Abstract
Parasites reduce host fitness, and so instigate counter adaptations by their hosts. In temporary social parasitism, usurpers must not only enter the colony unharmed, but also have their eggs reared by the host workers. We introduced parasitic Formica lugubris and Formica aquilonia queens into queen right and orphaned fragments of three host species, Formica cinerea, Formica picea and Formica fusca, and show that workers of all three host species kill over 40 % of the introduced queens within 10 days, regardless of the presence/absence of a resident queen, and parasite species. More parasite queens died in F. cinerea than in F. picea and F. fusca. There were no major differences in survival between the parasite species (except that F. lugubris survived longer than F. aquilonia in F. fusca colonies compared to F. picea colonies), but parasite queens survived longer in orphaned than in queen right fragments of F. fusca. Experimental introduction of parasite (F. aquilonia) eggs into orphaned colonies of F. fusca showed that none of the parasite eggs were reared until pupation; whereas on average, 12 % of the con-specific hetero-colonial eggs introduced in the same manner were reared until pupation. In all colonies that received parasite brood, all offspring consisted of worker-laid males, whereas the corresponding value was 50 % for colonies that received con-specific hetero-colonial brood. Thus, when the risks of entering host colonies and brood failure are combined, the rate of successful colony take-over is very low. Moreover, the host workers can to some extent alleviate the costs of parasitism by producing a final batch of own offspring.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Parasitism is defined as a symbiosis in which one organism (the parasite) benefits from another organism (the host) which suffers fitness loss (Bush 2001). Parasites are ubiquitous and most species are targeted by at least one, but more commonly several species of parasites (Bush 2001; Thomas et al. 2005). As a result, parasitism crucially affects adaptive evolution in all organisms (Schmid-Hempel 1998) and evokes host counter adaptations and hones host ability to prevent intrusion (Davies et al. 1989; Thompson 2005). This, in turn leads to refinement of parasite abilities to overcome host defence systems and initiates co-evolutionary arm races between hosts and their parasites (Davies et al. 1989; Thompson 2005). The genetic composition of both hosts and parasites has been proposed to influence both host defence, and parasite transmission and virulence (Hamilton 1980). In particular, the colonies of social insects present an especially attractive resource for both internal and external parasites owing to their high densities of genetically similar individuals combined with the accumulation of localized resources (Baer and Schmid-Hempel 1999; Hughes and Boomsma 2004, 2006).
Nest parasites of social insects may exploit hosts in many ways, from nest sharing, food and brood theft, slave-making, and nest take-over, to permanent social parasitism, i.e. inquilinism (social insects themselves have secondarily evolved into parasites and exploit host colonies of other social insects throughout their reproductive life) (Wojcik 1989; Lenoir et al. 2001; Buschinger 2009). To date, about 230 species of socially parasitic ants, either inquilines or temporary social parasites, have been identified among the ca. 12,500 described species of ants (Buschinger 1986; 2009).
In temporary social parasitism, newly mated parasite queens enter the host nest and use the available resources to establish a nest of their own (Buschinger 2009). These queens must bypass two lines of host defense: first they must gain entry into the colony and become integrated as colony members, and second they must have their first brood reared by the host workers. Upon colony usurpation, the host queen(s) are often killed by intruding parasite queens (in ants, Faber 1967; Santschi 1906; Buschinger 2009; Polistes wasps, e.g. Cervo 2006). Alternatively, parasite queens may enter orphaned host nests, which may facilitate usurpation given that acceptance threshold in these nests can be more permissive (Helanterä and Sundström 2007). If the nest take-over is successful the host colony becomes moribund, as workers eventually disappear due to ageing and fatalities (Wilson 1971; Schmid-Hempel 1998). Thus the fitness consequences for the host colony are dire, and selection should hone precise recognition abilities of the host species (Davies et al. 1989) both with respect to rejecting intruding queens and selectivity in the rearing of non-nest mate eggs (Achenbach and Foitzik 2009; Chernenko et al. 2011).
Parasite queens may enter host colonies on their own, or be carried into the nest by host workers, join a slave raid targeting the nest (Mori and Le Moli 1998; Buschinger 2009 and references therein), or form groups to attack host colonies and reduce individual parasite mortality (Raczkowski and Luque 2010). Hosts of slave-making ants have been observed to guard their nest and prevent parasites from entering (Mori et al. 1995; Foitzik et al. 2001), but the behavior of host workers towards parasite queens in temporary social parasites has to our knowledge not been described. More importantly, although one field observation (Santschi 1906) and two experimental studies (Faber 1967; Raczkowski and Luque 2010) have reported temporary social parasite queens entering host nests, no studies have, to our knowledge, assessed how often parasite queens successfully usurp host colonies in ants, and whether moribund orphaned host colonies are more vulnerable to intrusion than queen-right ones. A few studies have addressed brood acceptance and survival, and shown that eggs of the dulotic parasite Polyergus breviceps are rejected by workers of its hosts Formica occulta and Formica gnava (Johnson et al. 2005), sexual pupae of the slave-maker ant Protomognathus americanus are attacked by enslaved Temnothorax workers (Achenbach and Foitzik 2009), and workers of Formica fusca and Formica lemani reject eggs of their temporary social parasite Formica truncorum when offered for retrieval into their nest (Chernenko et al. 2011). However, when F. fusca and F. lemani were offered a mixture of parasite and con-colonial eggs, some parasite brood remained after 10 days (Chernenko et al. 2011). This begs the question to what extent host workers rear parasite brood until adulthood, when intermingled with their own brood as would be the case when a parasite queen has successfully entered a host colony.
