Abstract
Group-living species have to deal with higher risks of exposure to pathogens and of disease propagation between group members. Insect societies have therefore evolved behaviours that contribute to the social immunity of the whole colony. Here, we investigate how the sanitary strategies displayed by ants depend on colony size, which is known to influence their social organisation. Myrmica rubra ant colonies of different sizes were faced with waste items that were infected or not by the entomopathogen fungus Metarhizium brunneum. By keeping nest parameters and pathogen loads proportional to colony size, we show that the largest colonies less suffered from exposure to life-threatening spores. Indeed, the largest colonies showed the lowest mortality rates and were the fastest to reject all the infected waste items out from their nest. Unexpectedly, small-sized colonies displayed distinct sanitary strategies according to the pathogenicity of the waste items. When challenged with fungus-bearing items, they opted out for an “emergency strategy” in which workers first moved out from their nest carrying brood and reintegrate it after sanitization by waste-transporting individuals. This demonstrates the behavioural plasticity of ant colonies of which both the size and the sanitary threat determine the efficiency and the type of hygienic responses to hygienic challenges.
Significance statement
Insect societies have evolved several collective hygienic behaviours that contribute to their “social immunity”. Here, we found out that both internal and external factors determine the efficiency and the type of hygienic responses to waste items introduced in an ant colony. In particular, small-sized colonies demonstrated a behavioural plasticity with distinct sanitary strategies according to the pathogenicity of the waste items. Such a plasticity appears to be an adaptive way to limit exposure of larvae to pathogens and hence to preserve colony development. It also raises some questions about the origin of the differences in worker’s internal threshold allowing colonies to shift between sanitary strategies.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The ecological success and dominance of social insects in many biotopes arise from the benefits derived from cooperation between groupmates in terms of information sharing, food collection, brood care or nest defence (Hölldobler and Wilson 1990). However, eusociality implies a high level of genetic relatedness between nestmates as well as a high rate of social interactions that bring new constraints such as increased risks of disease transmission within the group (Shykoff and Schmid-Hempel 1991; Fefferman et al. 2007). In addition, the confined and dark space of the ant nest or the hive offers amenable conditions for the development of pathogens such as fungi (Schmid-Hempel 1998; McCallum et al. 2001). Social insects have therefore to trade-off between the benefits of social life and the costs associated with a greater exposure to pathogens (Fefferman et al. 2007). In addition to the physical barrier of the cuticle and the innate immune defences of individuals, colonies have thus evolved a range of individual and collective behaviours that participate to the “social immunity” of the whole colony (Cremer et al. 2007). Thanks to these mechanisms, eusocial insects can afford a relatively low investment into individual immunity compared to solitary or gregarious species (Evans et al. 2006; Harpur and Zayed 2013). However, similarities in several immune genes across a gradient of sociality suggest that the reduced immune repertoire of social insects may have predated the evolution of sociality (Barribeau et al. 2015; Sadd et al. 2015). Social immunity involves prophylactic behaviours that limit the level of colony exposure to pathogens, such as the allogrooming of congeners that entered the nest or the avoidance of contaminated substrate for nest digging (Hughes et al. 2002; Rosengaus et al. 1998; Staples and Milner 2000). The prophylaxis of a colony is however not restricted to social grooming and encompasses all the behaviours that prevent the intake or development of pathogens within the nest. For instance, ant workers sanitize nest material with antibiotic compounds by mixing it with the resin from coniferous trees (Chapuisat et al. 2007; Castella et al. 2008). Similarly, honeybees line their hives with propolis that has antifungal and antibacterial properties (Simone et al. 2009; Simone-Finstrom and Spivak 2010). Workers also remove from the nest waste items such as food remains, nestmates’ corpses or faeces (Jackson and Hart 2009; Diez et al. 2013) that may later become a source of infection inside the nest.
When prophylaxis fails to prevent the introduction of pathogens inside the nest, the colony can nevertheless reduce the establishment and the propagation of diseases by performing hygienic behaviours. Such hygienic behaviours mostly consist in the avoidance or the active removal of contaminated nestmates or corpses (Oi and Pereira 1993; Cremer et al. 2007). For instance, Apis mellifera honeybee workers are able to uncap cells of larvae infected by the Varroa jacobsoni mites and to remove them from the nest (Boecking and Spivak 1999; Spivak and Reuter 2001). Likewise, some ant species are known to selectively remove infected brood from the nest even before fungal outgrowth (e.g. in Cardiocondyla obscurior: Ugelvig et al. 2010) and to speed up necrophoric behaviour when corpses were fungus-contaminated (e.g. in Myrmica rubra: Diez et al. 2012). In some rare cases, corpses can also be buried inside or outside the nest (e.g. in Temnothorax lichtensteini: Renucci et al. 2011) or even cannibalized (e.g. in Solenopsis invicta: Howard and Tschinkel 1976). Finally, information about parasitic threats can be transmitted to the whole colony through an elaborate communication network between workers. For instance, the termite Zootermopsis angusticollis displays a striking vibratory behaviour conveying information about the presence of pathogens to nearby unexposed nestmates that will thereafter avoid the infected substrate (Rosengaus et al. 1999). Although costly in time and energy, such behaviours that allow the early detection and discrimination of pathogens are essential for maintaining hygiene in an insect society that can live for years or decades inside the same nesting site (Schmid-Hempel 1998; Cremer et al. 2007).
Since most of the prophylactic and hygienic strategies are based on between-nestmate interactions and cooperation, the size of a colony is expected to act upon the type, the rate of occurrence as well as the efficiency of its sanitary strategies (Hou et al. 2010; Waters et al. 2010). In this respect, together with the investment of workers in innate immunity, the occurrence of prophylactic behaviours such as self-grooming and allogrooming (Okuno et al. 2012) is known to increase with colony size. As for prophylaxis, the hygienic behaviours that are set up to face a pathogenic threat within the nest are expected to change as the colony grows in size (Cremer et al. 2007). Actually, theoretical models predict that larger colonies should be more efficient at managing sanitary risks (Naug and Camazine 2002; Pie et al. 2004; Fefferman et al. 2007). However, no study to date has directly demonstrated that larger colonies are more resistant to diseases or are better at detection or removal of disease-causing agents.
