Abstract
Schizocosa wolf spiders show tremendous diversity in courtship complexity, with different species employing varying numbers of components within and across sensory modalities. Using a comparative approach, we investigate the importance of each signaling modality in the courtship display of five Schizocosa species (three stridulating and two drumming) by assessing mating success under manipulated signaling environments. Irrespective of the degree of male ornamentation, the three stridulating species exhibit a dependence on the seismic, but not visual, signaling environment for mating success. Mating was independent of signaling environment for the two drumming species. We next ask whether the degree to which each species depends upon a signaling modality for mating (i.e., modality importance) is correlated with the estimated modality-specific signal complexity. We first calculate effect sizes for the influence of seismic versus visual signaling environments on the likelihood to mate for ten Schizocosa species and then use an element-counting approach to calculate seismic and visual signal complexity scores. We use a phylogenetic regression analysis to test two predictions: (1) the importance of seismic signaling is correlated with seismic signal complexity and (2) the importance of visual signaling is correlated with visual signal complexity. We find a significant relationship between visual signal importance and visual signal complexity, but no relationship between seismic signal importance and seismic signal complexity. Finally, we test the hypothesis that selection acts on complexity per se by determining whether seismic and visual signal complexity is correlated across species. We find support for this hypothesis in a significant relationship between seismic and visual signal complexity.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Complexity, or the intricate combination of many parts, is a fundamental attribute of biological systems, and arguably nowhere is it more conspicuous than in the courtship displays of certain animal groups. Yet the factors that drive this complexity remain unknown, as do the answers to such questions as whether courtship complexity (the combination of multiple display components) functions differently across different animal groups or in different behavioral contexts. Observed complexity in animal courtship displays can be thought of in terms of courtship elaboration and can incorporate multiple visual components in the form of distinct morphological structures, conspicuous coloration or pigment patterns, and/or vigorous multifaceted movements. Additionally, courting animals may incorporate the production and dissemination of chemical compounds, multicomponent acoustic signals, and/or air movements, among others. Many such multifaceted courtship displays incorporate signals or components that are categorized by their physical properties into different sensory channels or modalities (see Hebets 2011). Such displays are considered to be multimodal (Rowe 1999; Partan and Marler 1999).
Perhaps more striking than the diversity and ubiquitous nature of multimodal courtship displays is the frequency of observable disparity in the degree of complexity among closely related animal species (Darwin 1871). In some instances, such as within the North American wolf spider genus Schizocosa, this disparity is manifested as seemingly different investment in signaling modalities between closely related species, even sister taxa (see Fig. 1). Such divergence in traits associated with courtship displays among close relatives has led scientists to hypothesize that sexual communication, and likely sexual selection, plays a prominent role in morphological diversification and, ultimately, in speciation (Grant and Grant 1997; Shaw and Parsons 2002; Gerhardt and Huber 2002). The evidence for this hypothesis, however, is underwhelming (see Servedio 2012), and determining the extent to which sexual selection, versus natural selection, is involved in diversification and speciation remains a central goal of evolutionary biology (Ritchie 2007; Kraaijeveld et al. 2011). Species groups characterized by distinct and widespread variation in courtship displays and associated morphological traits provide ideal systems to tackle this goal. Here, we use just such a group, Schizocosa wolf spiders, to explore the evolution and function of complex, multimodal courtship signaling across numerous species.
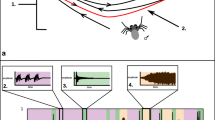
Bayesian consensus tree based on cytochrome c oxidase subunit 1 (COI) sequence data. Thickened bars represent species with tibial bristles present (from Stratton 2005). Waveforms are placed next to the species of focus and lines beneath each waveform depict one second of courtship. Although depicted as separate species here for clarity, COI has not revealed reciprocal monophyly for S. ocreata and S. rovneri. For a tree with posterior probabilities and branch lengths proportional to the expected number of substitutions per site see Supplementary Fig. 1
The genus Schizocosa consists of at least 63 species worldwide, 23 of which occur in North America (for discussion of relevance of non-Nearctic species which are likely to belong in other genera, see Stratton 2005). Across the reciprocally monophyletic North American Schizocosa clade, there exist extremes in terms of both courtship signal complexity and sexual dimorphism. Some species employ relatively simple, seismic-only courtship displays, while others incorporate not only more complex multicomponent seismic signals, but also visual displays that involve vigorous waving of ornamented forelegs (reviewed in Stratton 2005; Framenau and Hebets 2007; Vaccaro et al. 2010). Foreleg ornamentation, a sexually dimorphic trait, consists of some combination of pigmentation and/or brushes of setae (or hairs) on various segments of the first pair of walking legs in mature males (reviewed in Stratton 2005; Framenau and Hebets 2007). The first, and sometimes second, pair of walking legs is also the pair frequently waved during courtship dances. Among the 31 taxa included in a recent morphological phylogenetic analysis (23 described species plus additional populations of select species), foreleg ornamentation was hypothesized to have evolved independently five or six times, and to have been subsequently lost two or three times (Stratton 2005). While all described Schizocosa species incorporate a seismic signal in their courtship displays, only some possess additional courtship components in the form of foreleg ornaments and leg waves—making the displays of these species multimodal in nature (seismic plus visual).
Numerous studies have demonstrated female choice associated with male courtship displays across Schizocosa species (Stratton and Uetz 1981, 1983; McClintock and Uetz 1996; Scheffer et al. 1996; Hebets and Uetz 1999; Hebets and Uetz 2000; Uetz and Roberts 2002; Hebets 2003; Hebets 2005; Persons and Uetz 2005; Uetz and Norton 2007; Hebets 2007, 2008; Hebets et al. 2008a; Gibson and Uetz 2008; Hebets et al. 2011; Rundus et al. 2011; among others). Several of these studies have involved artificial manipulations of display components and subsequent assessment of female responses. A series of studies manipulated components of the visual signal using video playback and examined female receptivity displays (for review see Uetz and Roberts 2002; Hebets 2005; Uetz and Norton 2007; Hebets 2008), while others have studied female receptivity responses to isolated display components of live courting males (e.g., Scheffer et al. 1996; Hebets and Uetz 1999; reviewed in Uetz and Roberts 2002). Recent studies, however, have suggested that female receptivity responses may not always directly translate into mate choice (Hebets 2005, 2008), initiating a movement towards studies which enable direct female–male contact in signal ablation conditions where the successful transmission of seismic or visual signals can be manipulated independently (e.g., Taylor et al. 2006; Rundus et al. 2010; Rundus et al. 2011; Stafstrom and Hebets 2013). Such signal ablation studies have highlighted the importance of seismic signaling across numerous species, even those with conspicuous visual displays (Hebets 2005, 2008; Rundus et al. 2011). Additionally, such studies have highlighted the importance of inter-signal interactions (Hebets 2005; Hebets et al. 2011), and have demonstrated environment-dependent mate choice (Rundus et al. 2011).