The wood ants, Formica s. str., for example Formica lugubris and Formica aquilonia, are prime examples of temporary social parasites. Young mated queens of these species invade colonies of the subgenus Serviformica, where the parasite queens presumably kill the resident queens and rely on the host workers to rear the first worker brood (Collingwood 1979; Buschinger 1986; Czechowski et al. 2002; Seifert 2007; Buschinger 2009). In this study, we first test acceptance and survival of the parasite queens of the species F. lugubris and F. aquilonia in three potential host species, Formica cinerea, Formica picea and F. fusca, using behavioural assays where we introduce parasite queens into queen right and orphaned host nests. With this assay we assess whether parasite queens kill host queens, the extent to which parasite queens survive in the nests of the three host species, and whether parasite survival depends on the host species. Then, in a separate experiment we test brood survival until pupation of one parasite species, F. aquilonia in orphaned colonies of one host species F. fusca.
Material and methods
Experiment 1: acceptance of parasite queens
Study colonies
Colonies of F. cinerea, F. picea and F. fusca were collected in late April and early May in the vicinity of Tvärminne Zoological Station in southern Finland. At the time of collection, the queens had not resumed oviposition after hibernation. Upon collection, the queen right colonies of all three host species (16 colonies of each species) were split into two fragments each with 300–400 workers, one with one queen and one orphaned. In addition, workers and sexual brood was collected in late May and early June from eight colonies of F. aquilonia, and 10 colonies of F. lugubris in the same area. These samples contained workers and ca. 100 mature males and/or females.
All colonies were established in laboratory nests made of plastic boxes (30 × 30 × 40 cm), the walls of which were coated with fluon™ to prevent the ants from escaping, the bottom lined with peat, and a ceramic tile added to provide shelter and Sphagnum moss to maintain humidity. The nests were maintained at 25–27 °C, fed Bhatkar–Whitcomb diet (Bhatkar and Whitcomb 1970), and moistened daily throughout the experiments. The queen right host nests and their orphaned counterparts were left undisturbed until the experiments were started 2–4 weeks after collection. The colonies of F. aquilonia and F. lugubris provided mated young queens to be introduced into the experimental colonies. To obtain young parasite queens, we placed positively phototactic winged queens and males from different colonies in transparent boxes (50 × 60 × 60 cm), and collected wingless negatively phototactic queens each morning (alates, winged queens shed their wings soon after mating). Dissection of a subset of 20 queens of each species showed that they were all mated after this treatment.
Experimental design
The queen right and the corresponding orphaned fragments of F. cinerea, F. picea and F. fusca were used as prospective host colonies for the young queens of F. aquilonia and F. lugubris. To test the acceptance of parasite queens, we introduced one queen of either parasite species at a time into each queen right and orphaned host nest by leaving it on the nest surface in the nest box. This mimics the way parasite queens would encounter host nests also under natural conditions (Raczkowski and Luque 2010). The nests were then monitored daily and parasite queens removed either when found dead, or after 10 days. The condition of the resident queen (alive or dead) and the location of the parasite queen were noted (inside or outside the host nest). A new parasite queen was introduced the day after the previous one had been removed either because it was dead or 10 days had elapsed, the procedure being repeated twice (i.e. in total three parasite queens were introduced into each host fragment). Different sets of colonies were used for the two parasite species. One queen right fragment of F. cinerea for F. lugubris lacked an orphaned counterpart, and in 12 cases fewer than three queens were introduced into an orphaned fragment (four for F. picea and eight for F. cinerea).
Statistical procedures
We first used an ANOVA with a split-split-plot design (Snedecor and Cochran 1980), with the average proportion of dead parasite queens (number of dead queens divided by the total number of queens introduced into each nest fragment) as the response variable, host species as the main-plot factor, fragment type (queen-right or orphaned) with its interaction with host species as the sub-plot factor, and introduced parasite species with all remaining two-way and three-way interactions as the sub-sub-plot factor. This design was necessary in order to obtain the correct units of replication, and to account for the fact that colony identity necessarily differed between the different host and parasite species. The data were very close to a normal distribution (Wilk–Shapiro W 95 = 0.97, p = 0.042), but to ascertain the validity of the analysis we permuted the data across all replicates rechecking normality for each data set. Through this procedure, we identified one outlier, and after we removed this outlier, normality was restored (Wilk–Shapiro W 94 = 0.98, p = 0.28) Thus the use of a parametric ANOVA is legitimate to test for differences between main effects and their interactions. The full model, including the error terms is given in “Electronic supplementary material” (ESM) Table S1. This analysis was done with Statistix 9 (Analytical Software). We then used Kaplan–Meier survival analysis to test for species-specific responses of the host species towards the two parasite species. The response variable was the number of days the parasite queens remained alive in the host nest fragments, with the number of queens alive at the 10th day censored.