To address this question, we challenged Myrmica rubra colonies differing by their sizes with the entomopathogen fungus Metarhizium brunneum. Colonies of 50, 150 and 300 workers that were sampled out from the same mother colony were compared with regard to their mortality rate as well as to their dynamics of waste removal after being exposed to innocuous or fungus-infected items. In order to specifically investigate the effects of colony size, we kept density parameters constant by keeping the nest area, the number of larvae and the number of introduced waste items proportional to the number of workers inside the colony.
Material and methods
Maintenance of ant colonies
Colonies of M. rubra ants were collected in Belgium from the localities of Sambreville (50° 25′ 59.62″ N; 4° 37′ 22.12″ E) and Falisolle (50° 25′ 11.99″ N; 4° 37′ 50.41″ E) in dead wood and litter in semi-opened forests. In the laboratory, ants were reared in plaster nests (Janet type, 100 × 100 mm and 3 mm high) connected to a foraging arena (47 × 33 cm) of which the borders were coated with fluon (polytetrafluoroethylene) to prevent ants from escaping. Each mother colony used in the experiments contained 500 to 600 workers, at least 100 larvae and three queens, which is a colony composition commonly found in the field (Elmes 1973). Laboratory conditions were kept at 21 ± 1 °C and 50 ± 5% relative humidity, with a constant light period of 12 h per day. Each nest was provided with a sucrose solution ad libitum (0.3 M) and three mealworms (Tenebrio molitor) per week as a protein and lipid supply.
Entomopathogenic fungus
Metarhizium brunneum fungus is a generalist entomopathogen naturally occurring in M. rubra ant nests and in soils inhabited by this species (Evans et al. 2010). We used a commercial strain of the fungus (Strain F52 from Novozymes) that is produced in the form of rice grains coated with spores. This entomopathogenic fungus is commonly used in studies about disease resistance in insects and particularly in eusocial species such as ants (Chapuisat et al. 2007; Reber and Chapuisat 2012), bees (Butt et al. 1998) and termites (Yanagawa and Shimizu 2007).
Impact of the colony size on ants’ mortality and waste management
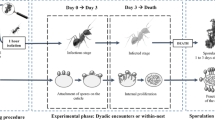
Nineteen mother colonies were used to study the impact of colony size on the ants’ survival as well as on the efficiency of waste management. Thirteen colonies were assigned to the experimental condition and were challenged with rice grains coated with M. brunneum spores. Six colonies were used as controls and were tested with spore-free rice grains. As M. rubra ant is not a granivorous ant species, rice grains were not consumed but were all rejected as waste items. From each mother colony, we randomly sampled out workers to form three subcolonies of different sizes (50, 150 and 300 workers) that were each placed in experimental squared nests with a queen and brood. For each subcolony, half of the workers were sampled in the foraging area, while the other half were directly taken inside the nest. The size of the nest as well as the size of the foraging area was adjusted to the number of ant workers in order to keep a constant ant density with 0.33 cm2 of nest area and 4 cm2 of foraging area per ant capita. Likewise, in each subcolony, larvae from the first two instars were introduced in order to account for 20% of the total colony size. As a result, each colony of 50 individuals was tested in a small plaster nest and foraging area (nest, 4 × 4 cm; foraging area, 20 × 10 cm) with ten larvae of the first two instars. Colonies of 150 workers were placed in medium-size nests (nest, 7 × 7 cm; foraging area, 30 × 20 cm) with 30 young larvae, whereas colonies of 300 individuals were placed with 60 larvae in larger setups (nest, 10 × 10 cm; foraging area, 40 × 30 cm). The ant nests were covered with a red filter, had a single entry and a central hole (1 cm diameter) through which the tested items were introduced. During 5 days, we let the colonies settle down inside their nest. Then, we counted daily the number of dead workers during 14 consecutive days in order to assess the mortality baseline in the absence of any pathogen threat (Fig. 1). On the evening of day 14, we added, in each subcolony, new workers and larvae from the same mother colony in order to compensate mortality and to recover the initial composition of the colonies. Then, on day 15, we introduced through the central hole of each nest either plain or fungus-infected rice grains. In order to get the same level of exposure to waste items per ant capita, we adjusted the number of introduced grains to the colony size. The level of exposure to waste items was fixed at 10% of the number of workers (i.e. 5, 15 and 30 grains in colonies of 50, 150 and 300 individuals, respectively). In order to limit a scaling effect of encounter rates due to a piling-up of grains, all these items were spaced allowing ants to walk easily between them. This was contributing to maintain, per ant capita, similar levels of exposure to sanitary risks as well as similar opportunities for ants to display clearing behaviour. Finally, the level of ant’s aggregation—that may have impacted the encounter rates with waste items—was of the same order of magnitude as suggested by the similar percentages of 1 cm2 squares that hosted no workers in the different colony sizes (12.5, 10.2 and 9% of empty nest squares for 50, 150 and 300 workers’ colonies, respectively).
As soon as all items were introduced inside the nest, the central hole was blocked by a plastic piece so that the items could only be rejected through the nest entry. We filmed each nest with a Logitech camera (Logitech Pro HD C920) placed 20 cm above the nest entrance until the last item was rejected. In addition, we counted for 22 extra days, the number of dead ants in order to draw survival curves and to calculate the daily mortality rate. To check whether death resulted from infection by M. brunneum fungus, all corpses were incubated at a temperature of 25 °C for post-mortem sporulation. Among the ants that died during the mortality baseline or in control colonies faced with plain rice grains, we never observed sporulating corpses. By contrast, most of the ants that died after being faced with infected items sporulated in the incubator (Colonies of 50 ants, 84% (N = 151); 150 ants, 88% (N = 364); 300 ants, 78% (N = 474)), thereby confirming the killing power of M. brunneum fungus in M. rubra ant colonies.
In total, the experiment lasted 36 days (Figs. 1 and 2) and allowed us to assess the impact of colony size as well as of item pathogenicity on colony mortality. In addition, we estimated the efficiency of waste management by comparing the dynamics of items’ rejection for the different colony sizes.
