled with accurate risk assessment of that predator (Ferrari et al. 2010b). For many organisms, chemicals are often the main source of information available to embryonic individuals (Hay 2009; Ferrari et al. 2010b). The cues used by those embryonic species to learn usually encompass the chemical signatures from predators (predator odors) and cues from injured conspecifics (Chivers et al. 1996; Brown and Smith 1998; McCarthy and Fisher 2000; Jacobsen and Stabell 2004). For a wide variety of aquatic species, this latent associative learning can result in immediate, overt antipredator responses when the threat is detected in later life stages (Mitchell and McCormick 2013; Nelson et al. 2013; Atherton and McCormick 2015). For instance, Polo-Cavia and Gomez-Mestre (2014) demonstrated this when larval western spadefoot toads (Spea hammondii) were able to recognize novel red swamp crayfish (Procambarus clarkii) predators after embryonic conditioning only if predator cues were paired with injured conspecific cues. This learning can also serve to inform the prey about novel, but closely related predators via a process of generalization (Ferrari and Chivers 2009b) and can help prey identify temporal patterns of risk to improve allocation of foraging and antipredator efforts (Ferrari et al. 2010a).
In addition to latent behaviors, developmental carry-over effects may reduce predation risk by altering growth, developmental rates and timing of life history transitions in subsequent life stages (Sih and Moore 1993; Warkentin 1995; Relyea 2001; Pechenik 2006; Tarvin et al. 2015). For example, Chivers et al. (1996) showed that exposure to the predators of damselfly larvae led damselfly embryos to delay hatching, leading them to hatch at large sizes, which increased their chances of survival with larval predators. Developmental carry-over effects can, therefore, have significant impacts on the functional development of individuals (Van Buskirk and Schmidt 2000, Warkentin 2007). While both behavioral and developmental carryover effects have been investigated, very few studies have looked at the interplay between the two. We know that, for a given life stage, prey can benefit from both behavioral and morphological defenses, with very few experiments investigating the integration of the two defense types (but see DeWitt 1998). Whether embryos have the ability to integrate that level of complexity so early is unknown. However, early life exposure might represent the optimal way to induce the development of specific traits that would be beneficial in the current set of conditions (Ferrari et al. 2015).
Carry-over effects may be an important mechanism for invasive species experiencing novel stressors. It is currently unknown if carry-over can influence invasion success or if invaders are capable of embryonic learning or adopting developmental carry-over effects to preemptively evaluate and respond to novel predators (Sih et al. 2010; Garcia et al. 2012; Pintor and Byers 2015). Here, we tested if the American bullfrog (Lithobates catesbeianus), a highly successful invasive species, was capable of carrying over experience with predators across life history stages. Bullfrogs exhibit complex life histories and behaviors and thus are an excellent candidate species to test for carry-over effects (Hayes and Jennings 1986). This large anuran has successfully established invasion ranges across four continents (Lever 2003) and is a serious conservation threat to native communities (Lowe et al. 2000; Pearl et al. 2004). Previous research found that bullfrogs in the northwestern US invasion range perceive largemouth bass, Micropterus salmoides, as a novel threat (Garcia et al. 2012). Invasive bullfrogs collected from habitats with no resident largemouth bass populations were unable to respond to largemouth bass chemical cues with appropriate anti-predator behaviors. This is interesting as both bullfrog and largemouth bass native and invasive ranges notably overlap in the US (Garcia et al. 2012). The lack of an innate response to a historic predator suggests that bullfrogs may rely more on flexible antipredator strategies rather than a fixed behavioral response. As such, learning about resident fish predators during the embryonic stage may have significant advantages for bullfrogs in newly invaded habitats.
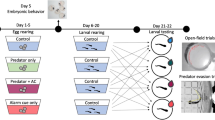
In this study, we tested the ability of American bullfrogs to display latent learning and developmental carry-over effects based on embryonic experience with various environmental cues using a factorial design. We conditioned embryos to one of three conditioning treatments: (1) exposure to a novel predator odor alone (largemouth bass), (2) exposure to injured conspecific cues paired with predator odor or (3) exposure to a water control. As larvae, we exposed them to one of four testing cues: (1) predator odor alone, (2) injured bullfrog cues alone, (3) a combination of injured bullfrog cues and predator odor or (4) a water control. We recorded their behavioral response to the cues (refuge use) along with morphological measurements to quantify differences in growth rate. Based on previous work on embryonic learning (Mathis et al. 2008), we predicted that larval bullfrogs conditioned as embryos to predator cues paired with injured conspecific cues would be capable of responding to the predators with increased refuge use, while those embryos not receiving the injured conspecific cues treatment would not. We predicted developmental carry-over effects would be a function of injured conspecific cue exposure alone or in combination with predator cues, and would be independent from any latent behaviors.