The factor and the strata (i.e. nesting within the factor) were host species and fragment type (queen right or orphaned), host species and parasite species, fragment type and host species, parasite species and host species. To compare factor levels we used Mantel-Cox pair wise differences for each stratum. This analysis was done in SPSS 19 (IBM).
Experiment 2: rearing of parasite brood
Study colonies
Thirty seven entire colonies of F. fusca each with over 400 workers but no queen, and 16 colonies of F. aquilonia, each with ca. 35 queens and ca. 500 workers, were collected in early April in the same area as the colonies for the first experiment, and established as described above. We used orphaned fragments because orphaning renders F. fusca less discriminating against con-specific hetero-colonial eggs than queen right ones (Helanterä and Sundström 2007), and therefore facilitates acceptance while avoiding the problem with alien brood being replaced by the large number of eggs inevitably laid by the host queen. Once most orphaned fragments of F. fusca contained eggs (after 18 ± 4 days, 20 of the 37 fragments contained worker-laid eggs), the experiments were started. The nests of F. aquilonia were maintained to provide a source of eggs to introduce into the F. fusca nests for rearing.
Experimental design
To test whether orphaned nests of F. fusca produce their own offspring rather than rear con-specific or parasite eggs, we introduced eggs laid by either F. aquilonia queens or orphaned hetero-colonial F. fusca workers into the orphaned nests of F. fusca, and allowed the host workers to rear brood until the onset of pupation. Three F. fusca fragments with eggs were used as a source of con-specific hetero-colonial eggs, and not used as recipients. At this stage, the F. aquilonia nests also had large numbers of eggs.
To start the experiment we introduced a mixture of eggs from four F. aquilonia nests into 18 F. fusca nests, and a mixture of hetero-colonial F. fusca eggs from the three orphaned donor nests into 16 F. fusca nests. Each recipient nest received 50 eggs. Before introduction, the eggs were examined under binocular microscope to ensure that they were undamaged. Eggs, already present in recipient nests were collected and checked, then returned to the fragment (ca. 100 eggs per colony in 54 % of the colonies; the remaining colonies had no eggs). We routinely handle eggs this way and no adverse effects have been observed (Chernenko et al. 2011, 2012). Owing to the limited number of orphaned F. fusca nests, we considered the establishment of a separate control with con-colonial worker-laid eggs unnecessary, as we routinely use orphaned fragments to rear worker-produced males into adulthood (Chernenko et al. 2011). We collected all brood when pupae were present (ca. 30 days), and stored these in 96 % alcohol for genetic analyses. Twelve colonies started to deteriorate early on and were discarded.
Genetic analyses
We genotyped 16 adult workers from each F. fusca colony as well as all brood at nine polymorphic DNA-microsatellite loci (FL12, FL20: Chapuisat 1996; FE13, FE17, FE19, FE21, FE51: Gyllenstrand et al. 2002; FY7, FY13: Hasegawa and Imai 2004). DNA-extraction and PCR amplification followed the protocols described in Chernenko et al. (2011). The PCR products were then diluted 1:26 with ddH2O, separated using automated capillary sequencer MegaBACE 1000 and sized against ET400-R standard, GE Healthcare. The genotypes were scored using the program Fragment Profiler v1.2 GE Healthcare and allele calling was confirmed manually. To distinguish eggs laid by host workers from the introduced eggs laid by the parasite queens, we first checked whether each brood item was diploid or haploid. Individuals heterozygous at least at one locus are necessarily diploid and therefore laid by F. aquilonia queens, whereas individuals with a homozygous genotype can be either diploid or haploid (i.e. males). Haploid male-destined eggs may be either parasite or worker laid. Brood items homozygous at all nine loci were considered haploid and potentially worker-laid. Given the observed population-wide allele frequencies at the nine loci, the combined probability that a diploid individual was homozygous at all nine loci and mistakenly assigned as haploid was 0.00034. For haploid brood we then selected one diagnostic locus (FY13), which allowed us to distinguish brood laid by parasite queens from that laid by the workers in the experimental colonies as the species shared no alleles at this locus (Eva Schultner personal communication). Haploid brood was considered produced in F. fusca colonies if it carried no parasite alleles. Brood items with missing information at the diagnostic locus were excluded from the analysis (N = 28 out of 174). To distinguish between con-colonial and hetero-colonial con-specific brood we compared offspring genotypes at the nine loci with worker genotypes in both the host colony and the source colonies. Brood with genotypes that matched both host and source colonies was considered as undetermined and excluded from the analysis (N = 12 out of 174), whereas brood with alleles present in host-colony workers, but not in the source-colony workers were considered con-colonial.
Statistical procedures
We used Relatedness 5.0.8 (Queller and Goodnight 1989) to estimate average within-colony relatedness. Nests were weighed equally, and standard errors were obtained by jack-knifing over colonies. We used an ANCOVA combined with a split-plot design (Snedecor and Cochran 1980) to analyse rearing preferences of the host colonies with the number of brood items of a specific type (own or alien) reared as the response variable. The data were normally distributed (Wilk–Shapiro W = 0.99, p = 0.44), so permitting the use of an ANOVA. The model included the following explanatory variables: colony as the replication variable, brood type reared (con-colonial versus hetero-colonial con-specific or parasite) as the main plot factor, egg origin (con-specific hetero-colonial vs. parasite) with the interaction egg origin × brood type reared as the sub-plot factors, and relatedness as a covariate. We also tested whether the presence of own eggs at the start of the experiment influenced the type of brood reared with an ANCOVA also with a split-plot design. The response variable, the replication variable, and the main plot factor, were the same, but the sub-plot factor was the presence/absence of own eggs and the interaction between it and the main-plot factor, within-colony worker relatedness was added as a co-variate. The full model including the error terms is given in ESM Table S2. This analysis was done with Statistix 9 (Analytical Software).