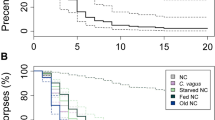
Survival curves (mean ± SD) of colonies of different sizes before and after the introduction of waste items (day 15) that were either fungus-infected rice grains (infected wastes (a)) or plain ones (control wastes (b)). Grey curve, 50-worker colonies (N = 13); Black dotted curve, 150-worker colonies (N = 13); Black curve, 300-worker colonies (N = 13). Survival curves were compared using a Cox proportional hazard regression model (***P < 0.001)
Statistical analyses
It was not possible to record data blind because our study involved different group sizes and items that were well identified by an observer’s eye. However, blinded method was used to analyse data. All data were analysed with Statistica software version 10 (©Statsoft). Since no data met normality conditions, non-parametric tests with a significance level of alpha = 0.05 were used. We used Cox proportional hazard regression models to investigate the effects of treatment (plain vs. infected grains), colony size (50 vs. 150 vs. 300 workers) and interaction on the survival curves of ant colonies, during the baseline phase (day 1 to day 14) as well as during the second phase of exposure to waste items (day 15 to day 36). Subgroup analysis of group-size effect was conducted with respect to a reference condition, which was the colony size of 300 individuals, as they were the conditions under which ants underwent the lowest mortality rates. Parameter estimates derived from the Cox models account for the expected increase in the relative hazard for each one-unit increase in a main effect, holding other effects constant. For interpretability, we computed hazard ratios (HR) by exponentiating the parameter estimates of the Cox models. These hazard ratios account for the probability of an event (death in our study) occurring in an experimental condition divided by the probability of the same event occurring in the reference condition.
With regard to the removal of waste items by ant workers, we compared the removal dynamics of plain vs infected rice grains for all colony sizes. Generalized linear mixed-effect models (GLMM) were used to investigate the main effects of colony size, item’s pathogenicity, mother colony and all relevant second-order interactions on the rejection times of the first and the last rice grain. Item’s pathogenicity and colony size were specified as fixed effects, whereas mother colony was considered as a nested random factor within colony size (Pinheiro and Bates 2000). Post hoc pairwise comparisons of rejection times were done by using Tukey tests.
Finally, we used Spearman’s correlation test to assess the relationship between the time spent to reject all items and the final mortality rate of the colonies.
Data availability statement
The datasets generated during and/or analysed during the current study are available in the Zenodo repository: https://zenodo.org/record/1003123#.WddimGi0OM8.
Results
Impact of colony size on ants’ mortality
For these mortality baselines (Table 1), we did not find any significant effect of interaction (colony size by treatment interaction effect, Wald χ 2 = 0.69; P = 0.71), colony size (colony size effect, Wald χ 2 = 3.27; P = 0.19) or treatment to which colonies will be further assigned—i.e. the pathogenicity of waste items (treatment effect, Wald χ 2 = 0.19; P = 0.66; Table 1).
Once colonies were exposed to waste items, there was a significant interaction effect between the treatment and colony size on ant survival (colony size by treatment interaction effect, Wald χ 2 = 81.51; P < 0.001; Table 1). When plain rice grains were introduced in control colonies, the ants’ mortality remained quite low with at most 3% of dead nestmates per colony after 22 days, regardless of colony size (colony size effect, Wald χ 2 = 2.39; P = 0.30; Fig. 2). In the absence of pathogenic wastes, larger colonies did not survive better than smaller ones (Cox model, 300 vs. 150 ants: P = 0.19; 300 vs. 50 ants: P = 0.99; 150 vs. 50 ants: P = 0.66; Table 2).
By contrast, when being exposed to fungus-infected waste items, the potential of ant colonies to resist to this sanitary threat significantly differed according to their size (colony size effect, Wald χ 2 = 201.58; P < 0.001). At the end of the experiment, the global mortality rates of the largest colonies were quite limited to a loss of 7% of their workers and differed from those of smaller colonies of 50 or 150 individuals that lost around 20% of their initial size (Fig. 2). The comparison of survival curves confirmed that larger colonies resisted significantly better to pathogen exposure than smaller ones (Cox model, 300 vs. 150 ants: P < 0.001; 300 vs. 50 ants: P < 0.001; 150 vs. 50 ants: P < 0.001; Table 2).
Hazard ratios (HR) of the cox models allowed us to compare the likelihood of dying for an ant tested in a given experimental condition with that of an ant tested in the reference condition. In the presence of fungus-infected wastes, the individual risks of dying were much higher for ants that stayed in small and medium colonies. For instance, when faced with a pathogen, the dying risk for an ant was almost threefold (HR = 2.86) and more than threefold (HR = 3.19) higher in colonies of 150 and 50 nestmates, respectively, in comparison with large colonies of 300 workers (Table 2).
Impact of colony size on waste management
We did not find any interaction effect between the pathogenicity of waste items and the colony size on the removal of the first waste item from the nest (GLMM, colony size by treatment interaction effect F (2.39) = 1.89, P = 0.17. Fig. 3). The rejection time of the first waste item was always shorter for infected grains than for plain grains (GLMM, treatment effect F (1.39) = 11.96, P < 0.001). In addition, we observed a significant main effect of the colony size (GLMM, F (2.39) = 26.5, P < 0.001) showing that the larger the colony size, the shorter the time to reject the first rice grain (Tukey post hoc tests for all pairwise comparisons between colony sizes, P < 0.001. Fig. 3). For instance, the first infected item was rapidly rejected from large colonies within the first minutes (mean 36.2 ± 11.3 min), whereas it took several hours for the small and medium-sized colonies (mean 1190 ± 207 min and 402 ± 152 min, respectively, Figs. 3 and 4).
