Abstract
Repeated pathogen exposure is a common threat in colonies of social insects, posing selection pressures on colony members to respond with improved disease-defense performance. We here tested whether experience gained by repeated tending of low-level fungus-exposed (Metarhizium robertsii) larvae may alter the performance of sanitary brood care in the clonal ant, Platythyrea punctata. We trained ants individually over nine consecutive trials to either sham-treated or fungus-exposed larvae. We then compared the larval grooming behavior of naive and trained ants and measured how effectively they removed infectious fungal conidiospores from the fungus-exposed larvae. We found that the ants changed the duration of larval grooming in response to both, larval treatment and their level of experience: (1) sham-treated larvae received longer grooming than the fungus-exposed larvae and (2) trained ants performed less self-grooming but longer larval grooming than naive ants, which was true for both, ants trained to fungus-exposed and also to sham-treated larvae. Ants that groomed the fungus-exposed larvae for longer periods removed a higher number of fungal conidiospores from the surface of the fungus-exposed larvae. As experienced ants performed longer larval grooming, they were more effective in fungal removal, thus making them better caretakers under pathogen attack of the colony. By studying this clonal ant, we can thus conclude that even in the absence of genetic variation between colony members, differences in experience levels of brood care may affect performance of sanitary brood care in social insects.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Organisms benefit greatly from the ability to respond to repeated extrinsic stimuli by improved task performance. Insects are known to gather experience and to be able to learn in different contexts (Dukas 2008), such as foraging (Dukas and Visscher 1994; Ravary et al. 2007) and thermal brood relocation (Weidenmüller et al. 2009). Experience could for instance result in (i) a faster reaction time to the stimulus (e.g., Weidenmüller et al. 2009), (ii) a higher probability to perform the task (e.g., Ravary et al. 2007), and (iii) a more efficient task performance (e.g., O’Donnell and Jeanne 1992; Dukas and Visscher 1994; Raine and Chittka 2007), or a combination of these.
Colonies of social insects (wasps, bees, ants, and termites) are a good model system to study the effects of experience in social groups, as they are characterized by a strong division of labor with different group members specializing on particular tasks (Oster and Wilson 1978). Task specialization, the long-term behavioral bias toward the performance of a task (Oster and Wilson 1978), can be affected by factors such as age (age polyethism; reviewed, e.g., in Hölldobler and Wilson 1990; Robinson 1992; Mersch et al. 2013) and genetic background (e.g., patriline: Robinson and Page 1988, 1989; Waddington et al. 2010; Schlüns et al. 2011; genetic colony composition: Arathi and Spivak 2001) and seems further modulated by experience (Theraulaz et al. 1998; Ravary et al. 2007). Response-threshold models propose that task specialization within groups arises from inter-individual variation in response thresholds for task-associated stimuli. Individuals with the lowest thresholds most likely become specialists, as they will be the first to respond and thereby decrease the stimulus level present in the nest, so that the higher thresholds of their nestmates will not be met (reviewed in Beshers and Fewell 2001). Furthermore, task performance of the acting individuals can further lower their future reaction threshold, increasing the propensity of future task performance and further increasing specialization (self-reinforcement: e.g., Theraulaz et al. 1998; Weidenmüller 2004; Westhus et al. 2013).
Whereas previous work studied the effect of experience on activities such as foraging, nursing, temperature control, and colony emigration in social insects (e.g., Dukas and Visscher 1994; Langridge et al. 2004, 2008; Weidenmüller 2004; Ravary et al. 2007; Dukas 2008; Weidenmüller et al. 2009; Muscedere et al. 2013; Westhus et al. 2013), it is not well understood how experience in anti-pathogen defense affects the performance and efficiency of sanitary behaviors in the colonies of social insects. Yet, this is an important factor particularly in the long-lived colonies of ants and termites, where individuals likely are repeatedly confronted with the same pathogens within their lifespan of several months to years (Schmid-Hempel 1998; Schmid-Hempel 2005). Repeated pathogen exposures can occur because individuals pick up the same infectious particles recurrently from their surroundings or by contraction from their sick nestmates inside the colony (Rosengaus and Traniello 1997; Hughes et al. 2002; Cremer et al. 2007; Konrad et al. 2012).
Repeated pathogen exposure may affect both the physiological immune system and the behavioral defense line, which in social insects can occur both at the individual and group level (e.g., Siva-Jothy et al. 2005; Cremer et al. 2007; Wilson-Rich et al. 2009). The physiological immune system of social insects (bumblebees: Sadd and Schmid-Hempel 2006; ants: Rosengaus et al. 2013) shows lower susceptibility to a previously encountered pathogen upon secondary contact (referred to as immune priming in invertebrates; Kurtz and Armitage 2006). At low exposure doses, an infection can even lead to a protective immune upregulation (termites: Rosengaus et al. 1999; ants: Konrad et al. 2012), and such low-level exposures may be contracted during social contact with a diseased nestmate (“social immunization”; Konrad et al. 2012).
Also, the behavioral anti-pathogen defenses of social insects were found to be upregulated after first pathogen exposure. Ant colonies that had previous contact to the fungal pathogen Metarhizium show increased expression of collective sanitary behaviors, such as allogrooming (Walker and Hughes 2009; Reber et al. 2011). During allogrooming, social insects efficiently remove infectious particles from the body surface of their nestmates (Rosengaus et al. 1998; Hughes et al. 2002; Yanagawa et al. 2008; Tragust et al. 2013a). To our knowledge, it has not been studied, however, if repeated pathogen encounter of particular individuals changes their propensity to perform sanitary brood care of diseased brood. Brood care is an integral part of sociality in insect societies and is of particular importance in the context of disease defense, as the brood is, on one hand, particularly susceptible to disease (Patterson and Briano 1993) and, on the other hand, very valuable, as it represents the next generation of workers and sexuals in the colony.