Materials and methods
Animal collection
We collected five freshly laid bullfrog egg masses (>24 h after deposition) from a seasonal pond at the William L. Finley National Wildlife Refuge near Corvallis, Oregon. The pond contained no resident fish populations. All embryos were transported to Oregon State University and held in a controlled-environment chamber at 15 °C and on a natural photoperiod. Using a split-clutch design, egg masses were divided and mixed, with 75 total eggs from three or more egg masses added to 5-L glass aquaria (N = 9). Aquaria were aerated and filled with 3 L of filtered, dechlorinated tap water. Conditions were standardized for all aquaria for the entirety of their embryonic period.
Cue preparation
Aquaria were randomly assigned to one of three embryonic conditioning treatments: control, largemouth bass chemical odor (fish cues), and injured conspecific cues paired with largemouth bass chemical odor (tadpole + fish cues). Fish were collected and held for 7 days prior to the initiation of the experiment and fed a combination of live crickets and worms. To generate the fish cues, we temporarily held one adult largemouth bass (30 cm total length) in a 19-L bucket of filtered, dechlorinated tap water for 45 min. To generate tadpole cues, one large bullfrog tadpole was emulsified, daily, in 100 ml of control water before each conditioning (10.7 cm ± 0.8 cm SD large tadpole total length). All cues were made immediately before being administered each day.
Embryonic conditioning
Embryos were exposed to conditioning treatments for two consecutive days (27–28 May 2014) with cues administered at 1300 hours each day. In all aquaria, 1 L of tank water was removed and replaced with treatment water. “Control” tanks received 1 L of filtered, dechlorinated tap water; “fish cue” tanks received 1 L fish cue water, and “tadpole + fish cues” tanks received 5 mL of the tadpole emulsion and 995 mL of fish cue water. Cue exposure stopped (29-May-2014) when hatching was imminent, indicated by embryonic tail straightening (developmental Gosner stage 18; Gosner 1960). Three embryos from each tank were collected daily during conditioning and at hatching, to assess development and growth rates over the conditioning period.
Larval rearing
Conditioning treatment groups and environmental conditions were maintained after hatching; all individuals were transferred to 9 30-L HDPE tubs containing 20 L of filtered, dechlorinated tap water. Larvae were fed algal pellets and a rabbit chow/fish flake mix (3:1) ad libitum with partial water changes performed every 5 days. Temperatures were raised to 18 °C on 30-June-2014 to reflect seasonal conditions.
Larval exposure trials
Individuals were tested for predator recognition 90 days after hatching. Following the 3 × 4 design, every treatment combination was replicated 8 times, for a total of 96 replicates. Embryonic conditioning treatments included exposure to a novel predator odor alone (largemouth bass), exposure to injured conspecific cues paired with predator odor or exposure to a water control. Larval exposure treatments included predator odor alone, injured bullfrog cues alone, a combination of injured bullfrog cues and predator odor or a water control. Experimental units (19 × 32 cm clear plastic tubs) contained one refuge consisting of an 8 × 6 cm piece of corrugated black plastic. Placement of the refuge rotated clockwise around the four corners of the unit across all 96 replicates. All units contained 1 L of control water (filtered and dechlorinated tap water) and 1 L of the randomly assigned larval exposure treatment water. Individual tadpoles were randomly selected from each embryonic conditioning treatment group, added to units, and given a 30-min acclimation period before behavioral observations began. Behavioral spot-checks and recording of refuge use were conducted every 20 min for a total of 12 observations per unit beginning at 1100 hours Each instance of refuge use across all 12 observations was recorded for all individuals. Observers were blind to assigned treatment combinations, and all experimental units were located behind observation blinds to limit observer disturbance.
Upon termination of the experiment, larvae were measured (total and snout-vent length), weighed, and developmentally staged (Gosner 1960). All individuals were humanely euthanized using MS-222 and preserved in 70% ethanol. All applicable institutional and national guidelines for the care and use of animals were followed.
Statistical analyses
We used a principal component analysis (PCA) to create a composite body size variable from total larval body length, larval snout-vent length, and larval weight. The PCA used a covariance matrix and the scores resulted from the non-rotated solution. The first axis (PC1) explained 91.5% of the variance and loaded mainly on total larval body length and secondarily on snout-vent length. The second axis (PC2) explained 8% of the variance and loaded mainly on snout-vent length. We ran two one-way ANOVAs to test the effect of embryonic conditioning treatment on PC1 and PC2 to inform subsequent analyses on potential interactions between morphological and behavioral responses. We also ran one-way ANOVAs on hatching size (embryonic body length) and developmental stage at hatching to assess direct effect of conditioning treatment.