Rejection dynamics of rice grains introduced in the centre of the nest of small (a), medium (b) and large colonies (c). Each point corresponds to the removal time (mean + SD) of rice grains that was successively rejected out of the nest. Black bars, colonies with infected rice grains (N = 13); Grey bars, colonies with plain rice grains (N = 6)
Rejection times of the first (a) and the last (b) infected rice grain depending on the colony size. Grey boxplots, 50-worker colonies (N = 13); Grey dotted boxplots, 150-worker colonies (N = 13); Black boxplots, 300-worker colonies (N = 13). Boxplots show the interquartile range and the median values, the bars above and below the box show the minimum and maximum values. Post hoc pairwise comparisons were made with Tukey test. Boxes with different letters are significantly different
With regard to the exhaustive removal of all waste items, there was a significant interaction between colony sizes and item pathogenicity (GLMM, colony size by item pathogenicity interaction F (2.39) = 21.13, P < 0.001, Fig. 3). Large colonies always cleaned up their nest from all plain rice grains more quickly than medium and small ones (Tukey post hoc tests, 300 vs. 150 ants and 300 vs. 50 ants: P < 0.001, Fig. 3), whereas the smallest colonies were faster than medium ones (Tukey post hoc tests, 150 vs. 50 ants: P < 0.001). In the case of spore-bearing grains, the time spent to reject all these infected items decreased in larger colony sizes (Tukey post hoc tests, 300 vs. 150 ants and 300 vs. 50 ants: P < 0.001), although being not significantly shorter between 150-worker and 50-worker colonies (Tukey post hoc test, 150 vs. 50 ants: P = 0.09, Figs. 3 and 4b). As a result, 20 h after the introduction of infected wastes, large colonies of 300 individuals had rejected all the items, whereas medium and small colonies had rejected only 33 and 20% of the rice grains, respectively.
Interestingly, most of the small colonies of 50 individuals (76.9%, N = 13) displayed an alternative strategy when facing infected items within their nest (Fig. 5). Instead of immediately rejecting infected grains as done by large colonies, workers first moved out of their nest and relocated themselves in the foraging area. Then, a few workers returned into the nest in order to remove the infected grains. Once all waste items were removed, nestmates reintegrated the sanitized nest a few hours later. Over the ten colonies which moved out from their nest, only one never reintegrated it. Such a nest moving lasted several hours (15.4 h ± 5.6 on average, N = 10). Of notice, the three colonies which did not move out underwent the highest mortality rates.
Finally, we found a significant main effect of the mother colony on the time spent to reject the first (GLMM, F (12.39) = 83.25; P < 0.001) and the last item (GLMM, F (12.39) = 142.69; P < 0.001). This suggests that some colonies were more efficient to detect and reject items than others, independently of their size. Nevertheless, same-sized colonies that were the fastest to remove items from their nest did not better survive to the fungus pathogen. Indeed, no significant correlation was found between the final mortality rate and the time spent by large and medium-sized colonies to reject all the items (Spearman tests, 150 ants R 2 = 0.11, P = 0.12; 300 ants R 2 = 0.24, P = 0.11). In the case of 50 workers, such a correlation was meaningless since, due to nest moving, the time spent to sanitize the nest from all infected items did not reflect the actual level of exposure to the fungus and hence the possible impact on ants’ mortality.
Discussion
A large colony size was found out to enhance the survival of ants as well as the efficiency of their hygienic responses. Large-sized colonies always rejected waste items earlier and more quickly than medium-sized colonies. To make up for their lower efficiency in waste removal, small-sized colonies followed an alternative “emergency” sanitary strategy of nest moving when challenged by an infectious source. Then, all ants exited the fungus-contaminated nest and re-entered it once it was sanitized by a few workers. Ant colonies therefore showed a phenotypic plasticity by adapting their hygienic response according to sanitary risks and to their colony size. Finally, there was a significant colonial effect on the dynamics of items’ rejection suggesting that some ant colonies are more hygienists and efficient in waste management than others.
The pathogenicity of Metarhizium brunneum spores was confirmed by the increased mortality of workers exposed to infected items in all colony sizes (Okuno et al. 2012; Diez et al. 2014, 2015). Nearly all the cadavers sporulated after a few days, thereby confirming that death mainly resulted from fungus infection. Less expectedly, even though the level of fungus exposure was kept proportional to the ant colony size, the largest colonies better survived, and hence, individuals underwent lower risks of dying from infection than those living in smaller colonies. The mortality rate started to increase around 1 week after the introduction of infected items when fungus hyphae are known to invade the ant body (Hänel 1982; Bos et al. 2012). For the workers that died later—i.e. more than 1 week after the introduction of fungus-infected items, they could have suffered from a low exposure to living spores that remained on ants’ cuticle and/or that were released on the nest substrate. Indeed, while M. brunneum fungus takes about 5 days to kill ant workers exposed to a strong lethal concentration of spores (Bos et al. 2012; Leclerc and Detrain 2016), a lower level of exposure slows down the infection process within the host (Verhoeff 1974; Okuno et al. 2012) and thus delays the ants’ death.
We found out that M. rubra ants were able to detect spores on wastes and reject them more quickly. Such a capability was already observed for the necrophoric behaviour of M. rubra workers that rejected sporulating cadavers faster than non-sporulating ones (Diez et al. 2012) as well as for leaf-cutting ants that rapidly eliminate pathogens from their fungus gardens by an increased grooming of alien spores as well as by the specific removal of infected fungus garden (Currie and Stuart 2001). We also demonstrated that larger colonies were better at detection and removal of waste items. Even though the total number of introduced items was higher, the largest colonies were the most efficient at rejecting wastes what may explain their lower susceptibility to fungal spores. Furthermore, other non-linear processes involved in social immunity such as the increase of allogrooming behaviours (Schmid-Hempel 1998; Okuno et al. 2012) and interaction rates (Gordon and Mehdiabadi 1999 ; Thomas and Elgar 2003) with colony size may have been beneficial to workers’ survival and may have socially facilitated waste removal in large-size colonies. Since the number of waste items per capita was similar for all colony sizes, such a faster removal in the largest colonies could be due to a more elaborate division of labour in which subgroups of workers perform specific tasks including necrophoresis (i.e. the removal of dead nestmates outside the nest. Julian and Cahan 1999) or waste rejection (Hart and Ratnieks 2002; Ballari et al. 2007; Waddington and Hughes 2010; but see Dornhaus et al. 2009). On the other hand, workers of small colonies are usually less specialized in specific tasks and thereby ensure flexibility of colonial responses to immediate needs (Karsai and Wenzel 1998; Bourke 1999; Anderson and Franks 2001). This is achieved at the expenses of a lower efficiency of individuals for specific tasks such as waste management (Holbrook et al. 2011). It has still to be investigated whether the impact of colony size on the rejection dynamics of waste items occurred through changes in the number of workers participating to hygienic tasks and/or through changes in the level of specialization of individuals in waste management tasks.