Social insects react to pathogen-exposed brood either by intensive brood grooming (ants: Ugelvig et al. 2010, Tragust et al. 2013b), by cannibalism (termites: Rosengaus and Traniello 2001), or by brood removal from the colony, the latter being termed “hygienic behavior” (bees: Rothenbuhler and Thompson 1956; Wilson-Rich et al. 2009; ants: Ugelvig et al. 2010; Tragust et al. 2013b). Whereas little is known for other social insects, such hygienic behavior is performed by middle-aged individuals in honeybees (Arathi et al. 2000). Moreover, the propensity to perform the task depends on the genotype, the genetic colony composition, and is higher in individuals with increased olfactory sensitivity (Arathi and Spivak 2001; Masterman et al. 2001; Gramacho and Spivak 2003; Arathi et al. 2006). In general, hygienic tasks do not seem to be equally performed by all individuals of a colony, but rather by a subset of specialized workers (Trumbo et al. 1997; Arathi et al. 2000). This task specialization promotes behavioral and spatial nest compartmentalization, which is predicted to decrease the risk of pathogen transmission (Schmid-Hempel and Schmid-Hempel 1993; Naug and Camazine 2002; Cremer et al. 2007), yet the factors underlying hygienic task specialization and division of labor are not well understood.
The aim of the current study was to test whether individual experience in sanitary brood care—gained by repeated contact of workers to pathogen-exposed brood—may affect the performance or effectiveness of their hygienic actions. As a model system, we used the parthenogenetically reproducing (thelytokous) ant Platythyrea punctata (Schilder et al. 1999) and the general insect pathogenic fungus Metarhizium robertsii (Bischoff et al. 2009), a natural pathogen of ants (Rath et al. 1992; Keller et al. 2003; St. Leger et al. 2011). We chose this ant species that forms small colonies by clonal reproduction to study the effect of experience on task performance in groups with a low level of division of labor (Hartmann and Heinze 2003) and in the absence of genetic variation among workers. In our experimental approach, we repeatedly confronted ant workers to Metarhizium-exposed or sham-treated larvae. We then compared ants before and after training, i.e., at first contact with the larvae (trial 1) and after nine consecutive contacts of the same type of larvae (all fungus-exposed or all controls; trial 9) to test whether trained as compared to naive ants may show (i) a faster response to the stimulus, (ii) increased grooming performance, and (iii) higher removal of fungal infectious particles, or a combination of these. This experimental design allowed us not only to determine whether experience may affect the performance or efficiency of sanitary brood care in the clonal ant P. punctata, but also to disentangle a general effect of repeated brood care (trial 1 vs. 9) from a specific effect of repeated pathogen exposure (interaction between trial and treatment).
Materials and methods
Ant colonies
Colonies of the thelytokous ant P. punctata were collected from two populations in Puerto Rico (2005, authorized by the Departamento de Recoursos Naturales y Ambientales DRNA), one from the Dominican Republic (2006, authorized by La Dirección General de Vida Sylvester y Biodiversidad) and one from Barbados (2007, authorized by the Ministry of Agriculture and Rural Development), as detailed in Kellner et al. 2013. Ants were reared in the laboratory under standard conditions (24 ± 2 °C, 60–65 % humidity, 12:12 h light/dark cycle; see Hartmann and Heinze 2003). All ants used in the experiments had eclosed in the laboratory and were therefore naive to the fungal pathogen.
Eight colonies with confirmed clonal colony structure (microsatellite analysis; Kellner and Heinze 2011) were used as source colonies to set up two subcolonies from each. All subcolonies consisted of 15 individually color-marked (enamel paint, PinRestore) adult ants with brood (ten larvae and pupae). The size of these subcolonies lies in the natural range of colony size of this ponerine ant species (Kellner and Heinze 2011). To ensure that only non-reproductive workers were used in the following experiment, the single reproductive individual that established in each subcolony within 2 weeks after set up was determined by observation of egg-laying. It remained within its respective subcolony over the whole course of the experiment. Twenty additional colonies were used as “larval donors” to provide brood for the experiments, as colonies of P. punctata readily accept alien brood (Kellner et al. 2010). We excluded the smallest and largest larval stages, i.e., L1 and L5, respectively, and preferentially used L3 larvae (mean weight 5.01 ± 1.11 mg standard deviation as revealed from weighing of 116 larvae in a preliminary experiment).
Fungal pathogen exposure
As a pathogen, we used the entomopathogenic fungus M. robertsii (strain ARSEF 2575 (Fang et al. 2006), previously named Metarhizium anisopliae, but now recognized as a sister species (Bischoff et al. 2009)). The non-sexually produced fungal transmission stage (conidiospores/conidia) was freshly harvested from malt agar plates 3 to 4 days before the start of each experiment (Konrad et al. 2012), suspended in the sterile surfactant Triton X-100 (0.05 %; Sigma) at a concentration of 106 conidiospores/ml and the fungal germination rate determined (>97 % in all cases). In the fungus exposure treatment, ant larvae were exposed to 500 conidiospores of the pathogenic fungus by applying 0.5 μl of the conidiospore suspension, while the sham control was treated with the same amount of Triton X solution. Treated larvae were air-dried on sterile plastic for 1 h before start of each experiment. This exposure dose was chosen to (i) provide a stimulus that likely approximates a natural exposure dose from surrounding soil (Keller et al. 2003: 104 colony forming units of M. anisopliae per gram soil) and to (ii) prevent tending ants from contracting the disease so we could study behavioral changes based on repeated hygienic brood care experience rather than sickness of the tending workers.