Here, we aim to add to an already impressive amount of data regarding mate choice and multimodal signal function across Schizocosa species by examining five additional species in the genus. We first examine the relative importance of seismic versus visual signaling in the mating success of each species by conducting mate choice trials in signaling environments in which seismic and/or visual signals cannot transmit effectively. We include new data on three species that produce their seismic signals predominantly through stridulation (Schizocosa rovneri, Schizocosa saltatrix, Schizocosa duplex) and two species that produce their seismic signals predominantly through percussion or drumming (Schizocosa retrorsa and Schizocosa avida) (see Stratton 2005). We use the results of these signal ablation mating trials in addition to results from previous studies for an additional five species (Schizocosa ocreata, Schizocosa uetzi, Schizocosa stridulans, Schizocosa floridana, and Schizocosa crassipes) to calculate effect sizes for the influence of the seismic signal and the influence of the visual signal on mating success. These calculated effect sizes represent proxies of the importance of each signaling modality for mating (e.g., proxies of receiver response) and provide a means by which we can compare across species. Next, we ask whether there is a correlation between the importance of a signaling modality and its degree of elaboration, or its complexity. To do this, we calculate complexity scores for the seismic signal and the visual signal for all ten species. Using our calculated effect sizes and our complexity scores, we test the hypothesis that modality-specific signal complexity is driven by modality-specific receiver responses. Specifically, we test the following predictions using phylogenetic comparative methods: (1) the importance of seismic signaling for mating (seismic signal effect size) is correlated with seismic signal complexity and (2) the importance of visual signaling for mating (visual signal effect size) is correlated with visual signal complexity. Finally, we ask whether there is a relationship between the degree of signal complexity across signaling modalities and test the following prediction: (3) seismic signal complexity is correlated with visual signal complexity.
Methods
Signal ablation and mating success
Spider collections and maintenance
Immature females and males, and some mature males of S. rovneri, S. saltatrix, S. duplex, S. retrorsa, and S. avida were collected from sites near Oxford, MS, USA (Electronic supplementary material (ESM) Table 1). All individuals were brought back to the laboratory and were housed individually in visually isolated cages. They were placed on a 12:12-h light/dark cycle, were provided a constant source of water, and were fed two to three crickets per week. We checked every individual for molts every 2 days and recorded the date of the final maturation molt.
Mating trials
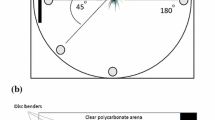
All signal ablation/isolation trials were run in a 2 × 2 full factorial design of visual signal present/absent (light/dark) and seismic signal present/absent (filter paper/granite) ultimately resulting in four signaling environments (visual/seismic: V+/S+, V+/S−, V−/S+, and V−/S−) for each of the five species. The design was nearly identical to those used previously for S. uetzi (Hebets 2005), S. stridulans (Hebets 2008), and S. crassipes (Stafstrom and Hebets 2013). Briefly, visual absent trials were run in a completely dark room during the animals normal light period (so that all animals were tested at the same time during their circadian rhythm and only the signaling environment varied) and were videotaped with a mini camcorder (Sony DCR-TRV38 MiniDV Handycam), using the Nightshot option. Visual present trials were run under both natural and artificial lighting—they were run on a bench top in the laboratory underneath a skylight. On any given day, a set of light and dark trials were paired and run back to back in random order. Seismic-absent trials were conducted on a piece of granite rock with a circular acetate arena, measuring 10.1 cm in diameter, glued to its surface (see Fig. 1, Hebets 2005). A single female and male pair were placed inside the arena and allowed to interact freely on the granite. Seismic signals are highly attenuated on granite (Elias et al. 2004) and previous studies have demonstrated that copulation frequency is greatly reduced on granite in spider species that are thought to rely heavily on seismic signaling (Elias et al. 2004; Hebets 2005, 2008; Rundus et al. 2011). For seismic-present treatments, the same size circular arena was placed within a 10.16 × 10.16 × 12.86-cm Amac Plastic Product box lined on the bottom with a piece of Whatman no. 1 filter paper—which provided a substrate for transmitting the seismic signal—cut to fit the circular arena. The bottom of both the rock and the plastic box were painted white to control for both visual contrast and odor. During a given set of observations, two seismic-present and two seismic-absent arenas were observed simultaneously either in the light or in the dark, resulting in four simultaneous mate choice trials. The sides and bottom of all arenas were swabbed with alcohol in between trials and each side of a piece of filter paper was only used once.
Individual females and males were randomly assigned to one of the four signaling environments. For most species, all females and males were only used once and females were at least 10-days post-maturation molt. Prior to the onset of a trial, females were placed in their assigned arena and were allowed to acclimate for at least 5 min. Males were then placed in the arena and trials were observed for 45 min. During the 45-min trial, we recorded the following in real time: time to first male courtship, number of male attempted mounts, number of female attacks, presence/absence of cannibalism, presence/absence of copulation, and the time to copulation/cannibalism. Due to the variation in courtship complexity across species, our ability to determine the presence/absence of courtship also varied (e.g., some species have predominantly seismic courtship which can be difficult to discern on granite). When relevant, we discuss the proportion of trials in which we were confident that courtship occurred.
All statistical tests were run with JMP 8.0. Data that were not normally distributed were analyzed with non-parametric statistics as noted. For all species, we ran a nominal logistic model with predictor variables of: seismic environment (S+/S−), visual environment (V+/V−), and an interaction between seismic and visual environments. We also included female age. Our response variables were copulation (present/absent) and cannibalism (present/absent).
Effect size calculations
For measures of effect size, we used the Chi-square values generated for each of our predictor variables in our nominal logistic regression models to calculate r (see Nakagawa and Cuthill 2007). Effect sizes were calculated using freely available software (http://www.lyonsmorris.com/ma1/index.cfm). We calculated effect sizes using a variety of additional methods and all values were within the range of 0.09 differences (data not shown).