Some mother colonies were consistently more efficient at removing wastes regardless of their size. Such intercolonial differences in the performance of hygienic behaviours and in the efficiency of waste removal could result from differences in the response thresholds of individual workers depending on their age or genetic background. Indeed, the tasks performed by workers change with their age, with young workers being more likely to perform interior tasks such as brood and queen care, whereas old ones rather perform exterior tasks such as foraging or waste management (Seeley 1985; Seeley and Kolmes 1991). Likewise, the response thresholds to stimuli, and hence, task performance can depend on workers’ genotype (Oldroyd and Fewell 2007; Smith et al. 2008) as already shown in honeybees (Page and Robinson 1991; Fewell and Page 1993), bumblebees (O'donnell et al. 2000), wasps (O’Donnell 1998) and ants (Snyder 1993; Julian and Fewell 2004). In honeybees, the ability to detect and remove dead or diseased brood from capped cells is a genotypic, heritable trait, and thus, removal dynamics can be modulated by selective breeding (Bigio et al. 2014). As a correlate, several empirical and theoretical studies showed that colonies with a large number of genetic lines have a more finely tuned task allocation and thus respond more efficiently to changing stimuli or priorities (Fewell and Page 1993; Page et al. 1995; Pruitt et al. 2013; Modelling: Cox and Myerscough 2003; Myerscough and Oldroyd 2004). In the case of polygynous and polyandrous species such as M. rubra ant species, it would be interesting to investigate whether hygienic colonies that quickly remove infected items from their nest are characterized by a higher genetic diversity and/or possess more workers belonging to hygienic genetic lines.
The present study also revealed an amazing alternative strategy exhibited by small-sized colonies challenged by a pathogenic threat of which workers first moved out of their nest and took away brood. Thereafter, a few individuals came back to the nesting site to remove all infected items, suggesting a self-reinforcement process in performing this task (Theraulaz et al. 1998). Indeed, M. rubra workers can become highly committed in necrophoresis (Diez et al. 2013) or brood transport during nest moving (Abraham and Pasteels 1980) over a short time frame to deal with changes in colony needs. Likewise, some individuals may have become short-term specialists in the removal of infected waste items, allowing to quickly sanitize the nest which was thereafter reintegrated by the colony. The shift of small colonies towards such an “emergency strategy” could be linked to changes in the task allocation of workers that may covary with group size (Mailleux et al. 2003; Thomas and Elgar 2003). Indeed, small-sized colonies should invest relatively more effort in promoting colony growth, such as foraging and brood care (Kolmes and Winston 1988; Schmid-Hempel and Schmid-Hempel 1993; Thomas and Elgar 2003). Therefore, nest moving appears to be an adaptive way to limit exposure of larvae to pathogens and hence to preserve colony development.
Nest evacuation is a common strategy displayed by ant colonies facing life-endangering situations such as nest flooding (Wilson 1986), intense predation (O’Shea-Wheller et al. 2015) or detection of an approaching army ant raid (Lamon and Topoff 1981; Chadab-Crepet and Rettenmeyer 1982; Droual 1983, 1984; McGlynn 2007). Likewise, in the case of a sanitary threat, movements to new nest sites that have been reported in ant colonies, infected by fungi, (Oi and Pereira 1993) or nematodes (Drees et al. 1992), probably reflect avoidance by workers from further contacts with the pathogens (but see Pontieri et al. 2014; Brütsch et al. 2014). The present study is however the first to report a strategy of transient nest evacuation in which the original nest is re-integrated by the colony after being sanitized by a few individuals and of which the occurrence of nest moving is determined by the size of the endangered ant colonies. As this “emergency” strategy was observed exclusively in the smallest colonies, we can hypothesize that there is a colony size threshold at which a shift occurs from the waste-removing to the nest-moving sanitary strategy. This assumption implies that for a given colony size, there is an optimal strategy to reduce the establishment as well as the propagation of diseases within the nest.
Of notice, while the majority of small-sized colonies showed a phenotypic plasticity by shifting to an emergency strategy, a few colonies did not opt out for this “nest moving” solution and acted as their larger sister colonies by directly rejecting the infected items. One may wonder whether this phenotypic plasticity arise from differences in workers’ internal threshold to stimuli (Myerscough and Oldroyd 2004; Pruitt et al. 2013). It would also be interesting to compare responses of small-sized colonies issued from same mother colony to test if this ability to use alternative sanitary strategies is a colonial—and possibly heritable trait.