Experimental procedure
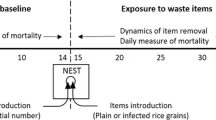
We randomly assigned one subcolony per source colony to the sham control and the other subcolony to fungus exposure. From each subcolony, eight intra-nest workers were taken from the brood chamber, isolated from the colony for 1 h, during which they were placed individually in an arena (petri dish, diameter 5.5 cm), where they encountered a larva (a Triton X-treated larva for ants from the control-subcolony and a fungus-exposed larva for ants from the fungus-subcolony). Despite their isolation from the colony, ant workers readily engaged in normal brood care behavior. We repeated this procedure with exactly the same eight ants per colony over five consecutive days, with two trials per day (morning and afternoon training session) on the first 4 days and one on the fifth day (morning session only), always using a new larva and arena, so that each ant was trained for nine consecutive trials (Fig. 1). Trials 1 and 9 could thus be compared without any potential confounding effect of time of day, which is a known factor influencing behavior (Blackmer and Byrne 2008). After each trial, the ants were placed back into their subcolony and left undisturbed until the next trial. We performed the experiments for the two subcolonies (sham control and fungus exposure) of each source colony at the same time and the eight experiments/colonies in consecutive order within a period of 8 weeks.
Experimental design. Platythyrea punctata ants were individually confronted with either one sham-treated control larva (blue group) or fungus-exposed larva (green group) in nine consecutive trials (each of 1 h). Behavioral observations were performed for naive ants at their first encounter with the respectively treated larva (trial 1; light colors) and for trained ants after repeated encounters (trial 9; dark colors). Conidiospore number of fungus-exposed larvae was counted after trial 1 and trial 9 to test for changes in conidiospore removal capacity of the ants due to experience
Behavioral observations
“Naive ants” (trial 1) and “trained ants” (trial 9) were video-recorded immediately after the larvae were placed into the arena containing the ants (Logitech QuickCam Sphere AF). Videos were analyzed blindly with respect to larval treatment, using the software BioLogic (http://sourceforge.net/projects/biologic/). We determined the time to first contact (mostly antennation behavior) of the ant to the larva, as well as the time to first performance of sanitary brood care (larval grooming; Ugelvig et al. 2010) after the start of the experiment. We also determined the frequency (number of events/hour) and duration of larval grooming and worker self-grooming during the complete hour of the trial. We did not detect any trophallaxis behavior between the adult ants and the larvae. In very rare cases, ants also showed mandible-opening behavior, which is generally classified as slightly aggressive behavior. Due to its rareness, it was not included in the analysis.
Conidiospore removal
To determine how many of the originally applied 500 conidiospores were removed by the ants during grooming, we washed off conidiospores from the surface of fungus-exposed larvae (as in Hughes et al. 2002) after the 1-h contact to either naive (trial 1) or trained (trial 9) ants and counted them. To account for potential differences in fungal attachment to the larval cuticle (Vestergaard et al. 1999), we determined the baseline number of conidiospores that could be washed off from additional fungus-exposed larvae for each source colony after 1 h in the absence of any ants, both at day 1 (trial 1) and day 5 (trial 9). Washes were performed by vortexing each larva for 2 min in 50-μl Triton X solution. After larval removal, the conidiospore suspension was concentrated to 10 μl and the number of conidiospores determined by counting five droplets of 1 μl each per sample under a stereomicroscope (at × 400 magnification).
Disease contraction by adult ants
At the end of the experiment, we determined whether adult ants had contracted the disease during contact with the larvae. To this end, all ants were killed by freezing for 6 min at −20 °C, which does not affect conidiospore germination (M. Klatt and CW unpublished data). To check for internal fungal infection, each ant was surface-sterilized (Lacey and Brooks 1997) and monitored for outgrowth of M. robertsii hyphae and conidiospores over 3 weeks under humid conditions at 24 °C.
Data analysis
We used the program R (v. 2.12.1) for all statistical analyses.
Behavioral observations
We analyzed a mean of five (2–8) ants per colony and treatment, at both their first and ninth trial, to compare the same ant in its naive and trained state (total of 80 ants, 40 of which were trained to sham-treated larvae and 40 to fungus-exposed larvae; total 160 h of observations). Ninety-three percent (74/80) of the ants (37/40 in both groups) had contact to the larva in both trials 1 and 9 of the experiment, and only these ants were subjected to further statistical analysis. We compared the occurrence of larval grooming performed by naive and trained ants toward sham-treated and fungus-exposed larvae with Fisher exact tests and corrected the significance level α to 0.025 due to multiple testing (Bonferroni correction). We analyzed the time to first larval contact and the time delay between first contact and first grooming using survival analysis (Cox proportional regression, generating Wald statistics), as data were censored (R package “survival” (Therneau 2012)). Larval treatment (sham control vs. fungus exposure) and experience of the ants (trial 1 vs. 9) were included as fixed effects, with individual ants nested within their source colony as random effect using the frailty function in coxph. Non-significant interaction terms were removed from the final models. For behavioral frequencies and durations, we performed linear mixed-effects models (LMM) to analyze the effects of treatment (sham control vs. fungus exposure) and experience (trial 1 vs. 9) as fixed effects and their interaction, with individual ants nested within their source colony as random effect, thereby controlling for repeated measures of the same individual (R package “nlme” (Pinheiro et al. 2012)). Data either showed normal error distribution and homogeneity of variance or reached it by log(x + 1)-transformation (applied for larval grooming frequency and frequency and duration of worker self-grooming). From the full model, the significance of the interaction and each fixed factor were tested by removing the factor of interest (likelihood-ratio test) to obtain a minimal adequate model only containing significant terms.
Conidiospore removal
We tested for a potential difference in the basal fungal attachment rates in the absence of ants in trials 1 and 9 by performing a LMM with trial as fixed factor (trial 1: n = 31, trial 9: n = 37) and source colony as a random effect. Following, we determined whether the number of conidiospores washed off from fungus-exposed larvae depended on experience (trial 1 vs. 9) and grooming duration performed by the ants during the respective trial (n = 24 ants, each in trial 1 and 9), by performing a LMM including trial as a fixed factor and grooming duration as covariate and individual ants and source colony as random effect. Conidiospore number was log-transformed to obtain a normally distributed error structure and homogeneity of variance. For display, we show the number of conidiospores removed by grooming (i.e., the applied 500 minus the count value of conidiospores still to be washed off after the experiment).
The data sets supporting the results of this article are available in the DRYAD repository (doi:10.5061/dryad.NNNNN).