Results
Experiment 1: acceptance of parasite queens
None of the resident queens died during the experiment, and all resident queens were always found inside the nests. A significantly greater fraction of parasite queens found inside the nest were dead (121 dead, 16 alive) as compared to those found outside the nest (70 dead, 37 alive; Fisher’s exact test two-tailed p < 0.0001). The average mortality of parasite queens differed between host species, with significantly higher parasite mortality in F. cinerea nests (100 %) than in the other two host species, but no significant difference in parasite mortality between F. picea and F. fusca (ANOVA, host species, F 2, 14 = 21.8, p < 0.0001, Tukey’s post-hoc test: p = 0.01, and p > 0.10, respectively; Fig. 1). Neither the presence/absence of a host queen nor the parasite species had a significant influence on parasite mortality (ANOVA, fragment type, F 1, 21 = 0.62, p = 0.44; parasite species, F 1, 40 < 0.01, p = 0.97, Table S1). None of the interactions were significant (host species × fragment type, F 2, 21 = 1.60, p = 0.22; host species × parasite species, F 2, 40 = 0.76, p = 0.47; fragment type × parasite species, F 1, 40 = 0.14, p = 0.71; host species × fragment type × parasite species, F 2, 40 = 0.30, p = 0.74).
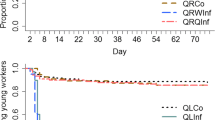
When the daily survival rate was examined we also found significant differences in parasite survival, both with respect to parasite species, host species, and the status of the host species (queen-right or orphaned). The two parasite species did not differ significantly in their overall survival rate, or their survival rate in F. cinerea colonies, but F. lugubris survived overall longer than F. aquilonia in F. fusca than in F. picea colonies (Table 1, Fig. 2). Orphaning had no effect on the survival of parasite queens in F. cinerea and F. picea, but in F. fusca parasite queens survived longer in orphaned than queen-right fragments (Table 1, Fig. 2).
Survival of queens of two parasite species (Formica aquilonia and F. lugubris) in different host species (F. cinerea, F. fusca and F. picea) orphaned and queen right fragments. The graph illustrates one of the four tested models in the Kaplan–Meier analysis with parasite species as the factor and host species as strata (nested within factor)
Egg rearing
Average worker relatedness was 0.62 ± 0.19 across all experimental F. fusca colonies, but the colony-specific estimates varied extensively (0.27–0.89) indicating a mixture of monogyne (single queen) and polygyne (multiple queen) colonies. We found brood at different developmental stages from eggs to pupae, but eggs and small larvae were present only in negligible numbers in six colonies. The remaining brood included medium larvae (10 colonies), large larvae (14 colonies) and pupae (11 colonies), in most cases the brood comprised a mixture of all three categories of brood but in three cases only medium larvae were found (Fig. 3). All brood was haploid and thus male-destined.
Overall colonies that received con-specific eggs reared on average a total of 15.7 ± 11.7 brood items (N = 11), and those that received parasite eggs reared 15.6 ± 7.6 items (N = 11). Of the introduced brood items, on average 6.3 ± 8.1 of the 50 eggs introduced remained in the colonies that received con-specific hetero-colonial eggs, whereas none remained in any of the colonies that received parasite eggs (Figs. 3 and 4). The colonies that received con-specific hetero-colonial eggs reared on average 8.6 ± 9.4 own offspring, whereas those that received parasite eggs reared 12.7 ± 6.2 own offspring (on average 0.58 ± 1.6 brood items per colony failed to amplify or did not provide reliable information). The overall number of own offspring reared was significantly higher than the number of introduced brood items reared, whereas the type of eggs introduced (con-specific vs. parasite) had no significant effect on the total number of brood items reared, i.e. own plus introduced brood (ANCOVA main plot factor, type of brood (con-specific vs. parasite) reared: F 1, 10 = 8.49, p = 0.015; sub-plot factor, type of eggs introduced F 1, 10 = 0.33, p = 0.57). The interaction between the main- and the sub-plot factors was also significant (F 1, 19 = 7.92, p = 0.011) because all parasite brood was replaced by own brood, whereas the colonies that received con-specific hetero-colonial brood retained a considerable fraction of these at the expense of rearing own offspring. Neither worker relatedness (covariate F 1, 19 = 0.11, p = 0.74), nor the presence of own eggs at the beginning of the experiment had any effect on the outcome (ANOVA main plot factor, type of brood reared: F 1, 10 = 7.51, p = 0.021; sub-plot factor, presence/absence of eggs F 1, 10 = 0.85, p = 0.37, interaction F 1, 10 = 0.58, p = 0.46).