References
Abraham M, Pasteels JM (1980) Social behaviour during nest-moving in the ant Myrmica rubra L. (Hym. Form.) Insect Soc 27(2):127–147. https://doi.org/10.1007/BF02229249
Anderson C, Franks NR (2001) Teams in animal societies. Behav Ecol 12(5):534–540. https://doi.org/10.1093/beheco/12.5.534
Ballari S, Farji-Brener AG, Tadey M (2007) Waste management in the leaf-cutting ant Acromyrmex lobicornis: division of labour, aggressive behaviour, and location of external refuse dumps. J Insect Behav 20(1):87–98. https://doi.org/10.1007/s10905-006-9065-9
Barribeau SM, Sadd BM, Du Plessis L, Brown MJ, Buechel SD, Cappelle K, Evans J (2015) A depauperate immune repertoire precedes evolution of sociality in bees. Gen Biol 16(1):83. https://doi.org/10.1186/s13059-015-0628-y
Bigio G, Toufailia HA, Hughes WO, Ratnieks FL (2014) The effect of one generation of controlled mating on the expression of hygienic behaviour in honey bees. J Apic Res 53(5):563–568. https://doi.org/10.3896/IBRA.1.53.5.07
Boecking O, Spivak M (1999) Behavioral defenses of honey bees against Varroa jacobsoni Oud. Apidologie (France) 30(2-3):141–158. https://doi.org/10.1051/apido:19990205
Bos N, Lefevre T, Jensen AB, D’Ettorre P (2012) Sick ants become unsociable. J Evol Biol 25(2):342–351. https://doi.org/10.1111/j.1420-9101.2011.02425.x
Bourke AFG (1999) Colony size, social complexity and reproductive conflict in social insects. J Evol Biol 12(2):245–257. https://doi.org/10.1046/j.1420-9101.1999.00028.x
Brütsch T, Felden A, Reber A, Chapuisat M (2014) Ant queens (Hymenoptera: Formicidae) are attracted to fungal pathogens during the initial stage of colony founding. Myrmecol News 20:71–76
Butt TM, Carreck NL, Ibrahim L, Williams IH (1998) Honey-bee-mediated infection of pollen beetle (Meligethes aeneus Fab.) by the insect-pathogenic fungus, Metarhizium anisopliae. Biocontrol Sci Tech 8(4):533–538. https://doi.org/10.1080/09583159830045
Castella G, Chapuisat M, Christe P (2008) Prophylaxis with resin in wood ants. Anim Behav 75(4):1591–1596. https://doi.org/10.1016/j.anbehav.2007.10.014
Chadab-Crepet R, Rettenmeyer CW (1982) Comparative behavior of social wasps when attacked by army ants or other predators and parasites. In: Breed MD, Michener CD, Evans HE (eds) Biology of social insects. Westview Press, Boulder
Chapuisat M, Oppliger A, Magliano P, Christe P (2007) Wood ants use resin to protect themselves against pathogens. Proc R Soc Lond B Biol Sci 274(1621):2013–2017. https://doi.org/10.1098/rspb.2007.0531
Cox MD, Myerscough MR (2003) A flexible model of foraging by a honey bee colony: the effects of individual behaviour on foraging success. J Theor Biol 223(2):179–197. https://doi.org/10.1016/S0022-5193(03)00085-7
Cremer S, Armitage SAO, Schmid-Hempel P (2007) Social immunity. Curr Biol 17:693–702
Currie CR, Stuart AE (2001) Weeding and grooming of pathogens in agriculture by ants. Proc R Soc Lond B Biol Sci 268(1471):1033–1039. https://doi.org/10.1098/rspb.2001.1605
Diez L, Deneubourg JL, Detrain C (2012) Social prophylaxis through distant corpse removal in ants. Naturwissenschaften 99(10):833–842. https://doi.org/10.1007/s00114-012-0965-6
Diez L, Le Borgne H, Lejeune P, Detrain C (2013) Who brings out the dead? Necrophoresis in the red ant, Myrmica rubra. Anim Behav 86(6):1259–1264. https://doi.org/10.1016/j.anbehav.2013.09.030
Diez L, Lejeune P, Detrain C (2014) Keep the nest clean: survival advantages of corpse removal in ants. Biol Lett 10:03–06
Diez L, Urbain L, Lejeune P, Detrain C (2015) Emergency measures: adaptive response to pathogen intrusion in the ant nest. Behav Process 116:80–86. https://doi.org/10.1016/j.beproc.2015.04.016
Dornhaus A, Holley JA, Franks NR (2009) Larger colonies do not have more specialized workers in the ant Temnothorax albipennis. Behav Ecol 20(5):922–929. https://doi.org/10.1093/beheco/arp070
Drees BM, Miller RW, Vinson BS, Georgis R (1992) Susceptibility and behavioral response of red imported fire ant (Hymenoptera: Formicidae) to selected entomogenous nematodes (Rhabditida: Steinernematidae & Heterorhabditidae). J Econ Entomol 85(2):365–370. https://doi.org/10.1093/jee/85.2.365
Droual R (1983) The organization of nest evacuation in Pheidole desertorum Wheeler and P. hyatti Emery (Hymenoptera: Formicidae). Behav Ecol Sociobiol 12(3):203–208. https://doi.org/10.1007/BF00290772
Droual R (1984) Anti-predator behaviour in the ant Pheidole desertorum: the importance of multiple nests. Anim Behav 32(4):1054–1058. https://doi.org/10.1016/S0003-3472(84)80221-3
Elmes GW (1973) Observations on density of queens in natural colonies of Myrmica rubra L.(Hymenoptera: Formicidae). J Anim Ecol 42(3):761–771. https://doi.org/10.2307/3136
Evans JD, Aronstein K, Chen YP, Hetru C, Imler JL, Jiang H, Hultmark D (2006) Immune pathways and defence mechanisms in honey bees Apis mellifera. Insect Mol Biol 15(5):645–656. https://doi.org/10.1111/j.1365-2583.2006.00682.x
Evans HC, Groden E, Bischoff JF (2010) New fungal pathogens of the red ant, Myrmica rubra, from the UK and implications for ant invasions in the USA. Fungal Biol 114(5-6):451–466. https://doi.org/10.1016/j.funbio.2010.03.007
Fefferman NH, Traniello JF, Rosengaus RB, Calleri DV (2007) Disease prevention and resistance in social insects: modeling the survival consequences of immunity, hygienic behavior, and colony organization. Behav Ecol Sociobiol 61(4):565–577. https://doi.org/10.1007/s00265-006-0285-y
Fewell JH, Page RE (1993) Genotypic variation in foraging responses to environmental stimuli by honey bees, Apis mellifera. Cell Mol Life Sci 49(12):1106–1112. https://doi.org/10.1007/BF01929923