To compare results from this study with prior results on other species, we calculated effect sizes from previous datasets. We used raw data for S. uetzi from Hebets (2005), for S. stridulans from Hebets (2008), and for the conspicuously brush-legged S. crassipes (Miller et al. 1998) from Stafstrom and Hebets (2013) to run nominal logistic regression models and calculate effect sizes as described above.
Raw data were not available for S. ocreata, so we took advantage of a recently published study which assessed female receptivity responses to isolated seismic versus visual courtship components. We note here that this study scored female receptivity, not actual copulation success. All prior data refer to copulation success. Nonetheless, a study using S. ocreata found similar results when comparing actual mating success and female receptivity scores between males with shaved versus intact brushes (Scheffer et al. 1996). To calculate the effect size for female responses to seismic versus visual signal courtship components in S. ocreata, we extrapolated information from Uetz et al. (2009). We approximated the proportion of females responding receptively to each modality in isolation. Based upon Fig. 4 (in Uetz et al. 2009), we estimated the percentage of females responding to seismic-only signals to be 50 % (12/24) and the percentage responding to visual alone to be ∼41 % (10/24). We then generated a fictitious dataset incorporating these values and ran a nominal logistic regression with predictor variables of: seismic signal, visual signal, and both signals and a response variable of: female receptive (1/0). We used the Chi-square values generated from this regression to calculate the effect size of a female’s response to seismic and visual signals.
Seismic signal recordings
To record seismic signals, males were placed in a circular arena (13 × 6 cm) floored with a filter paper substrate (Whatman no. 1, 185 mm), and walled with clear acetate to prevent males from escaping. The filter paper floor was elevated 2.5 cm by resting on a circular metal ring with rubber footings. This arena was located inside of a rectangular soundproof chamber (50 × 37 × 43 cm), which was lined with loaded vinyl PSA and soundproof foam (Super Soundproofing Co., San Marcos, CA, USA) and placed on a vibration isolation table (Minus K 50BM-8C, Minus K Technology, Inglewood, CA, USA) to prevent outside noise being recorded. To stimulate males to begin signaling, conspecific female chemical cues were introduced into the arena with the male. Female silk contains pheromones indicating receptivity to mate (Tietjen 1979). To collect female silk, females were restricted inside a small arena floored with filter paper (Whatman no. 1, 185 mm) substrate for approximately 2 h and the pheromone-laden filter paper was subsequently placed in the recording arena. Seismic recordings were made using a laser vibrometer (Polytec PDV100). For the vibrometer to measure substrate vibrations made by the signaling male, a square (0.5 × 0.5 cm) piece of reflective tape was attached to the filter paper in the center of the arena. Digital output from the vibrometer was recorded on an Apple iMac in Quicktime Pro. All vibration recordings were exported from Quicktime Pro as uncompressed AIFF files at 44.1-kHz sampling rate. For each species, we recorded the seismic signal of numerous males (typically >20), all of which appeared to incorporate like components (see Gibson and Uetz 2008; Rundus et al. 2011; Gibson and Uetz 2012). We used one exemplar from each species and each male was recorded for at least 5 min after beginning courtship. Audio files and waveforms used to quantify the seismic signal complexity were made from the first bout of courtship from each male. These files ranged from 10–30 s in length.
Quantification of signal complexity
Quantifying signal complexity is a non-trivial task and as such, numerous distinct approaches exist. For example, Chen and colleagues recently developed a clever adaptation of the Shannon–Wiener diversity index to quantify color pattern complexity in agamid lizards, scoring the number of pattern types as equivalent to species richness, and abundance of the pattern type as equivalent to species equitability (Chen et al. 2012). Similar “enumeration” or element-counting approaches are regularly used to measure complexity of acoustic communication in diverse animal taxa such as birds, cetaceans, primates, bats, etc. (reviewed in Botero et al. 2008); and a more recent elaboration using algebraic equations is used to quantify complex courtship displays in jumping spiders (Elias et al. 2012). Building upon this substantive body of prior work, we base our complexity metric on the number of discrete identifiable components unique to each species’ seismic and visual display. Fortunately, Schizocosa have been the focus of behavioral research for decades and relevant, quantifiable display components have already been identified; we borrow heavily from this prior work.
We generated a score of complexity, or the number of identifiable discrete signal components, for both seismic and visual courtship displays of ten species of Schizocosa. Our complexity scores are based upon our current knowledge of signal components and are likely to reflect components which could be subject to selection. Our assumption with this approach is that each quantifiable element can be perceived by females. This approach purposefully ignores information regarding the presence or absence of specific components that might reflect independent or shared evolutionary origins (such as independent production mechanisms)—information which we cannot yet integrate in a satisfactory manner.
Seismic signal complexity score
We examined the signal waveforms of all ten focal species and identified discrete components of the signal (see details in Table 1). An observer who was blind to both the species and to the experiment identified unique seismic signal elements. The blind scorer (JSG) simultaneously listened to audio files of each species’ seismic signal while visually inspecting the waveform from which the audio file was produced. Listening to the audio file permitted identification of components that were similar in amplitude modulation (as seen in the waveform), but distinct in frequency modulation. The blind scorer (JSG) had prior experience quantifying wolf spider seismic signals, but no direct experience with the majority of species used in this study (with the exception of S. ocreata; Gibson and Uetz 2008, 2012). For many species, the seismic signal had previously been described, and for those we incorporate the relevant information into our complexity score (e.g., Stratton and Uetz 1981; Elias et al. 2006b; Gibson and Uetz 2008). Seismic complexity scores ranged from one to four with S. rovneri and S. avida representing the least complex seismic signals and S. ocreata representing the most complex seismic signal (Table 1).
Visual signal complexity score
The movements involved in visual courtship displays of numerous Schizocosa have been well characterized (reviewed in Stratton 2005) and foreleg dimorphism has been quantified for all described species (Stratton 2005). We use total counts of the number of identifiable descriptors of visual movement displays (e.g., leg arch and leg wave/tap) and foreleg dimorphism (e.g., femur pigment, tibia pigment, tibia brushes, metatarsus brushes) (see Table 2). We did not include body bounces (e.g., S. rovneri), cheliceral strikes (e.g., S. ocreata), or push-up displays (e.g., S. retrorsa) in our visual complexity score as these components appear to be consequences of the production of the seismic component and we lack data on their potential function in the visual modality. Visual complexity scores ranged from zero to six, again with S. rovneri representing the least complex visual display and S. ocreata representing the most complex visual display (Table 2). We note that the seismic and visual complexity scores could go arbitrarily high—we included only elements that were observed in displays of focal species.