Number of own and introduced reared brood items as a function of worker relatedness depending on the origin of introduced eggs. Circles indicate own offspring reared, whereas triangles indicate introduced offspring reared. Open symbols indicate that the fragment received con-specific eggs and closed symbols indicate that the fragment received parasite eggs
Discussion
In this study, we show that when the combined effects of risks during colony take-over and the odds for having their eggs reared into adulthood are taken into account, queens of temporary parasites stand a very low chance of success upon entering a prospective host colony. Whereas all resident host queens survived throughout the experiment, the survival rate of intruders was on average 22 % for the duration of 10 days. Survival rate, however, differed among the host species, which suggests that the odds for a successful take-over also can depend on the prospective host species. Yet also the host species that was the least discriminating against intruding queens, F. fusca, reared none of the parasite brood offered.
None of the resident queens died during the experiment, which runs counter to two earlier studies (one field observation and one laboratory experiment) showing that parasite queens of Lasius reginae, Bothriomyrmex decapitans, B. rigicidus, and Monomorium santschii kill the host queens upon entering the nest (Santschi 1906; Faber 1967). Because the parasite queens were in our experiment left on the top of the recipient nest, some may not have entered the colony and therefore were not in a position to kill the host queen. However, a third of the queens that were alive at the end of the test, were found inside the host colony and therefore would have had an opportunity to kill the host queen. This suggests that neither parasite queens nor host workers kill host queens, at least within the time frame used in this study.
None of the parasite queens survived in F. cinerea, whereas, within the time period used in this study, on average 50 and 20 % of parasite queens survived in F. fusca and F. picea, respectively. Three factors may underpin such differences in the propensity to fend off intruding parasites: First, the chemical recognition cues may show different degrees of overlap between hosts and parasites, making recognition less or more difficult. However, based on cuticular hydrocarbons F. fusca and F. cinerea cluster together and relatively close to Formica s. str., whereas F. picea clusters together with Coptoformica (Martin et al. 2008a). Alternatively, chemical insignificance may facilitate intrusion (Lorenzi 2006 and references therein), but if so all potential host species should be equally vulnerable to intrusion, which is not the case. Second, given that territoriality and aggression tends to increase with average colony size across species (Stuart 1991; Crosland 1990; Tschinkel et al. 1995), intruders may be rejected more often in species with large colonies. Indeed, of the three host species F. cinerea has the largest average colony size (Collingwood 1979; Czechowski et al. 2002), and defend not only their nest, but also food resources and foraging areas against intruders (Dlusskij 1967; Czechowski and Marko 2005). In contrast, F. fusca and F. picea only defend their nests against intruders (Rosengren et al. 1986; Savolainen and Vepsäläinen 1989; Savolainen 1990; Czechowski and Vepsäläinen 1999). Third, given that theory predicts (Reeve 1989) and empirical data show and that a high incidence of parasite intrusions tends to ramp up defenses in the hosts, whether physical (Foitzik et al. 2001; Buczkowski and Silverman 2005; Fürst et al. 2012) or related to recognition ability (Martin et al. 2011), the species most commonly hosting parasites should show the strongest defense. However, contrary to this expectation, the species usually targeted by the parasites (Collingwood 1979; Czechowski et al. 2002), F. fusca, was the least aggressive in our experiments. Alternatively, F. fusca is more often targeted because the two parasites F. aquilonia and F. lugubris are better able to exploit this host without eliciting aggression. Finally, colony kin structure, chemical dissimilarity and parasite exposure may jointly affect overall aggression levels. We do not have joint data on cuticular chemistry, kin structure and parasite exposure on the colonies used in this study, so the extent to which the differences in parasite rejection are based on such joint effects remains open. Further studies designed to test the rate of parasite intrusion, and links between territoriality and between-species discrimination should add to our insights on this question.
We found no differences in the overall survival rate between the two parasite species F. lugubris and F. aquilonia, except that F. lugubris survived slightly better than F. aquilonia in F. fusca colonies. This agrees only partly with the suggestion by Hölldobler and Wilson (1990) that monogyne species, such as F. lugubris (Sundström et al. 2005) mainly exploit hetero-specific colonies because their young mated queens are less likely to be adopted into their natal colonies, whereas queens of obligately polygyne species, such as F. aquilonia (Sundström et al. 2005), are frequently adopted into their natal or non-natal con-specific nests (Pamilo et al. 1992; Crozier and Pamilo 1996). Thus, our results show some differences between the two parasite species, but whether this reflects a general tendency of monogyne species being more adept at being adopted than polygyne ones can only be resolved with data on additional species.
In the egg-rearing experiment, on average 57 % of the brood items retained at the end of the experiment were hetero-colonial con-specific. This suggests that colonies of the host species may be prone to intra-specific brood parasitism. Indeed, this is the case in honeybees and bumblebees (Lopez-Vaamonde et al. 2004; Härtel et al. 2006; Beekman and Oldroyd 2008; Chapman et al. 2009). Whether this also occurs in ants and whether orphaning in these cases indeed facilitates infiltration remains to be studied.