Gordon DM, Mehdiabadi NJ (1999) Encounter rate and task allocation in harvester ants. Behav Ecol Sociobiol 45(5):370–377. https://doi.org/10.1007/s002650050573
Hänel H (1982) The life cycle of the insect pathogenic fungus Metarhizium brunneum in the termite Nasutitermes exitiosus. Mycopathologia 80(3):137–145. https://doi.org/10.1007/BF00437576
Harpur BA, Zayed A (2013) Accelerated evolution of innate immunity proteins in social insects: adaptive evolution or relaxed constraint? Mol Biol Evol 30(7):1665–1674. https://doi.org/10.1093/molbev/mst061
Hart AG, Ratnieks FLW (2002) Waste management in the leaf-cutting ant Atta colombica. Behav Ecol 13(2):224–231. https://doi.org/10.1093/beheco/13.2.224
Holbrook CT, Barden PM, Fewell JH (2011) Division of labor increases with colony size in the harvester ant Pogonomyrmex californicus. Behav Ecol 22(5):960–966. https://doi.org/10.1093/beheco/arr075
Hölldobler B, Wilson EO (1990) The Ants. Harvard University Press, Cambridge
Hou C, Kaspari M, Vander Zanden HB, Gillooly JF (2010) Energetic basis of colonial living in social insects. Proc Natl Acad Sci 107(8):3634–3638. https://doi.org/10.1073/pnas.0908071107
Howard DF, Tschinkel WR (1976) Aspects of necrophoric behavior in the red imported fire ant, Solenopsis invicta. Behaviour 56(1):157–180. https://doi.org/10.1163/156853976X00334
Hughes WOH, Eilenberg J, Boomsma JJ (2002) Trade-offs in group living: transmission and disease resistance in leaf-cutting ants. Proc R Soc Lond B 269(1502):1811–1819. https://doi.org/10.1098/rspb.2002.2113
Jackson DE, Hart AG (2009) Does sanitation facilitate sociality? Anim Behav 77(1):e1–e5. https://doi.org/10.1016/j.anbehav.2008.09.013
Julian GE, Cahan S (1999) Undertaking specialization in the desert leaf-cutter ant Acromyrmex versicolor. Anim Behav 58(2):437–442. https://doi.org/10.1006/anbe.1999.1184
Julian GE, Fewell JH (2004) Genetic variation and task specialization in the desert leaf-cutter ant, Acromyrmex versicolor. Anim Behav 68(1):1–8. https://doi.org/10.1016/j.anbehav.2003.06.023
Karsai I, Wenzel JW (1998) Productivity, individual-level and colony-level flexibility, and organization of work as consequences of colony size. Proc Natl Acad Sci 95(15):8665–8669. https://doi.org/10.1073/pnas.95.15.8665
Kolmes SA, Winston ML (1988) Division of labour among worker honey bees in demographically manipulated colonies. Insect Soc 35(3):262–270. https://doi.org/10.1007/BF02224059
Lamon B, Topoff H (1981) Avoiding predation by army ants: defensive behaviours of three ant species of the genus Camponotus. Anim Behav 29(4):1070–1081. https://doi.org/10.1016/S0003-3472(81)80060-7
Leclerc JB, Detrain C (2016) Ants detect but do not discriminate diseased workers within their nest. Naturwissenschaften 103(7-8):70. https://doi.org/10.1007/s00114-016-1394-8
Mailleux AC, Deneubourg JL, Detrain C (2003) How does colony growth influence communication in ants? Insect Soc 50(1):24–31. https://doi.org/10.1007/s000400300004
McCallum H, Barlow N, Hone J (2001) How should pathogen transmission be modelled? Trends Ecol Evol 16(6):295–300. https://doi.org/10.1016/S0169-5347(01)02144-9
McGlynn TP (2007) Serial monodomy in ants: an antipredator strategy? Ecol Entomol 32(6):621–626. https://doi.org/10.1111/j.1365-2311.2007.00909.x
Myerscough MR, Oldroyd BP (2004) Simulation models of the role of genetic variability in social insect task allocation. Insect Soc 51(2):146–152. https://doi.org/10.1007/s00040-003-0713-1
Naug D, Camazine S (2002) The role of colony organization on pathogen transmission in social insects. J Theor Biol 215(4):427–439. https://doi.org/10.1006/jtbi.2001.2524
O’Donnell S (1998) Genetic effects on task performance, but not on age polyethism, in a swarm-founding eusocial wasp. Anim Behav 55(2):417–426. https://doi.org/10.1006/anbe.1997.0627
O’Shea-Wheller TA, Sendova-Franks AB, Franks NR (2015) Differentiated anti-predation responses in a superorganism. PLoS One 10(11):e0141012. https://doi.org/10.1371/journal.pone.0141012
O'donnell S, Reichardt M, Foster R (2000) Individual and colony factors in bumble bee division of labor (Bombus bifarius nearcticus Handl; Hymenoptera, Apidae). Insect Soc 47(2):164–170. https://doi.org/10.1007/PL00001696
Oi DH, Pereira RM (1993) Ant behavior and microbial pathogens (Hymenoptera, Formicidae). Fla Entomol 76(1):63–74. https://doi.org/10.2307/3496014
Okuno M, Tsuji K, Sato H, Fujisaki K (2012) Plasticity of grooming behaviour against entomopathogenic fungus Metarhizium anisopliae in the ant Lasius japonicus. J Ethol 30(1):23–27. https://doi.org/10.1007/s10164-011-0285-x
Oldroyd BP, Fewell JH (2007) Genetic diversity promotes homeostasis in insect colonies. Trends Ecol Evol 22(8):408–413. https://doi.org/10.1016/j.tree.2007.06.001
Page RE, Robinson GE (1991) The genetics of division of labour in honey bee colonies. Adv Insect Physiol 23:117–169. https://doi.org/10.1016/S0065-2806(08)60093-4
Page RE, Robinson GE, Fondrk MK, Nasr ME (1995) Effects of worker genotypic diversity on honey bee colony development and behavior (Apis mellifera L.) Behav Ecol Sociobiol 36(6):387–396. https://doi.org/10.1007/BF00177334
Pie MR, Rosengaus RB, Traniello JF (2004) Nest architecture, activity pattern, worker density and the dynamics of disease transmission in social insects. J Theor Biol 226(1):45–51. https://doi.org/10.1016/j.jtbi.2003.08.002
Pinheiro JC, Bates DM (2000) Linear mixed-effects models: basic concepts and examples. Mixed-effects models in S and S-Plus. Spriger Verlag, New York, p 3–56