Phylogenetic reconstruction
As a preliminary investigation of the phylogenetic structure of the genus Schizocosa, we used the mitochondrial gene cytochrome c oxidase subunit 1 (COI). COI has been previously used to examine phylogenetic relationships among Schizocosa species (Hebets and Vink 2007) and in other lycosid genera (Vink and Paterson 2003; Chang et al. 2007). We sequenced fragments of COI from 17 specimens representing 15 North American species (ESM Table 2). Species were identified using the following references (Dondale and Redner 1978; Uetz and Dondale 1979; Stratton 1991, 1997).
DNA was extracted from two to three legs using a ZR Genomic DNA™-Tissue MiniPrep kit (Zymo Research). The primers used to PCR amplify and sequence COI fragments were C1-J-1718-spider (5′-GGNGGATTTGGAAATTGRTTRGTTCC-3′) (Vink et al. 2005) plus C1-N-2776-spider (5′-GGATAATCAGAATANCGNCGAGG-3′) (Vink et al. 2005). PCR amplification was performed using i-StarTaq™ DNA Polymerase (iNtRON Biotechnology) in a Mastercycler® (Eppendorf) thermocycler with a cycling profile of 35 cycles of 94 °C denaturation (30 s), 48 °C annealing (30 s), 72 °C extension (1 min) with an initial denaturation of 3 min, and a final extension of 5 min. Excess primers and salts were removed from the resulting double-stranded DNA using a DNA Clean & Concentrator™ Kit (Zymo Research). Purified PCR fragments of DNA were sequenced in both directions at the Massey Genome Service (Massey University). Sequence data are deposited in GenBank (www.ncbi.nlm.nih.gov/Genbank/).
Sequences were edited and compared to each other using Sequencher 4.6 (Gene Codes Corporation). Sequencher was also used for the alignment of COI sequences because there was no evidence of insertions/deletions or stop codons and alignment was straightforward. Partitioned Bayesian analyses implemented in MrBayes version 3.1.2 (Ronquist and Huelsenbeck 2003) were used to estimate the COI phylogenetic tree topology. MrModeltest version 2.3 (Nylander 2008) implemented in PAUP* version 4.0b10 (Swofford 2002) was used to select the model parameters. Within MrModeltest, the Akaike information criterion was used for model selection (Posada and Buckley 2004). Based on the results of Brandley et al. (2005), the COI data were partitioned by codon with models selected for each codon; HKY (Hasegawa et al. 1985; Brandley et al. 2005) for the first and third codon positions and F81 + I (Felsenstein 1981) for the second codon positions.
Bayesian analyses were conducted by running two simultaneous, completely independent analyses, each with four heated chains and sampling every 1,000th tree. The analyses were run for 2 × 107 generations by which time the average standard deviation of split frequencies had dropped below 0.002, which indicated that the two tree samples had converged. Tracer version 1.5 (Rambaut and Drummond 2009) was also used to determine if the analyses had sufficient effective sample sizes. MrBayes was used to construct majority rule consensus trees, discarding the first 25 % of trees generated as burn-in. TreeView 1.6.6 (Page 1996) was used to view and save trees in graphic format.
Phylogenetic comparative methods
Branch lengths from the molecular phylogeny were transformed using non-parametric rate smoothing to create an ultrametric tree in TreeEdit (v 1.0a8). Although S. ocreata and S. rovneri are not reciprocally monophyletic, our analysis does not require that each tip on our tree represents a species, only that each tip represents molecular, behavioral, and/or morphological data that stem from the same population. These two species are represented in our ultrametric tree by sequences that are genetically similar to the populations for which the behavioral data was collected. Our analyses were re-run multiple times using numerous permutations of available data for S. ocreata and S. rovneri, including the removal of S. rovneri, and our results remained consistent. Using this ultrametric tree, phylogenetic least squares regression analyses were performed using the “nlme” and “ape” version 3.0-4 (Paradis et al. 2004) packages in R. Analyses were performed using a Brownian Motion (BM) model of character evolution. In each analysis, the dependent variable was the metric of complexity—seismic or visual. The complexity measures represent a continuous scale of complexity where each unit of change is equivalent, such that these variables are suitable for use in PGLS. The independent variable was the calculated effect size of the influence of seismic or visual signals on mating success.
We also calculated Blomberg’s K to test for phylogenetic signal in each of the four major variables included in the PGLS analyses (importance of visual and seismic signaling; complexity of visual and seismic signals). Values were calculated in R using the “picante” (Kembel et al. 2010) and ‘ape’ (Paradis et al. 2004) packages. Tests of the statistical significance of the K statistic are not meaningful at small sample sizes (Blomberg et al. 2003), and were therefore not performed. Values of K < 1 indicate low phylogenetic signal.
Results
Signal ablation and mating success
Schizocosa rovneri Uetz and Dondale 1979
A total of 66 female and male S. rovneri were run through mate choice trials and all individuals were used only once. Copulation frequency was dependent upon signaling environment (overall model, df = 4, χ 2 = 10.06, p = 0.039). Pairs were more likely to copulate in the seismic-present versus seismic-absent environment, but there was no effect of the visual environment and no interaction between the two (seismic, χ 2 = 8.6, p = 0.03; visual, χ 2 = 0.69, p = 0.41; seismic × visual, χ 2 = 0.03, p = 0.36; female age, χ 2 = 0.85, p = 0.36; Fig. 2a). The latency from first courtship to copulation was not dependent upon the signaling environment (F(3, 17) = 0.85, p = 0.49; Table 3).
The proportion of pairs in which males successfully copulated (black bars) and were cannibalized (white bars) for three Schizocosa species that produce seismic signals via stridulation. For all three species, pairs were significantly more likely to copulate in the presence versus absence of a seismic signal (S+ vs. S−). Different letters indicate significant differences. In S. saltatrix, males were more likely to be cannibalized in the light versus dark (V+ vs. V−)
In analyzing trials for which we were able to assess the presence/absence of courtship, there was no effect of the signaling environment on the likelihood to court (V+/S+, N = 12; V+/S−, N = 12; V−/S+, N = 4; V−/S−, N = 3; χ 2 = 3.5, p = 0.33). Regardless, we analyzed only trials for which a male courted and found a significant effect of signaling environment on copulation frequency (overall model, df = 3, χ 2 = 13.0, p = 0.005). As above, pairs were more likely to copulate in the presence versus absence of a seismic signal but there was no influence of the visual environment and no interaction between the two (seismic, χ 2 = 11.1, p = 0.0008; visual, χ 2 = 2.2, p = 0.14; visual × seismic, χ 2 = 0.05, p = 0.82).