Results
Performance of sanitary behavior
Occurrence of sanitary brood care
Ninety-two percent of the ants that had larval contact performed larval grooming (trial 1: sham control: 36/37, fungus exposure: 32/37; trial 9: sham control: 36/37, fungus exposure: 33/37), with no difference between larval treatments (Fisher exact test with α-level of 0.025; sham control vs. fungus exposure: P = 0.056) or experience level of the ants (trial 1 vs. trial 9: P = 1.000).
Time to response
Naive and trained ants (trials 1 and 9) of both treatment groups (sham control and fungus exposure) first approached larvae approximately 6 min after start of the experiment (Fig. 2a). Hence, there was no significant effect of treatment or experience in the time to first contact (Cox regression, treatment: Wald χ 2 = 0.71, df = 1, P = 0.400; trial: Wald χ 2 = 0.33, df = 1, P = 0.560). Ants of all groups then started grooming approximately another 6 min after the first contact (Fig. 2b). There was thus also no effect of larval treatment or experience of the ant on the delay period between the first contact to the larva and the first grooming (treatment: Wald χ 2 = 0.30, df = 1, P = 0.580; trial: Wald χ 2 = 0.60, df = 1, P = 0.440).
Time to response of brood care. Neither a the time to first contact of the ants to the larvae after start of the experiment nor b the delay between the first contact and performance of sanitary brood care (larval grooming) depended on larval treatment (sham control: blue; fungus exposure: green) or experience of the ants (naive ants in trial 1: light colors, trained ants in trial 9: dark colors). The numbers of cases across all replicates are shown as a heatmap with respective median values indicated as square in the color of the group
Frequency and duration of larval grooming
The frequency of larval grooming did differ neither across treatments (sham control vs. fungus exposure) nor between naive and trained ants (trial 1 vs. 9; LMM: treatment: LR1,4 = 2.807, P = 0.094; trial: LR1,5 = 0.118, P = 0.731; interaction treatment*trial: LR1,6 = 0.0003, P = 0.987; Fig. 3a). Grooming duration, however, was significantly affected by larval treatment (LMM: LR1,5 = 6.941, P = 0.008) with sham-treated larvae being groomed longer than fungus-exposed larvae. Moreover, trained ants groomed the larvae longer than naive ants, such that ants in the sham control increased larval grooming duration from trial 1 to 9 by 14 % and ants in the fungus treatment even by 32 % (Fig. 3b; LR1,5 = 4.473, P = 0.034). There was no significant interaction between treatment and trial (LR1,6 = 0.253, P = 0.615).
Frequency and duration of larval grooming. a Larval grooming frequency did differ neither between naive and trained ants (trial 1: light colors, trial 9: dark colors) nor across larval treatments (sham control: blue; fungus exposure: green). b Larval grooming duration, however, was significantly higher toward sham-treated (blue) than fungus-exposed (green) larvae and was performed significantly longer by trained (trial 9, dark colors) than naive (trial 1, light colors) ants. Bars depict mean ± SEM
Frequency and duration of worker self-grooming
The frequency of worker self-grooming was significantly affected by trial (LMM: trial: LR1,4 = 19.942, P < 0.0001) with naive workers self-grooming more frequently than trained ants, irrespective of larval treatment (LMM: LR1,5 = 0.041, P = 0.840), with no significant interaction between treatment and trial (LR1,6 = 0.715, P = 0.398; mean ± SE in events/hour; sham control: trial 1 56.6 ± 3.5, trial 9 35.6 ± 3.6; fungus exposure: trial 1 57.9 ± 6.8, trial 9 34.2 ± 2.9). Similarly, self-grooming duration was significantly affected by trial (LMM: LR1,4 = 4.683, P = 0.031) with naive workers self-grooming longer than trained ants but remained un-affected by larval treatment (LMM: LR1,5 = 0.112, P = 0.738). Again, no significant interaction was found between treatment and trial (LR1,6 = 0.718, P = 0.397; mean ± SE in min; sham control: trial 1 5.9 ± 0.7, trial 9 4.0 ± 0.5; fungus exposure: trial 1 5.6 ± 0.8, trial 9 4.6 ± 0.7).
Conidiospore removal
We found that the basal attachment rate of the fungal conidiospores to the larvae, as measured in the absence of any workers, showed a non-significant trend of being higher in trial 9 vs. trial 1 (LMM: LR1,4 = 3.712, P = 0.054). Despite this tendency toward stronger fungal attachment, trained ants removed a significantly higher number of conidiospores from the fungus-exposed larvae than naive ants (mean ± SE of proportion conidiospores removed in trial 1 63.1 ± 0.07 % and trial 9 76.2 ± 0.05 %; LMM: LR1,5 = 4.243, P = 0.039). Moreover, ants expressing longer larval grooming duration removed a higher number of conidiospores than ants that groomed the larvae shorter (Fig. 4; LMM: LR1,5 = 20.937, P < 0.0001). There was no significant interaction between the experience of the ants (trial 1 vs. 9) and their grooming duration (LR1,6 = 0.098, P = 0.754), indicating that trained ants did not remove more conidiospores per unit time than naive ants.
Conidiospore removal depending on larval grooming duration. Both naive (trial 1, light green) and trained ants (trial 9, dark green) removed significantly increasing numbers of conidiospores from the fungus-exposed larva with increasing larval grooming duration. Trained ants removed significantly more fungal conidiospores from the larvae than naive ants. Trend lines are shown for both groups (trial 1 and trial 9) in the respective color
Disease contraction by adult ants
None of the ants tending fungus-exposed larvae (n = 40) showed any fungal outgrowth of M. robertsii after surface sterilization, and the same was true for the ants tending sham-treated larvae (n = 40). We could therefore not detect any signs of internal infections of ants handling fungus-exposed brood.