By contrast, none of the parasite eggs were reared until pupation. Thus, although some parasite brood may survive until the first larval instar in orphaned colonies of F. fusca (Chernenko et al. 2011), such brood is not reared to adulthood. This shows that parasite brood is recognized, but only after some time. A similar discrimination delay for parasite brood was also found in F. occulta and F. gnava (Johnson et al. 2005), F. lemani (Chernenko et al. 2011) and three species of Temnothorax (Achenbach and Foitzik 2009). This delay suggests that discrimination may be imprecise at early developmental stages, so that attempts at removing parasite brood may lead to the accidental removal of own brood with prohibitive costs of discrimination in its wake (Reeve 1989; Sherman et al. 1997; Lenoir et al. 2001). Only multiple encounters of individual brood items, or a later larval stage may therefore allow reliable discrimination against parasites, so that such brood is removed later to minimize the costs of accidentally rejecting descendant brood. Indeed several studies have shown that the cuticular chemistry can change during brood development (Lenoir et al. 2001 and references therein). In tentative support of this interpretation, our results suggest a decline in the proportion of parasite versus descendant brood with increasing brood development (Fig. 3), but this should be verified with a properly controlled experiment. The pattern is very different in Polistes wasps, in which parasite queens actively manipulate colony recognition cues and so come to be accepted in the host colony by the emerging host workers (Lorenzi 2006 and references therein). Similarly, queens of the parasitic hornet Vespa dybowskii do not employ chemical mimicry to have their brood accepted, but whether they manipulate the host profile remains to be studied (Martin et al. 2008b).
Social parasitism in social insects, especially temporary social parasitism, represents an interesting parallel to brood parasitism in birds (Davies et al. 1989; Cervo 2006; Kilner and Langmore 2011). In both cases, four main outcomes of parasitism are expected—successful resistance, the evolution of defense portfolios, acceptance of the parasite, and tolerance of the parasite (Kilner and Langmore 2011). Nevertheless, in contrast to social insects, quite a few bird species accept parasitic offspring after hatching even though they demonstrate profound discrimination earlier in the breeding cycle (Kilner and Langmore 2011). Such a disparity may emanate from different fitness costs of erroneously accepting parasite brood. If the host queen is killed, parasitized ant colonies are moribund (Hölldobler and Wilson 1990; Buschinger 2009), whereas avian hosts only stand to lose 1 year of reproduction (Payne 1977; Davies 2000). As a result also, the recognition systems may have evolved to a different degree of precision. Indeed, with the exception of highly specialized host–parasite pairs, avian hosts accept both intra- and inter-specific brood parasites at relatively high rates (Payne 1977; Rothstein 1982; 1990; Davies 2000; Lyon and Eadie 2008; Kilner and Langmore 2011), whereas social insects sport finely tuned intra- and inter-specific discrimination capabilities based on cuticular hydrocarbons (reviewed in Lenoir et al. 2001). Thus the chemical recognition cues used by social insects are continuously honed by selection in an arms race of recognition and detection with hosts evolving more distinct profiles and parasites developing a profile that overlap with that of their hosts (Lenoir et al. 2001), so preventing immediate reliable discrimination. Alternatively, parasites sport an “odourless” chemical profile at the time of usurpation, which aids them in usurping host colonies (“cuticular chemical insignificance” Lenoir et al. 1999).
Social parasites, like avian brood parasites, also need to breach two defenses: entering the host colony (or successfully laying an egg in the host nest) and have their brood reared by the host. Here, we show that in the case of social parasites, both the rates of successful usurpation and successful brood survival are very low. The colonies that nevertheless are overtaken by parasite queens can turn to male production and so allow the workers to gain direct fitness benefits before the colony dies (Chernenko et al. 2011). Thus, the defense targets of avian and social insect hosts are fundamentally different. In avian hosts, the cost of parasitism may be lower than erroneously rejecting their own brood (Davies 2000; Davies and Welbergen 2008; Welbergen and Davies 2009), which selects for a more permissive attitude towards suspicious brood. By contrast, hosts of social parasites stand to lose their queen and with her their entire reproductive future, and so invest in both prevention of parasites from entering and post-infection defense.