Schizocosa saltatrix (Hentz 1844)
A total of 72 female and male S. saltatrix were run through mate choice trials. Copulation frequency was dependent upon signaling environment (overall model, df = 4, χ 2 = 11.89, p = 0.02; Fig. 2b). Specifically, the likelihood to copulate was not dependent on the visual environment, but was dependent on the seismic environment and there was no interaction between the two (visual, χ 2 = 0.12, p = 0.73; seismic, χ 2 = 8.87, p = 0.003; visual × seismic, χ 2 = 0.23, p = 0.63; female age, χ 2 = 2.69, p = 0.1; Fig. 2b). The latency from first courtship to copulation was not dependent on signaling environment (Kruskal–Wallis Test, df = 3, χ 2 = 3.12, p = 0.37; Table 3).
Due to the lack of obvious visual courtship displays, we were unable to confidently determine the presence/absence of courtship in seven of the trials and thus, they were removed from the following analysis. The likelihood to court was not dependent on signaling environment (V+/S+, N = 18; V+/S−, N = 17; V−/S+, N = 14; V−/S−, N = 16; χ 2 = 2.2, p = 0.53). Regardless, we analyzed only trials in which a male was known to court. For only trials in which a male obviously courted, copulation frequency did depend on signaling environment (overall model, χ 2 = 8.9, p = 0.03). As above, the likelihood to copulate was dependent on the seismic environment, but not the visual environment nor an interaction between the two (visual, χ 2 = 0.002, p = 0.96; seismic, χ 2 = 8.5, p = 0.004; visual × seismic, χ 2 = 0.15, p = 0.69).
A shortage of S. saltatrix males forced us to use males in multiple trials, but males were never used in the same signaling environment. A total of 34 different males were used in the 72 trials. Ten males were used once, 14 males twice, 6 males three times, and 4 males four times. To account for the re-use of males, we conducted an additional least squares regression analysis including male as a random factor. This analysis revealed the same results as above (visual, F = 0.44, p = 0.51; seismic, F = 8.56, p = 0.005; visual × seismic, F = 0.36, p = 0.56). Since males were never used multiply in the same treatment and because we found no effect of individual male on the likelihood to copulate (df = 33, χ 2 = 33.5, p = 0.44), we do not remove multiple males from analyses in the supplementary results (see below).
Schizocosa duplex Chamberlin 1925
A total of 66 female and male S. duplex were run through mate choice trials. All individuals were used only once. As we did not know the age for all females, female age was not included in this model. As with all other species, however, all females were known virgins. Copulation frequency was dependent upon signaling environment (overall model, df = 3, χ 2 = 8.17. p = 0.04). Specifically, copulation frequency was dependent upon the seismic environment (seismic, χ 2 = 4.2, p = 0.04), but not the visual environment nor an interaction between the seismic and visual environment (visual, χ 2 = 1.8, p = 0.18; visual × seismic, χ 2 = 1.24, p = 0.27; Fig. 2c). We were unable to determine the time of the initial courtship for three males, but in analyzing the other remaining trials, the latency from the first courtship to copulation did not vary across signaling environments (Kruskal–Wallis Test, df = 3, χ 2 = 3.1, p = 0.37; Table 3).
The likelihood to court for male S. duplex was not dependent upon the signaling environment (overall model, df = 3, χ 2 = 6.0, p = 0.11). However, we observed a trend for males to be less likely to court in the visual-present treatments. Thus, we conducted an additional analysis to test the effect of the visual environment and found that males were indeed less likely to court in the visual-present (63 %) versus visual-absent (85 %) treatments (χ 2 = 4.3, p = 0.04). When analyzing only trials in which a male courted, we did not find an effect of signaling environment on copulation frequency (overall model, df = 3, χ 2 = 5.0, p = 0.18). However, pairs still tended to mate more in the seismic-present versus seismic-absent treatments and when looking for an effect of the seismic environment specifically, we found copulation frequency to be dependent upon the seismic signaling environment (χ 2 = 4.4, p = 0.04).
Schizocosa retrorsa (Banks 1911)
A total of 86 female and male S. retrorsa were run through mate choice trials across the four signaling environments. Copulation success was not dependent upon signaling environment (overall modal, df = 4, χ 2 = 1.3, p = 0.86; Fig. 3a). The latency from courtship to copulation did not vary across signaling environments (Kruskal–Wallis Test, df = 3, χ 2 = 3.0, p = 0.39; Table 3) and there were no pre-copulatory cannibalism events (N = 87, 0 %; Fig. 3a).
The likelihood to court for S. retrorsa was not dependent upon signaling environment (df = 3, χ 2 = 6.1, p = 0.11). Nonetheless, we analyzed only trials in which a male courted. In these trials, copulation frequency was still not dependent upon signaling environment (N, V+/S+ = 14, V+/S− = 11, V−/S+ = 9, V−/S− = 6; df = 3, χ 2 = 1.1, p = 0.78).
Schizocosa avida (Walkenaer 1837)
A total of 61 female and male S. avida were run through mate choice trials across signaling environments and all females and males were used only once. Our overall model was significant (overall model, df = 4, χ 2 = 11.43, p = 0.02), but we found no effect of signaling environment (visual, χ 2 = 0.9, p = 0.76; seismic, χ 2 = 0.03, p = 0.86; visual × seismic, χ 2 = 1.37, p = 0.23; Fig. 3b). However, we found a significant influence of female age (χ 2 = 9.77, p = 0.002). Female age ranged from 13 to 88 days old and females that copulated were significantly younger than those that did not (Wilcoxon Test, χ 2 = 8.5, p = 0.004). The latency to copulation was not dependent upon signaling environment (Kruskal–Wallis Test: df = 3, χ 2 = 2.9, p = 0.41; Table 3). Due to the difficulty of accurately determining the presence/absence of courtship in the dark treatments for S. avida, we were not able to analyze variability in courtship as a function of signaling environment.