Discussion
We tested whether repeated contact to either sham-treated or fungus-exposed larvae would affect the performance of sanitary brood care in the clonal ant P. punctata. The experience of individual ants did not affect their probability or time to respond. Independent of their experience and larval treatment, ants thus (i) were equally likely to contact and groom the larvae, (ii) showed no difference in time to detection (first contact; Fig. 2a) and reaction (first contact to first grooming; Fig. 2b), and (iii) performed larval grooming at equal frequencies (Fig. 3a). However, we found a plastic response for the duration of grooming behavior. Ants groomed sham-treated larvae longer than fungus-exposed ones and trained ants in their ninth trial performed longer grooming than naive ants in both treatments (Fig. 3b), thereby removing a larger number of infectious conidiospores from the brood (Fig. 4). Repeated brood care therefore led to a more effective anti-pathogen defense in P. punctata. Yet, this increased sanitary brood care came along with reduced individual worker hygiene, as trained ants performed less self-grooming.
Our results contrast to previous findings in Camponotus ants, where experience led to a faster response in brood thermoregulation (Weidenmüller et al. 2009). Yet, they are in line with previous studies on P. punctata and other ants that—whilst not testing for experience but reaction to first pathogen contact—also found that ants did not differentiate between healthy and contaminated larvae during transport of larvae into their brood chamber (Ugelvig et al. 2010; Tragust et al. 2013b), even if the larval exposure dose used in the current experiment was thousandfold lower. Whereas it is known that social insects can contract Metarhizium infections from high-dose-exposed nestmates (Rosengaus and Traniello 1997; Hughes et al. 2002; Konrad et al. 2012), none of the ants in our low-dose experiment showed any signs of infection with M. robertsii or died from the disease.
Many studies report an upregulation of grooming in response to pathogen exposure (adults: Rosengaus et al. 1998; Jaccoud et al. 1999; Walker and Hughes 2009; Reber et al. 2011; brood: Ugelvig et al. 2010; Tragust et al. 2013b; fungus garden: Currie and Stuart 2001). The lower grooming of fungus-exposed brood was therefore unexpected, particularly since P. punctata ants do show increased grooming of Metarhizium-exposed brood when the applied exposure dose is thousandfold higher and the ants are in a colony context rather than in isolation (Tragust et al. 2013b). This suggests that the reduction in grooming toward fungus-exposed compared to sham-treated larvae in our experiment likely is a result of either the low exposure dose or the fact that ants have been removed from their colony context to be trained individually. Our experimental setting may thus have rather reflected the situation of an ant encountering the larvae outside of the nest, where avoidance of infectious items is common in social insects (Epsky and Capinera 1988; Diehl-Fleig and Lucchese 1991; Mehdiabadi and Gilbert 2002; Fouks and Lattorff 2011).
Despite these different basal grooming levels in the two treatments, brood grooming duration significantly increased by repeated brood care for both the sham-treated larvae (by 14 %) and the fungus-exposed larvae (by 32 %; Fig. 3b), whilst worker self-grooming was reduced. The absence of a significant interaction between trial and treatment for grooming duration indicates that both treatment groups reacted similarly to experience and that the presence of a pathogen neither caused nor interfered with prolonged brood grooming. It thus seems that experienced ants prophylactically upregulate their expression of sanitary brood care at the expense of individual hygiene. It was shown that ant colonies that have previously encountered Metarhizium fungi increase allogrooming toward adult nestmates that are either sham-treated (Reber et al. 2011) or fungus-exposed (Walker and Hughes 2009) and fungus-growing ants whose fungus garden has experienced pathogen threat increase self-grooming (Morelos-Juárez et al. 2010). The novelty of our study is that P. punctata ants intensify brood grooming after repeated brood care even in the absence of any previous pathogen encounter, thereby deviating from Pheidole ants, where nursing-experienced individuals did not perform more brood care than their naive nestmates (Muscedere et al. 2013).
While we can exclude that the presence of the fungal pathogen M. robertsii at the used low exposure level triggered the prolonged larval grooming by experienced ants, we cannot disentangle whether the intensified grooming may have been caused by repeated confrontation with the brood per se or with the detergent Triton X. The detergent is required to bring the hydrophobic fungal conidiospores into suspension and was thus the closest control to our fungus-exposure treatment. Some studies have reported that treatment with a detergent elicits the same grooming intensity as fungus exposure (Graystock and Hughes 2011; Reber et al. 2011; Tragust et al. 2013b; but see, e.g., Rosengaus et al. 1998; Ugelvig and Cremer 2007; Walker and Hughes 2009; Ugelvig et al. 2010; Tragust et al. 2013b), suggesting a potential effect of the detergent on the performance of sanitary behaviors. Intensified grooming in our experiment could thus be the result of either repeated nursing itself or repeated contact to a potentially irritating compound that may even act as a “danger signal” (Matzinger 1994), causing potential damage to the host cells through its detergent properties. Our study moreover revealed that fungal attachment might be affected by the storage time of the conidiospore suspension, highlighting that this should be strictly controlled for in experimental work. The cause may be an increase in RNA and protein synthesis during soaking, leading to faster swelling and germination when later coming in contact with the insect cuticle (Dillon and Charnley 1985; Hassan et al. 1989; Dillon and Charnley 1990).
As expected from previous work (Rosengaus et al. 1998; Hughes et al. 2002; Yanagawa et al. 2008; Tragust et al. 2013a), ants removed higher conidiospore numbers when grooming the larvae longer (Fig. 4). Experienced ants, which increased larval grooming duration (Fig. 3b), thus removed the fungal pathogen more effectively than naive ants. Such improved performance through experience is not self-evident, given that, e.g., honeybee undertakers did not improve in cadaver removal with experience (Trumbo and Robinson 1997) and brood-care-experienced Pheidole dentata ants were not more efficient nurses (Muscedere et al. 2013). Higher conidiospore removal by brood-care-experienced individuals is likely key to reduce the infection probability of exposed larvae (as is known for adults; Walker and Hughes 2009), as well as the transmission of the pathogen within the colony. Furthermore, social immunity, i.e., sanitary care by nestmates, can be beneficial for the helper, as it can bestow a survival benefit upon future exposure to the same pathogen (Traniello et al. 2002; Ugelvig and Cremer 2007; Hamilton et al. 2011; Konrad et al. 2012).