References
Achenbach A, Foitzik S (2009) First evidence for slave rebellion: enslaved ant workers systematically kill the brood of their social parasite Protomognathus americanus. Evol 63(4):1068–1075
Baer B, Schmid-Hempel P (1999) Experimental variation in polyandry affects parasite loads and fitness in a bumble-bee. Nat 397(6715):151–154
Beekman M, Oldroyd BP (2008) When workers disunite: intraspecific parasitism in eusocial bees. Annu Rev Entomol 53:19–37
Bhatkar A, Whitcomb WH (1970) Artificial diet for rearing various species of ants. Fla Entomol 53(4):229–232
Buczkowski G, Silverman J (2005) Context-dependent nestmate discrimination and the effect of action thresholds on exogenous cue recognition in the Argentine ant. Anim Behav 69(3):741–749
Buschinger A (1986) Evolution of social parasitism in ants. Trends Ecol Evol 1(6):155–160
Buschinger A (2009) Social parasitism among ants: a review (Hymenoptera: Formicidae). Myrmecol News 12:219–235
Bush AO (2001) Parasitism: the diversity and ecology of animal parasites. Cambridge University Press, Cambridge
Cervo R (2006) Polistes wasps and their social parasites: an overview. Ann Zool Fennici 43:531–549
Chapman NC, Nanork P, Gloag RS, Wattanachaiyingcharoen W, Beekman M, Oldroyd BP (2009) Queenless colonies of the Asian red dwarf honeybee Apis florea are infiltrated by workers from other queenless colonies. Behav Ecol 20(4):817–820
Chapuisat M (1996) Characterization of microsatellite loci in Formica lugubris B and their variability in other ant species. Mol Ecol 5(4):599–601
Chernenko A, Helanterä H, Sundström L (2011) Egg recognition and social parasitism in Formica ants. Ethol 117(12):1081–1092
Chernenko A, Helanterä H, Sundström L (2012) Colony kin structure and queen recruitment in the ant Formica fusca (Hymenoptera: Formicidae). Myrmecol News 16:93–100
Collingwood CA (1979) The Formicidae (Hymenoptera) of Fennoscandia and Denmark. Fauna Entomologica Scnadinavica. Scandinavian Science Press Ltd., Klampenborg
Crosland MWJ (1990) The influence of the queen, colony size and worker ovarian development on nestmate recognition in the ant Rhytidoponera confusa. Anim Behav 39(3):413–425
Crozier RH, Pamilo P (1996) Evolution of social insect colonies. Oxford University Press, Oxford
Czechowski W, Marko B (2005) Competition between Formica cinerea Mayr (Hymenoptera: Formicidae) and co-occurring ant species, with special reference to Formica rufa L.: direct and indirect interferences. Pol J Ecol 53(4):467–487
Czechowski W, Vepsäläinen K (1999) Plesiobiosis between Formica fusca L. and Formica aquilonia Yarr. (Hymenoptera, Formicidae). Ann Zool Wars 49:125–127
Czechowski W, Radchenko A, Czechowska W (2002) The ants of Poland. Museum & Institute of Zoology, Warsaw
Davies NB (2000) Cuckoos, cowbirds and other cheats. T. & A. D. Poyser, London
Davies NB, Welbergen JA (2008) Cuckoo–hawk mimicry? An experimental test. Proc R Soc B 275(1644):1817–1822
Davies NB, Bourke AF, de L Brooke M (1989) Cuckoos and parasitic ants: interspecific brood parasitism as an evolutionary arms race. Trends Ecol Evol 4(9):274–278
Dlusskij GM (1967) Murav'i roda Formika. Nauka, Moscow
Faber W (1967) Beiträge zur Kenntnis sozialparasitischer Ameisen, 1: Lasius (Austrolasius n. sg.) reginae n.sp., eine neue temporär sozialparasitische Erdameise aus Österreich (Hym. Formicidae). Pflanzenschutz-Ber 36:73–107
Foitzik S, DeHeer CJ, Hunjan DN, Herbers JM (2001) Coevolution in host–parasite systems: behavioural strategies of slave-making ants and their hosts. Proc R Soc B 268(1472):1139–1146
Fürst MA, Durey M, Nash DR (2012) Testing the adjustable threshold model for intruder recognition on Myrmica ants in the context of a social parasite. Proc R Soc B 279(1728):516–522
Gyllenstrand N, Gertsch PJ, Pamilo P (2002) Polymorphic microsatellite DNA markers in the ant Formica exsecta. Mol Ecol Notes 2(1):67–69
Hamilton WD (1980) Sex versus non-sex versus parasite. Oikos 35(2):282–290
Härtel S, Neumann P, Kryger P, von der Heide C, Moltzer G-J, Crewe RM, van Praagh JP, Moritz RFA (2006) Infestation levels of Apis mellifera scutellata swarms by socially parasitic Cape honeybee workers (Apis mellifera capensis). Apidologie 37(4):462–470
Hasegawa E, Imai S (2004) Characterization of microsatellite loci in red wood ants Formica (s. str.) spp. and the related genus Polyergus. Mol Ecol Notes 4(2):200–203
Helanterä H, Sundström L (2007) Worker policing and nest mate recognition in the ant Formica fusca. Behav Ecol Sociobiol 61:1143–1149
Hölldobler B, Wilson EO (1990) The ants. Belknap Press of Harvard University Press, Massachusetts
Hughes WOH, Boomsma JJ (2004) Genetic diversity and disease resistance in leaf-cutting ant societies. Evol 58(6):1251–1260
Hughes WOH, Boomsma JJ (2006) Does genetic diversity hinder parasite evolution in social insect colonies? J Evol Biol 19(1):132–143
Johnson CA, Topoff H, Vander Meer RK, Lavine B (2005) Do these eggs smell funny to you?: an experimental study of egg discrimination by hosts of the social parasite Polyergus breviceps (Hymenoptera: Formicidae). Behav Ecol Sociobiol 57(3):245–255
Kilner RM, Langmore NE (2011) Cuckoos versus hosts in insects and birds: adaptations, counter-adaptations and outcomes. Biol Rev 86(4):836–852