Variation in individual refuge use was assessed using a generalized linear model with a binomial logit link to accommodate the structure of the response variable. The model was built as a time series of observations with embryonic conditioning and larval exposure as predictor variables and larval length (PC1) as a covariate. All statistical analyses were conducted using R version 3.2.3 (R Core Team 2015) and packages, car and ggplot2 (Wickham 2009; Fox and Weisberg 2011). The datasets supporting these results have been uploaded as part of the supplementary material.
Results
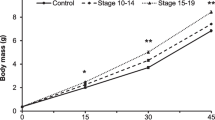
Our one-way ANOVA quantified the effects of embryonic conditioning on larval body size. We found a significant relationship between PC1 (total larval length) and embryonic conditioning (F 2,92 = 4.3, p = 0.016); there was no significant relationship between PC2 (snout-vent length) and embryonic conditioning (F 2,92 = 0.4, p = 0.7). Embryos conditioned to predator odor and bullfrog injury cues grew into longer bodied larvae (8–10% longer) relative to individuals exposed to predator odor only or controls (tad + fish: x̄ = 24.2 mm, SD = 3.8 mm; fish: x̄ = 21.9 mm, SD = 3.4 mm; control: x̄ = 22.4 mm; SD = 2.8 mm). There was no significant difference in larval body length across the predator odor only or control treatments (Fig. 1). All larvae measured at 90 days since hatching were at developmental Gosner stage 25 (Gosner 1960). There was no significant difference in embryo body length or developmental stage during cue administration or at hatching across the three embryonic conditioning treatments (embryo length: F 2,49 = 2.6, p = 0.08; embryo developmental stage F 2,49 = 1.8, p value = 0.17).
Boxplot of 90 days post-hatch larval length (PC1, accounting for 91.5% of the variation in larval total length and snout-vent length) for American bullfrog tadpoles (n = 96) that received water (control), largemouth bass and injured tadpole cues (tadpole + fish) or largemouth bass odor only (fish). Letters indicate significant difference at α = 0.05
We found a significant interaction between embryonic conditioning and larval exposure on mean refuge use (Wald test, X 2 = 23.4, df = 6, p < 0.001; Table 1). A behavioral response was found only when predation risk intensity varied across the embryonic and larval exposure treatments (Fig. 2). For example, individuals conditioned to high predation risk (predator odor with bullfrog injury cues) as embryos showed increased likelihood of refuge use as larvae when exposed to predator odor alone (log odds: 2.245; 95% CI: 0.947–4.109). Further, predator odor conditioned individuals showed increased likelihood of refuge use when exposed to the combination of predator odor with bullfrog injury cues (log odds: 1.612; 95% CI: 0.715–2.655; Fig. 3). Individuals conditioned to either control water or injured bullfrog cues alone did not increase refuge use regardless of exposure cue.
Log odds of refuge use probability of American bullfrog tadpoles (Lithobates catesbeianus) as result of embryonic conditioning and experimental exposure with 95% confidence intervals (n = 96; a value >0 indicates higher probability of refuge use of treatment compared to control exposure; a value <0 indicates lower probability of refuge use of treatment compared to control exposure). Exposure treatments to one of three variations from the control: (1) predator odor (fish—largemouth bass, Micropterus salmoides), (2) injured tadpole cues (tadpole), and (3) a combination of injured tadpole cues and predator odor (tadpole + fish). These individuals received one of three conditioning treatments as embryos: (1) predator cue (fish), (2) a combination of injured bullfrog cues and predator cues (tadpole + fish) and (3) a water control
The observed and predicted refuge use of American bullfrog tadpoles (Lithobates catesbeianus) over time for combinations of embryonic conditioning and experimental exposure treatment (n = 96). Points represent observed data and the gray curve and shaded area represent the model estimated refuge use and associated 95% confidence interval. Exposure treatments consist of the following: (1) predator odor (fish—largemouth bass, Micropterus salmoides), (2) injured tadpole cues (tadpole), (3) a combination of injured tadpole cues and predator odor (tadpole + fish), and (4) control water. These individuals received one of three conditioning treatments as embryos: (1) predator cue (fish), (2) a combination of injured bullfrog cues and predator cues (tadpole + fish) and (3) a water control
Discussion
The degree to which carry-over effects influenced American bullfrog development and behavior was dependent on the intensity and predictability of perceived risk across the embryonic and larval environments. Developmental carry-over effects occurred in individuals conditioned as embryos to high risk scenarios, specifically the combination of predator odor and injured bullfrog cues. These effects occurred regardless of larval exposure regimes (Fig. 4). Behavioral carry-over, however, was found only when embryonic conditioning cues differed from larval exposure cues, with larvae behaving with increased refuge use only when perceiving variability in risk across the embryo/larval transition. In consistently low predation risk scenarios (predator odor alone or controls), we found no evidence for developmental or behavioral defenses. This indicates a complex trade-off between a fixed developmental strategy and a more flexible behavioral strategy in risky environments.