Supplemental results
For all of the above species, we compared cannibalism rates across signaling environments. We also conducted analyses to insure that female and male ages and weights did not vary across signaling treatments. All results of these analyses can be found in the Electronic supplementary material.
Effect size
For S. rovneri, S. uetzi, S. stridulans, S. floridana, and S. saltatrix, the effect of the seismic signaling environment was much higher than the effect of the visual signaling environment (see ESM Table 3). The remaining species (S. ocreata, S. crassipes, S. duplex, S. retrorsa, and S. avida) showed similar effect sizes between the two modalities (<0.1 difference) (see ESM Table 3). The ranges for seismic effect size was 0.03–0.65 (S. avida–S. stridulans) and for visual effect size was 0.05–0.36 (S. saltatrix–S. crassipes) (see ESM Table 3).
Phylogenetic reconstruction and phylogenetic comparative methods
The Bayesian consensus tree supports many of the hypothesized relationships from Stratton 2005 (Fig. 1). We note that while we depict them as separate species in Fig. 1, COI does not separate out S. ocreata and S. rovneri (see also Hebets and Vink 2007).
The importance of seismic signaling did not correlate with seismic signal complexity, failing to support our first prediction (Table 4). We did find support, however, for our second and third predictions: (2) visual signal importance was correlated with visual signal complexity and (3) seismic signal complexity was correlated with visual signal complexity (Table 4).
Only the strength of seismic preference had a K value over 1 (K = 1.34), potentially indicating strong phylogenetic signal. K values for strength of visual preference, visual complexity, and seismic complexity were considerably less than 1 (K = 0.26, 0.12, and 0.13 respectively). Although no tests for significance could be performed, these values suggest that for the strength of visual preference, seismic complexity, and visual complexity scores, there is little or no phylogenetic signal.
Discussion
Using artificial environments in which we impeded the transmission of seismic and visual signals independently for five species of Schizocosa wolf spider, we determined that seismic signaling is important for the successful mating of S. rovneri, S. saltatrix, and S. duplex—all species which produce seismic signals through stridulation. In contrast, the visual signaling environment had no impact on the mating success of any species, despite the incorporation of numerous visual courtship components into many displays. The mating frequencies of S. retrorsa and S. avida—both drumming species—were independent of the signaling environment. For almost all Schizocosa species studied to date, irrespective of the degree to which males are ornamented and incorporate visual movements into their courtship displays, the presence/absence of visual courtship components has no influence on mating success (Hebets 2005; Taylor et al. 2006; Hebets 2008; Rundus et al. 2011; but see Stafstrom and Hebets 2013). Even in species where the visual signaling environment does influence mating success, the seismic environment remains significant (Stafstrom and Hebets 2013). In other words, there is no species studied yet for which the visual, but not seismic, signaling environment is important for mating success. Seismic signaling is hypothesized to be the ancestral signaling state for Schizocosa (Stratton 2005) and it remains the dominant modality in terms of facilitating mating success for most species in the genus.
The efficacy of seismic signal transmission is likely to have played an important role in the evolution of Schizocosa courtship signaling. Different species within the genus tend to be found predominantly on particular substrate types: e.g., S. retorsa: pine litter and red clay or sand (Hebets et al. 1996), S. ocreata: complex leaf litter (Dondale and Redner 1978; Stratton and Uetz 1981), S. stridulans: complex leaf litter (Stratton 1997), and S. avida: grass (Dondale and Redner 1978; personal observation). Similar to the signals of other spiders (e.g., jumping spiders Elias et al. 2004), the seismic courtship signals of Schizocosa transmit more or less effectively across these different substrate types (Hebets et al. 2008a; Elias et al. 2010; Gordon and Uetz 2011). The seismic signals of S. ocreata and S. stridulans are important for mating success and appear well suited to their natural leaf litter substrate, as signals match the average transmission characteristics of their signaling environment (Elias et al. 2010; Gordon and Uetz 2011). Nonetheless, selection for signal efficacy is not the entire story. In contrast to the signal–substrate matches discussed above, the seismic signal of S. retrorsa is not well matched to its natural signaling substrates, at least not in terms of signal attenuation. Mating frequency remains high on red clay (a natural signaling substrate) in spite of high seismic signal attenuation (Hebets et al. 2008a). This discrepancy between seismic signal transmission efficacy (in terms of attenuation) and mating success in S. retrorsa is hypothesized to be driven by selection for spectral properties of seismic displays—a hypothesis that remains to be tested directly.
Perhaps surprisingly, no studies have yet manipulated Schizocosa seismic signal structure directly (e.g., signaling rate, signal frequencies, etc.) and assessed female mate choice. Such an experimental approach is common in other acoustically displaying taxa (e.g., frogs: Gerhardt 1982; birds: reviewed in Douglas and Mennill 2010; crickets: Beckers and Wagner 2011), but appears to have lagged behind in animals that couple their complex acoustic signals to a substrate (but see examples in Bell 1980; Rodriguez et al. 2006). The dearth of such studies is likely due to the challenges associated with vibrational playbacks (reviewed in Cocroft and Rodriguez 2005), including inadequate techniques for playbacks for animals that move while communicating seismically. Similarly, while the condition dependence of putative visual ornaments have been the focus of much Schizocosa research (Uetz et al. 2002; Hebets et al. 2008b; Shamble et al. 2009; Rundus et al. 2011), the condition dependence of and reliance on particular seismic signal components for mating decisions have received relatively little attention (but see Rundus et al. 2011; Gibson and Uetz 2012). In the only two species previously studied, S. ocreata (Gibson and Uetz 2008) and S. floridana (Rundus et al. 2011), seismic signaling is condition dependent, and females appear to assess these seismic courtship components and utilize them for making mate choice decisions (Gibson and Uetz 2008; Rundus et al. 2011).
Male courtship rate, as frequently measured by the number of visual courtship components (e.g., leg taps or leg arches) per unit time, has consistently emerged as being highly predictive of male mating success across numerous Schizocosa species, including representatives of both stridulating and drumming species (S. uetzi: Shamble et al. 2009; S. retrorsa: Rundus et al. 2010; S. stridulans: Hebets et al. 2011; S. floridana: Rundus et al. 2011; Rosenthal and Hebets 2012; S. crassipes: unpublished data). Interestingly, the visual components used by researchers to quantify courtship rate typically have a seismic counterpart (see examples in salticids: Elias et al. 2006a; Elias et al. 2012). We suggest that it may be the seismic component of courtship that is the target of previously documented female choice for courtship rate. Given the recognized importance of seismic signaling, future studies should focus upon seismic signal manipulations and playbacks in order to quantify the details of female preferences for seismic signal components.