Pontieri L, Vojvodic S, Graham R, Pedersen JS, Linksvayer TA (2014) Ant colonies prefer infected over uninfected nest sites. PLoS One 9(11):e111961. https://doi.org/10.1371/journal.pone.0111961
Pruitt JN, Grinsted L, Settepani V (2013) Linking levels of personality: personalities of the “average” and “most extreme” group members predict colony-level personality. Anim Behav 86(2):391–399. https://doi.org/10.1016/j.anbehav.2013.05.030
Reber A, Chapuisat M (2012) No evidence for immune priming in ants exposed to a fungal pathogen. PLoS One 7:353–353
Renucci M, Tirard A, Provost E (2011) Complex undertaking behavior in Temnothorax lichtensteini ant colonies: from corpse-burying behavior to necrophoric behavior. Insect Soc 58(1):9–16. https://doi.org/10.1007/s00040-010-0109-y
Rosengaus RB, Maxmen AB, Coates LE, Traniello JFA (1998) Disease resistance: a benefit of sociality in the dampwood termite Zootermopsis angusticollis (Isoptera: Termopsidae). Behav Ecol Sociobiol 44(2):125–134. https://doi.org/10.1007/s002650050523
Rosengaus RB, Jordan C, Lefebvre ML, Traniello JFA (1999) Pathogen alarm behavior in a termite: a new form of communication in social insects. Naturwissenschaften 86(11):544–548. https://doi.org/10.1007/s001140050672
Sadd BM, Barribeau SM, Bloch G, De Graaf DC, Dearden P, Elsik CG, Robertson HM (2015) The genomes of two key bumblebee species with primitive eusocial organization. Gen Biol 16(1):76. https://doi.org/10.1186/s13059-015-0623-3
Schmid-Hempel P (1998) Parasites in social insects. Princeton University Press, Princeton
Schmid-Hempel P, Schmid-Hempel R (1993) Transmission of a pathogen in Bombus terrestris, with a note on division of labour in social insects. Behav Ecol Sociobiol 33:319–327
Seeley TD (1985) Honeybee ecology: a study of adaptation in social life. Princeton University Press, Princeton. https://doi.org/10.1515/9781400857876
Seeley TD, Kolmes SA (1991) Age polyethism for hive duties in honey bees—illusion or reality? Ethology 87:284–297
Shykoff JA, Schmid-Hempel P (1991) Parasites and the advantage of genetic variability within social insect colonies. Proc R Soc Lond B Biol Sci 243(1306):55–58. https://doi.org/10.1098/rspb.1991.0009
Simone M, Evans JD, Spivak M (2009) Resin collection and social immunity in honey bees. Evolution 63(11):3016–3022. https://doi.org/10.1111/j.1558-5646.2009.00772.x
Simone-Finstrom M, Spivak M (2010) Propolis and bee health: the natural history and significance of resin use by honey bees. Apidologie 41(3):295–311. https://doi.org/10.1051/apido/2010016
Smith CR, Toth AL, Suarez AV, Robinson GE (2008) Genetic and genomic analyses of the division of labour in insect societies. Nat Rev Genet 9(10):735–748. https://doi.org/10.1038/nrg2429
Snyder LE (1993) Non-random behavioural interactions among genetic subgroups in a polygynous ant. Anim Behav 46(3):431–439. https://doi.org/10.1006/anbe.1993.1212
Spivak M, Reuter G (2001) Resistance to American foulbrood disease by honey bee colonies Apis mellifera bred for hygienic behavior. Apidologie 32(6):555–565. https://doi.org/10.1051/apido:2001103
Staples JA, Milner RJ (2000) A laboratory evaluation of the repellency of Metarhizium anisopliae conidia to Coptotermes lacteus (Isoptera: Rhinotermitidae). Sociobiology 36:133–148
Theraulaz G, Bonabeau E, Deneubourg JL (1998) Response threshold reinforcements and division of labour in insect societies. Proc R Soc Lond B Biol Sci 265(1393):327–332. https://doi.org/10.1098/rspb.1998.0299
Thomas ML, Elgar MA (2003) Colony size affects division of labour in the ponerine ant Rhytidoponera metallica. Naturwissenschaften 90(2):88–92. https://doi.org/10.1007/s00114-002-0396-x
Ugelvig LV, Kronauer DJ, Schrempf A, Heinze J, Cremer S (2010) Rapid anti-pathogen response in ant societies relies on high genetic diversity. Proc R Soc Lond B Biol Sci 277(1695):2821–2828. https://doi.org/10.1098/rspb.2010.0644
Verhoeff K (1974) Latent infections by fungi. Annu Rev Phytopathol 12(1):99–110. https://doi.org/10.1146/annurev.py.12.090174.000531
Waddington SJ, Hughes WOH (2010) Waste management in the leaf-cutting ant Acromyrmex echinatior: the role of worker size, age and plasticity. Behav Ecol Sociobiol 64(8):1219–1228. https://doi.org/10.1007/s00265-010-0936-x
Waters JS, Holbrook CT, Fewell JH, Harrison JF (2010) Allometric scaling of metabolism, growth, and activity in whole colonies of the seed-harvester ant Pogonomyrmex californicus. Am Nat 176(4):501–510. https://doi.org/10.1086/656266
Wilson EO (1986) The organization of flood evacuation in the ant genus Pheidole (Hymenoptera: Formicidae). Insect Soc 33(4):458–469. https://doi.org/10.1007/BF02223951
Yanagawa A, Shimizu S (2007) Resistance of the termite, Coptotermes formosanus Shiraki to Metarhizium anisopliae due to grooming. Biol Control 52:75–85
Acknowledgements
We thank the anonymous referees for their comments as well as Nell Foster for improving the English of this MS.
Funding
This study was funded by a Ph.D. grant to J-B.L. from FRIA (Fonds pour la Recherche dans l’Industrie et dans l’Agriculture) and by a research credit (CDR J.0092.16) from FRS-FNRS (Fonds de la Recherche Scientifique). C.D. is Research Director from the Belgian National Fund for Scientific Research (FNRS).
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by S. Cremer
Electronic supplementary material
ESM 1
(DOCX 138 kb)
Rights and permissions
About this article
Cite this article
Leclerc, JB., Detrain, C. Impact of colony size on survival and sanitary strategies in fungus-infected ant colonies. Behav Ecol Sociobiol 72, 3 (2018). https://doi.org/10.1007/s00265-017-2415-0
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00265-017-2415-0