A conceptual model illustrating the relationship between carry-over effects and embryonic/larval predation risk gradients. American bullfrogs (Lithobates catesbeianus) exposed to Largemouth bass (Micropterus salmoides) cues display developmental (longer larval lengths) and behavioral (refuge use) carry-over responses, which varied based on the timing, intensity and predictability of the predator cue exposure. High predation risk is representative of the predator odor and bullfrog injury cue treatment (tadpole + fish) and the mid-level predation risk treatment is the predator odor only (fish) treatment. Control and bullfrog injury cue only treatments elicited no behavioral or developmental responses
Predation risk intensity
Bullfrog embryos exposed to chemical cue combinations indicative of a high predation risk environment exhibited a developmental carry-over response. Individuals conditioned to predator odor and bullfrog injury cues as embryos hatched into larva that grew 10% longer compared to individuals conditioned to lower risk environments (predator odor only or control treatments). This was irrespective of larval exposure, resulting in a fixed life history strategy based on environmental conditions experienced only during the embryonic stage (Figs. 1, 4). Remarkably, this body size difference was quantified in individuals that experienced a relatively truncated conditioning period (bullfrog embryos were exposed to conditioning treatments for 2 days prior to hatching). Size differences can be directly attributed to a carry-over effect as no treatment differences in body size or length at hatching were detected.
Predation risk predictability
Uncertainty in predation risk can lead to the selection for flexible rather than fixed antipredator defenses (Roff 1992; Tollrian and Harvell 1999). Only when individuals were exposed to risk during both the embryonic and larval stages did we find evidence for a learned response. In contrast to most embryonic learning studies, this response occurred when predation risk intensity varied across the embryonic and larval exposure treatments. Individuals that were conditioned as embryos to a high risk environment (the combination of predator odor and injured bullfrog cues) exhibited increased refuge use only when exposed as larvae to a lower risk scenario (predator odor only). Inversely, individuals conditioned to predator odor only behaviorally responded as larvae when exposed to high risk environments (the combination of predator odor and injured bullfrog cues; Figs. 2, 4). Predation scenarios with low predictability, or variable risk profiles across life history stages, resulted in a more flexible antipredator strategy.
Fitness consequences
Each of these predator avoidance strategies, developmental and behavioral, have associated trade-offs in terms of energetics, survival, growth, and time until metamorphosis (Roff 1992). Predator-induced plasticity in morphological traits has been documented in multiple aquatic taxa (DeWitt 1998; Tollrian and Harvell 1999; Hettyey et al. 2015), particularly anurans (Smith and Van Buskirk 1995; Van Buskirk 2000; Relyea 2004; McIntyre et al. 2004). The effectiveness of changes to tail morphology can be highly predator specific (Van Buskirk and McCollum 2000; Relyea 2001; Van Buskirk 2002; Wilson et al. 2005) and further quantification of the functional implications of longer body lengths regarding swim speed and escape performance is needed. However, functional tradeoffs likely exist, with induced defenses associated with greater energetic expenditures, reduced development rates and longer time until metamorphosis (Van Buskirk 2000).
Behavioral carry-over effects that influence antipredator behaviors can also be costly for amphibian larvae (Skelly and Werner 1990; Epp and Gabor 2008a; van Allen et al. 2010). Refuge use, in particular, reduces an individual’s time spent foraging, and thus decreases growth and development rates (Wilbur and Collins 1973; Werner 1986; Relyea and Werner 1999). However, hiding can be an extremely effective strategy for minimizing predation risk, providing threat-sensitive protection in unpredictable predation environments. These trade-offs between increased survival in the presence of predators and decreased growth and development rates would result in strong selection for accurate risk assessment across all life history stages (Richardson 2001).
Particular treatment combinations resulted in larvae capable of producing both developmental and behavioral antipredator responses (Fig. 4). Scenarios in which individuals were conditioned to high risk cues as embryos and exposed to low risk cues during the larval stage resulted in individuals exhibiting both longer larval body lengths and greater time spent in refuge. While no statistical interactions between larval length and refuge use response were detected, we predict that individuals would suffer reduced growth and development rates over time as function of increased time spent in refuge and increased costs associated with induced morphological defenses (Benard and Fordyce 2003). Further study is needed to quantify fitness consequences associated with adopting both antipredator strategies simultaneously.