Courtship displays can be plastic and environment dependent. Males may remove elements from their courtship repertoire under conditions where these components may not transmit effectively (e.g., visual courtship components in the dark: S. ocreata (Taylor et al. 2005) and Rabidosa rabida (Wilgers and Hebets 2011)) and/or may increase their use of modality-specific components in environments where signals in alternate modalities are attenuated (e.g., increased use of visual signals when courting on rocks or soil) (Gordon and Uetz 2011). Detailed quantifications of courtship behavior were beyond the scope of this study and our data unfortunately cannot directly address the potential for courtship plasticity in our focal species. Although modality-specific signaling environments remained consistent (filter paper or granite; light or dark), the possibility remains that males could have altered their visual signaling more or less when courting on granite, or their seismic signal when courting in the dark. More natural signaling substrates (e.g., leaf litter, pine litter, sand, etc.) and/or prior signaling experience could also influence courtship deployment. For example, S. rovneri males are known to adjust their signaling behavior after receiving modality-specific feedback cues from receptive females (Sullivan-Beckers and Hebets 2011). Plasticity of courtship behavior remains an open and exciting research direction and one that will be imperative for a complete understanding of courtship signal evolution.
A majority of past research on Schizocosa wolf spiders has focused upon male ‘ornaments’, such as the enlarged brushes of hairs found on the forelegs of males of many species, likely due to our own perceptual biases. Yet despite the sexual dimorphism observed between female and male Schizocosa and the widespread incorporation of seemingly visual courtship components, the presence of visual signaling does not appear to be of crucial importance in facilitating mating for most Schizocosa species studied to date (Hebets 2005; Taylor et al. 2006; Hebets et al. 2008a; Rundus et al. 2011; Stafstrom and Hebets 2013; present study). Recent work in fact suggests that much visual signaling in Schizocosa may not be a direct target of female mate choice, but instead is important only through its interaction with other traits (Hebets et al. 2011; Stafstrom and Hebets 2013). Wolf spiders have been at the forefront of studies documenting inter-signal interactions between signal components (Hebets 2005; Hebets et al. 2011; Rosenthal and Hebets 2012; Wilgers and Hebets 2012b) and such studies have highlighted the complex nature of female decision making when it comes to mate choice (e.g., Wilgers and Hebets 2012a). We suggest that numerous such interactions remain to be discovered and that holistic approaches to complex signal function will facilitate our understanding of the role of ‘ornamentation’ within Schizocosa.
Complexity and signal evolution
Our comparative analyses across ten Schizocosa species provide evidence that the evolution of courtship complexity in this genus is driven, at least in part, by receiver responses (i.e., by sexual selection). Specifically, we document a positive relationship between the importance of visual signaling and visual signal complexity, suggesting a significant role of female mate choice in the evolution of visual signal form. In contrast, seismic signal complexity does not appear to be influenced by female choice, as we observed no relationship between the importance of seismic signaling and seismic signal complexity. We suggest that seismic signal form is influenced more by the signaling environment than female choice. Finally, across our focal species, the degree to which one modality is elaborated corresponds to the degree to which the second modality is elaborated. This final result of similar complexity scores among signaling modalities hints at the potential for selection to act on complexity per se.
Seismic signal complexity does not appear to be under strong selection from female choice in Schizocosa wolf spiders—seismic signal effect sizes did not correlate with seismic signal complexity scores. Nonetheless, as discussed previously, this modality remains crucial for mating across most Schizocosa species. We hypothesize that the details of seismic signal form are largely shaped by selection for successful signal transmission (see earlier discussion). Direct tests of this hypothesis would require comparative quantifications of component-specific signal transmission across signaling environments in combination with analyses of background noise. Similar studies have been conducted on bird song across habitat types (e.g., Tobias et al. 2010) and borrowing techniques from such studies would be prudent for future Schizocosa research. Quantifying the details of seismic signal divergence (i.e., differences in frequency, rate, etc.) between closely related species may also shed light on evolutionary selection pressures influencing seismic signal form and ultimately, species divergence (e.g., Uy and Safran 2013).
In contrast to seismic signals, visual signal effect sizes were correlated with visual signal complexity, suggesting a prominent role of sexual selection in visual signal evolution. The two conspicuously brush-legged species incorporated into our analysis, S. ocreata and S. crassipes, had high complexity scores and correspondingly high visual effect sizes. Preliminary data on an additional brush-legged species, S. bilineata, finds an identical pattern (Bern 2011). For these brush-legged species, visual signaling is important in female mating decisions and several detailed studies support this assertion (e.g., McClintock and Uetz 1996; Scheffer et al. 1996; Persons and Uetz 2005; Uetz and Norton 2007; Stafstrom and Hebets 2013). Future studies are now necessary to determine the mechanism underlying the increased importance of visual signaling in these species (e.g., pre-existing biases (Endler and Basolo 1998; Basolo 1990), sensory drive (Endler 1992; reviewed in Boughman 2002), female preferences for motor performance (Byers et al. 2010), etc.). Additionally, the inclusion of more species, the use of more robust phylogenetic reconstructions, and more detailed quantifications of complexity are necessary to corroborate our findings.
The complexity scores of seismic and visual signals were highly correlated across our focal Schizocosa species—raising the possibility that selection acts on courtship complexity per se. Our results cannot speak to the source(s) of such selection, but prior researchers have also hypothesized that females select for complexity (Elias et al. 2012). One potential explanation could be an association between courtship complexity and motor performance. A courtship display incorporating numerous components surely requires additional motor control, and evidence is increasing for the hypothesis that female mate choice is based upon motor performance (see Byers et al. 2010). Alternatively, more components across signaling modalities could facilitate successful signal transmission in variable signaling environments (e.g., back-up hypotheses, Candolin 2003; Hebets and Papaj 2005), or could result from selection for increased accuracy of receiver responses (e.g., redundancy, Møller and Pomiankowski 1993; Johnstone 1996). Despite our current inabilities to explain the pattern we observe, our results provide a solid foundation for future studies across additional taxa to address specific hypotheses regarding the evolution of complex courtship displays.