Lenoir A, Fresneau D, Errard C, Hefetz A (1999) The individuality and the colonial identity in ants: the emergence of the social representation concept. In: Detrain C, Deneubourg JL, Pasteels J (eds) Information processing in social insects. Birkhauser, Basel, pp 219–237
Lenoir A, D'Ettorre P, Errard C, Hefetz A (2001) Chemical ecology and social parasitism in ants. Annu Rev Entomol 46:573–599
Lopez-Vaamonde C, Koning JW, Brown RM, Jordan WC, Bourke AFG (2004) Social parasitism by male-producing reproductive workers in a eusocial insect. Nat 430(6999):557–560
Lorenzi MC (2006) The result of an arms race: the chemical strategies of Polistes social parasites. Ann Zool Fennici 43:550–563
Lyon BE, Eadie JM (2008) Conspecific brood parasitism in birds: a life-history perspective. Annu Rev Ecol Evol Syst 39(1):343–363
Martin SJ, Helanterä H, Drijfhout FP (2008a) Evolution of species-specific cuticular hydrocarbon patterns in Formica ants. Biol J Linn Soc 95(1):131–140
Martin SJ, Takahashi J, Ono M, Drijfhout FP (2008b) Is the social parasite Vespa dybowskii using chemical transparency to get her eggs accepted? J Insect Physiol 54(4):700–707
Martin SJ, Helanterä H, Drijfhout FP (2011) Is parasite pressure a driver of chemical cue diversity in ants? Proc R Soc B 278(1705):496–503
Mori A, Le Moli F (1998) Mating behavior and colony founding of the slave-making ant Formica sanguinea (Hymenoptera: Formicidae). J Insect Behav 11(2):235–245
Mori A, D'Ettorre P, Le Moli F (1995) Host nest usurpation and colony foundation in the European amazon ant; Polyergus rufescens Latr. (Hymenoptera: Formicidae). Insect Soc 42(3):279–286
Pamilo P, Chautems D, Cherix D (1992) Genetic differentiation of disjunct populations of the ants Formica aquilonia and Formica lugubris in Europe. Insect Soc 39(1):15–29
Payne RB (1977) The ecology of brood parasitism in birds. Annu Rev Ecol Syst 8(1):1–28
Queller DC, Goodnight KF (1989) Estimating relatedness using genetic-markers. Evol 43(2):258–275
Raczkowski JM, Luque GM (2011) Colony founding and social parasitism in Lasius (acanthomyops). Insect Soc 58(2):237–244
Reeve HK (1989) The evolution of conspecific acceptance thresholds. Am Nat 133(3):407–435
Rosengren R, Pamilo P, Cherix D (1986) Insular ecology of the red wood ant Formica truncorum Fabr. II. Distribution, reproductive strategy and competition. Ann Zool Fenn 59:63–94
Rothstein SI (1982) Mechanisms of avian egg recognition: which egg parameters elicit responses by rejecter species? Behav Ecol Sociobiol 11(4):229–239
Rothstein SI (1990) A model system for coevolution: avian brood parasitism. Annu Rev Ecol Syst 21(1):481–508
Santschi F (1906) A propos de moeurs parasitiques temporaires des fourmis du genre Bothriomyrmex. Ann Soc Entomol Fr 75:363–392
Savolainen R (1990) Colony success of the submissive ant Formica fusca within territories of the dominant Formica polyctena. Ecol Entomol 15(1):79–85
Savolainen R, Vepsäläinen K (1989) Niche differentiation of ant species within territories of the wood ant Formica polyctena. Oikos 56(1):3–16
Schmid-Hempel P (1998) Parasites in social insects. Princeton University Press, Princeton
Seifert B (2007) Die Ameisen Mittel- und Nordeuropas. Lutra Verlags- und Vertriebsgesellschaft, Görlitz/Tauer
Sherman PW, Reeve HK, Pfennig DW (1997) Recognition systems. In: Krebs JR, Davies NB (eds) Behavioural ecology: an evolutionary approach. Wiley–Blackwell, Oxford
Snedecor GW, Cochran WG (1980) Statistical methods, 8th edn. Iowa State University Press, Iowa
Stuart RJ (1991) Nestmate recognition in leptothoracine ants: testing for effects of queen number, colony size and species of intruder. Anim Behav 42(2):277–284
Sundström L, Seppa P, Pamilo P (2005) Genetic population structure and dispersal patterns in Formica ants—a review. Ann Zool Fenn 42(3):163–177
Thomas F, Renaud F, Guégan J-F (2005) Parasitism and ecosystems. Oxford University Press, Oxford
Thompson JN (2005) Coevolution: the geographic mosaic of coevolutionary arms races. Curr Biol 15(24):992–994
Tschinkel WR, Adams ES, Macom T (1995) Territory area and colony size in the fire ant Solenopsis invicta. J Anim Ecol 64(4):473–480
Welbergen JA, Davies NB (2009) Strategic variation in mobbing as a front line of defense against brood parasitism. Curr Biol 19(3):235–240
Wilson EO (1971) Insect societies. The Belknap press of Harvard University Press, Cambridge
Wojcik DP (1989) Behavioral interactions between ants and their parasites. Fla Entomol 72(1):43–51
Acknowledgements
This study was supported by the Academy of Finland (project nos. 121216, 206505, 213821, 121078 and 135970). The authors wish to thank Hannele Luhtasela-El-Showk and Martina Ozan for help in the field, David R. Nash for discussions and valuable comments.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by S. Cremer
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOC 49 kb)
Rights and permissions
About this article
Cite this article
Chernenko, A., Vidal-Garcia, M., Helanterä, H. et al. Colony take-over and brood survival in temporary social parasites of the ant genus Formica . Behav Ecol Sociobiol 67, 727–735 (2013). https://doi.org/10.1007/s00265-013-1496-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00265-013-1496-7