A decrease in antipredator behavior in response to increased frequency and severity of risk, albeit counter-intuitive at first, is in fact an adaptive response. The Risk Allocation Hypothesis (Lima and Bednekoff 1999; Ferrari et al. 2009) posits that prey cannot continuously increase the intensity of their antipredator response to increased risk, since they will eventually reach an unacceptable level of decreased foraging gain. Rather, the model posits that prey may be able to predict the pattern of risk and instead, allocate high antipredator response during periods of time when risk is perceived as high, and high foraging effort during periods when risk is perceived as lower. The model produces counter-intuitive results, as one individual’s high risk situation might be another one’s low risk situation. In our experiment, the high embryonic/high larval risk tadpoles displayed lower apparent level of refuge use. These results fit within the risk allocation framework.
Invading populations experience novel species interactions, abiotic conditions and physiological demands as they expand their range. These stressors can result in failed introductions if individuals are incapable of mitigating these dangers (Williamson et al. 1986; Rodriguez-Cabal et al. 2013). Our results indicate that invasive American bullfrogs are exhibiting nuanced abilities to detect predation risk based on cue exposure intensity and predictability across life history stages. Carrying over the developmental and behavioral effects of early predator exposure appears to improve bullfrog antipredator response and could increase invasion success. In habitats with consistent predation by largemouth bass, we predict that bullfrog larvae will develop longer bodies and tails relative to populations with variation in risk exposure, and perhaps be capable of escaping predators with improved swim speed and escape performance (Van Buskirk and McCollum 2000). In environments with temporal variation in predation events leading to inconsistent cue exposure and unpredictable risk regimes, we expect to see bullfrog larvae adopting behavioral antipredator responses, such as increased refuge use.
Our study suggests that embryonic learning can provision bullfrogs with specific antipredator behaviors. Larval refuge use response was predicated on that individual being conditioned to some degree of risk, be it predator odor alone or in combination with bullfrog injury cues. The lack of observed plasticity in larval bullfrog behavior is unusual as many amphibian larvae have the ability to respond to novel waterborne predator cues (Garcia et al. 2004; Epp and Gabor 2008b; Ferland-Raymond et al. 2010). However, novel species interactions involving invasive populations add complex elements of evolutionary history (Garcia et al. 2012; Pujol-Buxó et al. 2013; Hettyey et al. 2016). The relative benefits of embryonic learning over general behavioral plasticity for an invasive species are unknown and require future study.
Cue habituation may explain some of our behavioral responses. Habituation to predator stimuli is often highly selective (Blumstein 2016; Hemmi and Merkle 2009), and thus we would expect to see a reduction in refuge use after being conditioned to largemouth bass cue. While we did see minimal refuge use in treatments that exposed individuals to a consistent cue regime, individuals increased refuge use when exposed to largemouth bass cue combined with bullfrog injury cues. Similarly, conditioning to both predator odor and bullfrog injury cues resulted in increased refuge when larvae were exposed to predator cues alone. As such, we posit that embryos may have been desensitized to their conditioning cues, thus resulting in the lack of response to the same cue exposure in the larval stage. Altered cue regimes (addition or removal of tadpole injury cues) may have provided a level of novelty necessary to trigger the observed antipredator response (Winandy and Denoël 2013).
Invasive species are an excellent group in which to study carry-over effects and we would benefit from a stronger understanding of how embryonic learning and developmental carry-over impact species invasions. Invaders capable of accurately assessing risk within and across appropriate life stages can result in enhanced survivorship, increased establishment rates, and invasion success. Further, comparison of native and invasive populations of our most prolific invaders in their ability to learn and respond to novel predators would increase our awareness of population divergence in antipredator strategies. We posit that future invasion studies, and reviews of past research, must consider the influence of stress conditioning in early life history stages. Instead of asking if the chicken or the egg came first, we should be asking if, in fact, the egg knows more than we think it does.
Author contribution statement
TG and MF conceived and designed the study. TG, JU and EB performed data collection. TG and EB developed the statistical analysis. TG, JU, EB and MF contributed to the writing of the manuscript.