We can conclude that repeated brood care in P. punctata ants modulates their brood care activities, increasing their potential to fight fungal disease. As experience-dependent behavioral modulation is reversible and for instance depends on the time delay between task performances (Plowright and Plowright 1988; Theraulaz et al. 1998; Westhus et al. 2013), absence of stimuli could again lead to decreased intensity of brood care. Such experience-modulated expression of sanitary brood care likely allows colonies to respond flexibly to incoming pathogens. Response threshold models postulate that inter-individual variation in response thresholds for task-associated stimuli can lead to division of labor in the colony and individuals with lower response thresholds are more likely to become specialists for the task (reviewed in Beshers and Fewell 2001). Task specialization (i.e., the tendency of some workers to perform tasks more frequently or longer than their nestmates) may be more pronounced in larger ant societies (Bourke 1999; Thomas and Elgar 2003; Jeanson et al. 2007) and also in societies with higher genetic diversity (reviewed by Oldroyd and Fewell 2007). Yet, already in the absence of large colonies and genetic variation, as in the studied clonal ant P. punctata, repeatedly performed brood care makes ants better caretakers and may promote divergence between group members.
References
Arathi HS, Spivak M (2001) Influence of colony genotypic composition on the performance of hygienic behaviour in the honeybee, Apis mellifera L. Anim Behav 62:57–66
Arathi HS, Burns I, Spivak M (2000) Ethology of hygienic behaviour in the honey bee Apis mellifera L. (Hymenoptera: Apidae): behavioural repertoire of hygienic bees. Ethology 106(4):365–379
Arathi H, Ho G, Spivak M (2006) Inefficient task partitioning among nonhygienic honeybees, Apis mellifera L., and implications for disease transmission. Anim Behav 72:431–438
Beshers SN, Fewell JH (2001) Models of division of labor in social insects. Annu Rev Entomol 46:413–440
Bischoff JF, Rehner SA, Humber RA (2009) A multilocus phylogeny of the Metarhizium anisopliae lineage. Mycologia 101:512–530
Blackmer JL, Byrne DN (2008) Flight behaviour of Bemisia tabaci in a vertical flight chamber: effect of time of day, sex, age and host quality. Physiol Entomol 18(3):223–232
Bourke AFG (1999) Colony size, social complexity and reproductive conflict in social insects. J Evol Biol 12:245–257
Cremer S, Armitage SAO, Schmid-Hempel P (2007) Social immunity. Curr Biol 17:R693–R702
Currie CR, Stuart AE (2001) Weeding and grooming of pathogens in agriculture by ants. Proc R Soc Lond B Biol Sci 268:1033–1039
Diehl-Fleig E, Lucchese ME (1991) Reacoes comportamentais de operarias de Acromyrmex striatus (Hymenoptera, Formicidae) na presenca de fungos entomopatogenicos. Rev Bras Entomol 35:101–107
Dillon RJ, Charnley AK (1985) A technique for accelerating and synchronising germination of conidia of the entomopathogenic fungus Metarhizium anisopliae. Arch Microbiol 142:204–206
Dillon RJ, Charnley AK (1990) Initiation of germination in conidia of the entomopathogenic fungus, Metarhizium anisopliae. Mycol Res 94:299–304
Dukas R (2008) Evolutionary biology of insect learning. Annu Rev Entomol 53:145–160
Dukas R, Visscher PK (1994) Lifetime learning by foraging honey bees. Anim Behav 48:1007–1012
Epsky ND, Capinera JL (1988) Efficacy of the entomogenous nematode Steinernema feltiae against a subterranean termite, Reticulitermes tibialis (Isoptera: Rhinotermidtidae). J Econ Entomol 81:1313–1317
Fang W, Pei Y, Bidochka MJ (2006) Transformation of Metarhizium anisopliae mediated by Agrobacterium tumefaciens. Can J Microbiol 52:623–626
Fouks B, Lattorff HMG (2011) Recognition and avoidance of contaminated flowers by foraging bumblebees (Bombus terrestris). PLoS ONE 6:e26328
Gramacho KP, Spivak M (2003) Differences in olfactory sensitivity and behavioral responses among honey bees bred for hygienic behavior. Behav Ecol Sociobiol 54:472–479
Graystock P, Hughes WOH (2011) Disease resistance in a weaver ant, Polyrhachis dives, and the role of antibiotic-producing glands. Behav Ecol Sociobiol 65:2319–2327
Hamilton C, Lejeune BT, Rosengaus RB (2011) Trophallaxis and prophylaxis: social immunity in the carpenter ant Camponotus pennsylvanicus. Biol Lett 7:89–92
Hartmann A, Heinze J (2003) Lay eggs, live longer: division of labor and life span in a clonal ant species. Evolution 57(10):2424–2429
Hassan AEM, Dillon RJ, Charnley AK (1989) Influence of accelerated germination of conidia on the pathogenicity of Metarhizium anisopliae for Manduca sexta. J Invertebr Pathol 54:277–279
Hölldobler B, Wilson EO (1990) The ants. Harvard University Press, Cambridge, Massachusetts
Hughes WOH, Eilenberg J, Boomsma JJ (2002) Trade-offs in group living: transmission and disease resistance in leaf-cutting ants. Proc R Soc Lond B Biol Sci 269:1811–1819
Jaccoud DB, Hughes WOH, Jackson CW (1999) The epizootiology of a Metarhizium infection in mini-nests of the leaf-cutting ant Atta sexdens rubropilosa. Entomol Exp Appl 93:51–61
Jeanson R, Fewell JH, Gorelick R, Bertram SM (2007) Emergence of increased division of labor as a function of group size. Behav Ecol Sociobiol 62:289–298
Keller S, Kessler P, Schweizer C (2003) Distribution of insect pathogenic soil fungi in Switzerland with special reference to Beauveria brongniartii and Metharhizium anisopliae. Biol Control 48:307–319