References
Atherton JA, McCormick MI (2015) Active in the sac: damselfish embryos use innate recognition of odours to learn predation risk before hatching. Anim Behav 103:1–6. doi:10.1016/j.anbehav.2015.01.033
Benard MF, Fordyce JA (2003) Are induced defenses costly? Consequences of predator-induced defenses in western toads, Bufo boreas. Ecology 84:68–78. doi:10.1890/0012-9658(2003)084[0068:AIDCCO]2.0.CO;2
Blumstein DT (2016) Habituation and sensitization: new thoughts about old ideas. Anim Behav 120:255–262. doi:10.1016/j.anbehav.2016.05.012
Brown GE, Smith RJF (1998) Acquired predator recognition in juvenile rainbow trout (Oncorhynchus mykiss): conditioning hatchery-reared fish to recognize chemical cues of a predator. Can J Fish Aquat Sci 55:611–617. doi:10.1139/cjfas-55-3-611
Chivers DP, Wisenden BD, Smith R, Jan F (1996) Damselfly larvae learn to recognize predators from chemical cues in the predator’s diet. Anim Behav 52:315–320. doi:10.1006/anbe.1996.0177
DeWitt TJ (1998) Costs and limits of phenotypic plasticity: tests with predator-induced morphology and life history in a freshwater snail. J Evol Biol 11:465. doi:10.1007/s000360050100
Epp KJ, Gabor CR (2008) Innate and learned predator recognition mediated by chemical signals in Eurycea nana. Ethology 114:607–615. doi:10.1111/j.1439-0310.2008.01494.x
Ferland-Raymond B, March RE, Metcalfe CD, Murray DL (2010) Prey detection of aquatic predators: assessing the identity of chemical cues eliciting prey behavioral plasticity. Biochem Syst Ecol 38:169–177. doi:10.1016/j.bse.2009.12.035
Ferrari MCO, Chivers DP (2009a) Temporal variability, threat sensitivity and conflicting information about the nature of risk: understanding the dynamics of tadpole antipredator behaviour. Anim Behav 78:11–16. doi:10.1016/j.anbehav.2009.03.016
Ferrari MCO, Chivers DP (2009b) Sophisticated early life lessons: threat-sensitive generalization of predator recognition by embryonic amphibians. Behav Ecol 20:1295–1298. doi:10.1093/beheco/arp135
Ferrari MCO, Chivers DP (2009c) Latent inhibition of predator recognition by embryonic amphibians. Biol Lett 5:160–162. doi:10.1098/rsbl.2008.0641
Ferrari MCO, Chivers DP (2010) The ghost of predation future: threat-sensitive and temporal assessment of risk by embryonic woodfrogs. Behav Ecol Sociobiol 64:549–555. doi:10.1007/s00265-009-0870-y
Ferrari MCO, Chivers DP (2013) Adaptive responses of embryonic amphibians to predation risk. In: East ML, Dehnhard M (eds) Chemical signals in vertebrates, 12th edn. Springer, New York, pp 229–244
Ferrari MCO, Manek AK, Chivers DP (2010a) Temporal learning of predation risk by embryonic amphibians. Biol Lett 6:308–310. doi:10.1098/rsbl.2009.0798
Ferrari MCO, Wisenden BD, Chivers DP (2010b) Chemical ecology of predator–prey interactions in aquatic ecosystems: a review and prospectus. The present review is one in the special series of reviews on animal–plant interactions. Can J Zool 88:698–724. doi:10.1139/Z10-029
Ferrari MCO, Brown GE, Messier F, Chivers DP (2009) Threat-sensitive generalization of predator recognition by larval amphibians. Behav Ecol Sociobiol 63:1369–1375
Ferrari MCO, McCormick MI, Allan BJM et al (2015) Living in a risky world: the onset ond ontogeny of an integrated antipredator phenotype in a coral reef fish. Sci Rep 5:15537
Fox J, Weisberg S (2011) An R companion to applied regression, 2nd edn. Sage, Thousand Oaks
Garcia TS, Stacy J, Sih A (2004) Larval salamander response to UV radiation and predation risk: color change and microhabitat use. Ecol Appl 14:1055–1064
Garcia TS, Thurman LL, Rowe JC, Selego SM (2012) Antipredator behavior of American Bullfrogs (Lithobates catesbeianus) in a novel environment. Ethology 118:867–875. doi:10.1111/j.1439-0310.2012.02074.x
Gosner KL (1960) A simplified table for staging anuran embryos and larvae with notes on identification. Herpetologica 16:183–190
Hay ME (2009) Marine chemical ecology: chemical signals and cues structure marine populations, communities, and ecosystems. Ann Rev Mar Sci 1:193–212. doi:10.1146/annurev.marine.010908.163708
Hayes MP, Jennings MR (1986) Decline of ranid frog species in Western North America: are Bullfrogs (Rana catesbeiana) responsible? J Herpetol 20:490–509
Hemmi JM, Merkle T (2009) High stimulus specificity characterizes anti-predator habituation under natural conditions. Proc R Soc B Biol Sci 276:4381–4388. doi:10.1098/rspb.2009.1452
Hettyey A, Tóth Z, Thonhauser KE et al (2015) The relative importance of prey-borne and predator-borne chemical cues for inducible antipredator responses in tadpoles. Oecologia 179:699–710. doi:10.1007/s00442-015-3382-7
Hettyey A, Thonhauser KE, Bókony V et al (2016) Naive tadpoles do not recognize recent invasive predatory fishes as dangerous. Ecology 97:2975–2985. doi:10.1002/ecy.1532
Jacobsen HP, Stabell OB (2004) Antipredator behaviour mediated by chemical cues: the role of conspecific alarm signalling and predator labelling in the avoidance response of a marine gastropod. Oikos 104:43–50. doi:10.1111/j.0030-1299.2004.12369.x
Lever C (2003) Naturalized reptiles and amphibians of the world. Oxford University Press, Oxford and New York
Lima SL, Dill LM (1990) Behavioral decisions made under the risk of predation: a review and prospectus. Can J Zool 68:619–640. doi:10.1139/z90-092
Lima SL, Bednekoff PA (1999) Temporal Variation in Danger Drives Antipredator Behavior: the Predation Risk Allocation Hypothesis. Am Nat 153:649–659
Lowe C, Browne S, Boudjelas M, De SM (2000) 100 of the world’s worst invasive alien species: a selection from the global invasive species database. Aliens 12:12
Marshall D, Pechenik J, Keough M (2003) Larval activity levels and delayed metamorphosis affect post-larval performance in the colonial ascidian Diplosoma listerianum. Mar Ecol Prog Ser 246:153–162. doi:10.3354/meps246153
Mathis A, Ferrari MC, Windel N et al (2008) Learning by embryos and the ghost of predation future. Proc R Soc B Biol Sci 275:2603–2607. doi:10.1098/rspb.2008.0754
McCarthy TM, Fisher WA (2000) Multiple predator-avoidance behaviours of the freshwater snail Physella heterostropha pomila: responses vary with risk. Freshw Biol 44:387–397. doi:10.1046/j.1365-2427.2000.00576.x
McIntyre PB, Baldwin S, Flecker AS (2004) Effects of behavioral and morphological plasticity on risk of predation in a Neotropical tadpole. Oecologia 141:130–138. doi:10.1007/s00442-004-1652-x
Mitchell MD, McCormick MI (2013) Ontogenetic differences in chemical alarm cue production determine antipredator responses and learned predator recognition. Behav Ecol Sociobiol 67:1123–1129. doi:10.1007/s00265-013-1537-2
Nelson AB, Alemadi SD, Wisenden BD (2013) Learned recognition of novel predator odour by convict cichlid embryos. Behav Ecol Sociobiol 67:1269–1273. doi:10.1007/s00265-013-1554-1
Pearl CA, Adams MJ, Bury RB, McCreary B (2004) Asymmetrical Effects of Introduced Bullfrogs (Rana catesbeiana) on native Ranid Frogs in Oregon. Copeia 2004:11–20
Pechenik JA (2006) Larval experience and latent effects—metamorphosis is not a new beginning. Integr Comp Biol 46:323–333. doi:10.1093/icb/icj028
Pintor LM, Byers JE (2015) Do native predators benefit from non-native prey? Ecol Lett 18:1174–1180. doi:10.1111/ele.12496
Polo-Cavia N, Gomez-Mestre I (2014) Learned recognition of introduced predators determines survival of tadpole prey. Funct Ecol 28:432–439. doi:10.1111/1365-2435.12175
Pujol-Buxó E, San Sebastián O, Garriga N, Llorente GA (2013) How does the invasive/native nature of species influence tadpoles’ plastic responses to predators? Oikos 122:19–29. doi:10.1111/j.1600-0706.2012.20617.x
R Core Team (2015) R: A Language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria
Relyea RA (2001) Morphological and behavioral plasticity of larval anurans in response to different predators. Ecology 82:523–540
Relyea RA (2004) Fine-tuned phenotypes: tadpole plasticity under 16 combinations of predators and competitors. Ecol Ecol 85:172–179
Relyea RA, Werner EE (1999) Quantifying the relation between predator-induced behavior and growth performance in larval anurans. Ecology 80:2117–2124
Richardson JML (2001) A comparative study of activity levels in larval anurans and response to the presence of different predators. Behav Ecol 12:51–58. doi:10.1093/oxfordjournals.beheco.a000378