Kellner K, Heinze J (2011) Absence of nepotism in genetically heterogeneous colonies of a clonal ant. Ethology 117(6):556–564
Kellner K, Barth B, Heinze J (2010) Colony fusion causes within-colony variation in a parthenogenetic ant. Behav Ecol Sociobiol 64:737–746
Kellner K, Seal JN, Heinze J (2013) Sex at the margins: parthenogenesis vs facultative and obligate sex in a Neotropical ant. J Evol Biol 26:108–117
Konrad M, Vyleta ML, Theis FJ, Stock M, Tragust S, Klatt M, Drescher V, Marr C, Ugelvig LV, Cremer S (2012) Social transfer of pathogenic fungus promotes active immunisation in ant colonies. PLoS Biol 10(4):e1001300. doi:10.1371/journal.pbio.1001300
Kurtz J, Armitage SAO (2006) Alternative adaptive immunity in invertebrates. Trends Immunol 27:493–496
Lacey LA, Brooks WM (1997) Initial handling and diagnosis of diseased insects. In: Lacey LA (ed) Manual of techniques in insect pathology. Academic Press, London, pp 1–16
Langridge EA, Franks NR, Sendova-Franks AB (2004) Improvement in collective performance with experience in ants. Behav Ecol Sociobiol 56:523–529
Langridge EA, Sendova-Franks AB, Franks NR (2008) How experienced individuals contribute to an improvement in collective performance in ants. Behav Ecol Sociobiol 62:447–456
Masterman R, Ross R, Mesce K, Spivak M (2001) Olfactory and behavioral response thresholds to odors of diseased brood differ between hygienic and non-hygienic honey bees (Apis mellifera L.). J Comp Physiol A 187:441–452
Matzinger P (1994) Tolerance, danger, and the extended family. Annu Rev Immunol 12:991–1045
Mehdiabadi NJ, Gilbert LE (2002) Colony-level impacts of parasitoid flies on fire ants. Proc Biol Sci 269:1695–1699
Mersch DP, Crespi A, Keller L (2013) Tracking individuals shows spatial fidelity is a key regulator of ant social organization. Science 340(6136):1090–1093
Morelos-Juárez C, Walker TN, Lopes JFS, Hughes WOH (2010) Ant farmers practice proactive personal hygiene to protect their fungus crop. Curr Biol 20:R553–R554
Muscedere ML, Djermoun A, Traniello JFA (2013) Brood-care experience, nursing performance, and neural development in the ant Pheidole dentata. Behav Ecol Sociobiol 67(5):775–784
Naug D, Camazine S (2002) The role of colony organization on pathogen transmission in social insects. J Theor Biol 215:427–439
O’Donnell S, Jeanne RL (1992) Forager success increases with experience in Polybia occidentalis (Hymenoptera: Vespidae). Insect Soc 39:451–454
Oldroyd BP, Fewell JH (2007) Genetic diversity promotes homeostasis in insect colonies. Trends Ecol Evol 22:408–413
Oster GF, Wilson EO (1978) Caste and ecology in the social insects. Princeton University Press, Princeton, NJ
Patterson R, Briano J (1993) Potential of three biological control agents for suppression of Solenopsis invicta, the red imported fire ant. Proceedings of the 1st International Conference on Insect Pests on Urban Environments. Exeter, UK. pp. 35–43
Pinheiro J, Bates D, DebRoy S, Sarkar D and the R Development Core Team (2012) nlme: linear and nonlinear mixed effects models. R package version 3.1-110
Plowright RC, Plowright CMS (1988) Elitism in social insects: a positive feedback model. In: Jeanne RL (ed) Interindividual behavioral variability in social insects. Westview Press, Boulder, Colorado, pp 419–432
Raine NE, Chittka L (2007) Pollen foraging: learning a complex motor skill by bumblebees (Bombus terrestris). Naturwissenschaften 94:459–464
Rath AC, Koen TB, Yip HY (1992) The influence of abiotic factors on the distribution and abundance of Metarhizium anisopliae in Tasmanian pasture soils. Mycol Res 96(5):378–384
Ravary F, Lecoutey E, Kaminski G, Châline N, Jaisson P (2007) Individual experience alone can generate lasting division of labor in ants. Curr Biol 17:1308–1312
Reber A, Purcell J, Buechel S, Buri P, Chapuisat M (2011) The expression and impact of antifungal grooming in ants. J Evol Biol 24:954–964
Robinson GE (1992) Regulation of division of labor in insect societies. Annu Rev Entomol 37:637–665
Robinson GE, Page REJ (1988) Genetic determination of guarding and undertaking in honey-bee colonies. Nature 333:356–358
Robinson GE, Page RE Jr (1989) Genetic determination of nectar foraging, pollen foraging, and nest-site scouting in honey bee colonies. Behav Ecol Sociobiol 24(5):317–323
Rosengaus RB, Traniello JFA (1997) Pathobiology and disease transmission in dampwood termites [Zootermopsis angusticollis (Isoptera: Termopsidae)] infected with the fungus Metarhizium anisopliae (Deuteromycotina: Hypomycetes). Sociobiology 30(2):185–195
Rosengaus RB, Traniello JFA (2001) Disease susceptibility and the adaptive nature of colony demography in the dampwood termite Zootermopsis angusticollis. Behav Ecol Sociobiol 50:546–556
Rosengaus RB, Maxmen A, Coates L, Traniello JFA (1998) Disease resistance: a benefit of sociality in the dampwood termite Zootermopsis angusticollis (Isoptera: Termopsidae). Behav Ecol Sociobiol 44:125–134
Rosengaus RB, Traniello JFA, Chen T, Brown JJ (1999) Immunity in a social insect. Naturwissenschaften 86:588–591
Rosengaus RB, Malak T, MacKintosh C (2013) Immune-priming in ant larvae: social immunity does not undermine individual immunity. Biol Lett 9:20130563