Rodriguez-Cabal MA, Williamson M, Simberloff D (2013) Overestimation of establishment success of non-native birds in Hawaii and Britain. Biol Invasions 15:249–252. doi:10.1007/s10530-012-0285-y
Roff DA (1992) The evolution of life histories: theory and analysis. Chapman and Hall, New York
Sih A, Moore R (1993) Delayed hatching of salamander eggs in response to enhanced larval predation risk on JSTOR. Am Nat 142:947–960
Sih A, Bolnick DI, Luttbeg B et al (2010) Predator-prey naïveté, antipredator behavior, and the ecology of predator invasions. Oikos 119:610–621. doi:10.1111/j.1600-0706.2009.18039.x
Skelly DK, Werner EE (1990) Behavioral and life-historical responses of larval American Toads to an odonate predator. Ecology 71:2313–2322. doi:10.2307/1938642
Smith DC, Van Buskirk J (1995) Phenotypic design, plasticity, and ecological performance in two tadpole species. Source Am Nat Am Nat 145:211–233
Tarvin RD, Silva Bermúdez C, Briggs VS, Warkentin KM (2015) Carry-over effects of size at metamorphosis in red-eyed treefrogs: higher survival but slower growth of larger metamorphs. Biotropica 47:218–226. doi:10.1111/btp.12198
Tollrian R, Harvell CD (1999) The ecology and evolution of inducible defenses. Princeton University Press, Princeton
van Allen BG, Briggs VS, McCoy MW, Vonesh JR (2010) Carry-over effects of the larval environment on post-metamorphic performance in two hylid frogs. Oecologia 164:891–898. doi:10.1007/s00442-010-1728-8
Van Buskirk J (2000) The costs of an inducible defence in anuran larvae. Ecol 81:2813–2821
Van Buskirk J (2002) A comparative test of the adaptive plasticity hypothesis: relationships between habitat and phenotype in Anuran Larvae. Am Nat 160:87–102. doi:10.1086/340599
Van Buskirk J, McCollum SA (2000) Influence of tail shape on tadpole swimming performance. J Exp Biol 203:2149–2158
Van Buskirk J, Schmidt BR (2000) Predator-induced phenotypic plasticity in larval newts: trade-offs, selection, and variation in nature. Ecol 81:3009–3028
Warkentin KM (1995) Adaptive plasticity in hatching age: a response to predation risk trade-offs. Proc Natl Acad Sci 92:3507–3510. doi:10.1073/pnas.92.8.3507
Warkentin KM (2007) Oxygen, gills, and embryo behavior: mechanisms of adaptive plasticity in hatching. Comp Biochem Physiol Part A Mol Integr Physiol 148:720–731. doi:10.1016/j.cbpa.2007.02.009
Werner EE (1986) Amphibian metamorphosis: growth rite, predation risk and the optimal size at transformation. Am Nat 128:319–341
Wickham H (2009) ggplot2: elegant graphics for data analysis. Springer, New York. doi:10.1007/978-0-387-98141-3
Wilbur HM, Collins JP (1973) Ecological aspects of Amphibian Metamorphosis: nonnormal distributions of competitive ability reflect selection for facultative metamorphosis. Science 182:1305–1314. doi:10.1126/science.182.4119.1305
Williamson MH, Brown KC, Holdgate MW et al (1986) The analysis and modelling of british invasions. Philos Trans R Soc B Biol Sci 314:505–522. doi:10.1098/rstb.1986.0070
Wilson RS, Kraft PG, Van Damme R (2005) Predator-specific changes in the morphology and swimming performance of larval Rana lessonae. Funct Ecol 19:238–244. doi:10.1111/j.1365-2435.2005.00958.x
Winandy L, Denoël M (2013) Cues from Introduced fish alter shelter use and feeding behaviour in adult Alpine Newts. Ethology 119:121–129. doi:10.1111/eth.12043
Acknowledgements
We would like to acknowledge Angie Soken, Randy Wildman, Dave Paoletti and Greyson Paoletti for help with animal collection. Danielle Nelson and Lindsey Thurman assisted with experimental set-up and animal care. Animals were collected under Oregon Department of Fisheries and Wildlife Service Special Use Permit No. 008-14 and Oregon State University Animal Care and Use Protocol No. 4575.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Communicated by Joel trexler.
Rights and permissions
About this article
Cite this article
Garcia, T.S., Urbina, J.C., Bredeweg, E.M. et al. Embryonic learning and developmental carry-over effects in an invasive anuran. Oecologia 184, 623–631 (2017). https://doi.org/10.1007/s00442-017-3905-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00442-017-3905-5