Rothenbuhler WC, Thompson VC (1956) Resistance to American foulbrood in honey bees. I. Differential survival of larvae of different genetic lines. J Econ Entomol 49:470–475
Sadd BM, Schmid-Hempel P (2006) Insect immunity shows specificity upon secondary pathogen exposure. Curr Biol 16:1206–1210
Schilder K, Heinze J, Gross R, Hölldobler B (1999) Microsatellites reveal clonal structure of populations of the thelytokous ant Platythyrea punctata (F. Smith) (Hymenoptera; Formicidae). Mol Ecol 8:1497–1507
Schlüns EA, Wegener BJ, Robson SKA (2011) Genetic polyethism and nest building in the weaver ant Oecophylla smaragdina (FABRI-CIUS, 1775) (Hymenoptera: Formicidae). Myrmecol News 15:7–11
Schmid-Hempel P (1998) Parasites in social insects. Princeton University Press, Princeton, New Jersey
Schmid-Hempel P (2005) Natural insect host-parasite systems show immune priming and specificity: puzzles to be solved. Bio Essays 27:1026–1034
Schmid-Hempel P, Schmid-Hempel R (1993) Transmission of a pathogen in Bombus terrestris, with a note on division of labour in social insects. Behav Ecol Sociobiol 33:319–327
Siva-Jothy MT, Moret Y, Rolff J (2005) Insect immunity: an evolutionary ecology perspective. Adv In Insect Phys 32:1–48
St. Leger RJ, Wang C, Fang W (2011) New perspectives on insect pathogens. Fungal Biol Rev 25:84–88
Theraulaz G, Bonabeau E, Deneubourg J-L (1998) Response threshold reinforcement and division of labour in insect societies. Proc R Soc Lond B Biol Sci 265:327–332
Therneau T (2012) A package for survival analysis in S. R package version 2.36-14
Thomas ML, Elgar MA (2003) Colony size affects division of labour in the ponerine ant Rhytidoponera metallica. Naturwissenschaften 90:88–92
Tragust S, Mitteregger B, Barone V, Konrad M, Ugelvig LV, Cremer S (2013a) Ants disinfect fungus-exposed brood by oral uptake and spread of their poison. Curr Biol 23(1):76–82
Tragust S, Ugelvig LV, Chapuisat M, Heinze J, Cremer S (2013b) Pupal cocoons affect sanitary brood care and limit fungal infections in ant colonies. BMC Evol Biol 13:225. doi:10.1186/1471-2148-13-225
Traniello JFA, Rosengaus RB, Savoie K (2002) The development of immunity in a social insect: evidence for the group facilitation of disease resistance. PNAS 99(10):6838–6842
Trumbo ST, Robinson GE (1997) Learning and task interference by corpse-removal specialists in honey bee colonies. Ethology 103:966–975
Trumbo ST, Huang Z-Y, Robinson GE (1997) Division of labor between undertaker specialists and other middle-aged workers in honey bee colonies. Behav Ecol Sociobiol 41(3):151–163
Ugelvig LV, Cremer S (2007) Social prophylaxis: group interaction promotes collective immunity in colonies. Curr Biol 17(22):1967–1971
Ugelvig LV, Kronauer DJC, Schrempf A, Heinze J, Cremer S (2010) Rapid anti-pathogen response in ant societies relies on high genetic diversity. Proc R Soc B Biol Sci 277(1695):2821–2828
Vestergaard S, Butt T, Bresciani J, Gillespie AT, Eilenberg J (1999) Light and electron microscopy studies of the infection of the western flower thrips Frankliniella occidentalis (Thysanoptera: Thripidae) by the entomopathogenic fungus Metarhizium anisopliae. J Invertebr Pathol 73:25–33
Waddington SJS, Santorelli LA, Ryan FRF, Hughes WOH (2010) Genetic polyethism in leaf-cutting ants. Behav Ecol 21:1165–1169
Walker TN, Hughes WOH (2009) Adaptive social immunity in leaf-cutting ants. Biol Lett 5(4):446–448
Weidenmüller A (2004) The control of nest climate in bumblebee (Bombus terrestris) colonies: interindividual variability and self reinforcement in fanning response. Behav Ecol 15(1):120–128
Weidenmüller A, Mayr C, Kleineidam CJ, Roces F (2009) Preimaginal and adult experience modulates the thermal response behavior of ants. Curr Biol 19(22):1897–1902
Westhus C, Kleineidam CJ, Roces F, Weidenmüller A (2013) Behavioral plasticity in the fanning response of bumblebees: the impact of experience and rate of temperature increase. Anim Behav 85(1):27–34
Wilson-Rich N, Spivak M, Fefferman NH, Starks PT (2009) Genetic, individual, and group facilitation of disease resistance in insect societies. Annu Rev Entomol 54:405–423
Yanagawa A, Yokohari F, Shimizu S (2008) Defense mechanism of the termite, Coptotermes formosanus Shiraki, to entomopathogenic fungi. J Invertebr Pathol 97:165–170
Acknowledgments
We thank Katrin Kellner for colony establishment and characterization, Mike Bidochka for the fungal strain, Meghan Vyleta for fungal strain characterization, Martina Klatt and Simon Tragust for help in the laboratory, Dimitri Missoh for developing the software BioLogic, and Mark Brown and Raphaël Jeanson for discussion and help with data analysis. The study was funded by the European Research Council (ERC Starting Grant to SC; Marie Curie IEF to LVU) and the German Research Foundation DFG (to SC and to JH), and CW received funding by the doctoral school Diversité du Vivant (Cotutelle project to CD and SC).
Ethical standards
The performed experiments comply with European laws.
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by D. Naug
Rights and permissions
About this article
Cite this article
Westhus, C., Ugelvig, L.V., Tourdot, E. et al. Increased grooming after repeated brood care provides sanitary benefits in a clonal ant. Behav Ecol Sociobiol 68, 1701–1710 (2014). https://doi.org/10.1007/s00265-014-1778-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00265-014-1778-8