Our phylogenetic reconstruction is largely in agreement with previously hypothesized relationships among North American Schizocosa (Stratton 2005). These relationships highlight a pattern observed in other taxa in which closely related species appear quite divergent in courtship displays (Fig. 1). Schizocosa ocreata and S. rovneri, for example, although not reciprocally monophyletic based upon COI (see also Hebets and Vink 2007), represent the extremes of our calculated range of total courtship complexity. We note that microsatellite data suggests that populations of these two species are genetically distinct (Fowler-Finn 2009), yet a recently discovered population consists of male forms indistinguishable from S. ocreata and S. rovneri (Hebets and Vink 2007). Thus, there exists a single supposedly interbreeding population, presumably sharing a signaling environment, composed of males polymorphic in their degree of courtship complexity. Future studies using this mixed population could be invaluable in connecting signal divergence to speciation. Similar differences among closely related species are observed in S. bilineata and S. crassipalpata, hypothesized sister species (Stratton 2005) that share a microhabitat in certain regions of North America. Characterizations of their courtship signals also show that their modality-specific courtship signal complexity differs dramatically (Bern 2011). Such observations of divergence in courtship signal form between closely related taxa suggest that signal divergence, potentially driven by sexual selection, may facilitate species divergence. Recent work utilizing data from Schizocosa wolf spiders as one of many case studies proposes that divergence in female mating preference is the best predictor of signal divergence (Rodriguez et al. 2013), but the cause(s) underlying divergent preferences remains an open question.
Our complexity analyses are preliminary and may underestimate the true number of signaling components distinguishable by females. Additionally, signal complexity is only important in so far as it is detectable by intended receivers and a complexity metric that incorporates receiver-based sensory physiology would be welcome. We also express concern with using variables that are not heritable traits in a PGLS analysis. However, our concern is not great in reference to our analyses since the Blomberg’s K score, our measure of phylogenetic signal, was comparatively small for all variables involved in significant interactions. These low scores indicate that the relationships may be independent of the phylogeny. Ultimately, while we acknowledge our small sample sizes, the inadequacies of our complexity estimates (e.g., they do not take receiver perceptual abilities into consideration), and issues concerning the applicability of the PGLS analysis, we nonetheless find strong patterns concerning the evolution of signal complexity in Schizocosa wolf spiders. We suspect that future studies will corroborate our findings.
Conclusions
The genus Schizocosa represents rapid diversification in courtship signal complexity among closely related species (with hypothesized divergence since the last glaciations, Stratton 2005). At least 10 of the North American species had been the focus of prior behavioral studies (Stratton and Uetz 1981; Hebets et al. 1996; Hebets and Uetz 1999; Hebets 2003; Hebets 2007; Hebets et al. 2008a; Vaccaro et al. 2010; Rundus et al. 2011) and this study adds significantly to this growing literature by examining the mating frequency of five additional species across variable signaling environments. Our experimental design enabled us to document the dominance of seismic signaling across the genus, and our comparative approach facilitated progress towards answering questions about evolutionary selection pressures driving signal complexity both within and between sensory modalities. Our seismic signal analyses suggest a strong of role of the signaling environment in seismic signal evolution. In contrast, while our complexity analyses cannot separate cause from effect, our results are consistent with female choice influencing the evolution of visual signal complexity. We also found a correlation between seismic and visual signal complexity, suggesting selection for complexity itself. We are confident that improved estimates of signal complexity, continued incorporation of a phylogenetic framework, and data on additional species will not only inform us regarding the evolution of complex communication, but will enable elegant tests of the causes and consequences of signal divergence. We end by encouraging others working in diverse taxonomic groups to utilize approaches similar to those taken herein. Acquiring similar functional and comparative data on complex signaling across taxonomic groups will bring us closer to a unified theory of complexity.
References
Basolo AL (1990) Female preference predates the evolution of the sword in swordtail fish. Science 250(4982):808–810
Beckers OM, Wagner WE (2011) Mate sampling strategy in a field cricket: evidence for a fixed threshold strategy with last chance option. Anim Behav 81(3):519–527. doi:10.1016/j.anbehav.2010.11.022
Bell PD (1980) Transmission of vibrations along plant stems: implications for insect communication. J N Y Entomol Soc 88:210–216
Bern MD (2011) Exploring sources of selection on the multimodal courtship displays of two sister species of wolf spiders: Schizocosa crassipalpata and Schizocosa bilineata. University of Nebraska, Lincoln
Blomberg SP, Garland T, Ives AR (2003) Testing for phylogenetic signal in comparative data: behavioral traits are more labile. Evolution 57(4):717–745. doi:10.1111/j.0014-3820.2003.tb00285.x
Botero CA, Mudge AE, Koltz AM, Hochachka WM, Vehrencamp SL (2008) How reliable are the methods for estimating repertoire size? Ethology 114(12):1227–1238. doi:10.1111/j.1439-0310.2008.01576.x
Boughman JW (2002) How sensory drive can promote speciation. Trends Ecol Evol 17(12):571–577
Brandley MC, Schmitz A, Reeder TW (2005) Partitioned Bayesian analyses, partition choice, and the phylogenetic relationships of scincid lizards. Syst Biol 54:373–390
Byers J, Hebets E, Podos J (2010) Female mate choice based upon male motor performance. Anim Behav 79:771–778
Candolin U (2003) The use of multiple cues in mate choice. Biol Rev 78(4):575–595
Chang J, Song D, Zhou K (2007) Incongruous nuclear and mitochondrial phylogeographic patterns in two sympatric lineages of the wolf spider Pardosa astrigera (Araneae; Lycosidae) from China. Mol Phylogenet Evol 42:104–121
Chen I, Stuart-Fox DM, Hugall AF, Symonds MRE (2012) Sexual selection and the evolution of complex color patterns in dragon lizards. Evolution 66:3605–3614
Cocroft RB, Rodriguez RL (2005) The behavioral ecology of insect vibrational communication. Bioscience 55(4):323–334. doi:10.1641/0006-3568(2005)055[0323:tbeoiv]2.0.co;2
Darwin C (1871) The descent of man, and selection in relation to sex. J. Murray, London
Dondale CD, Redner JH (1978) Revision of nearctic wolf spider genus Schizocosa (Arachneida Lycosidae). Can Entomol 110(2):143–181
Douglas SB, Mennill DJ (2010) A review of acoustic playback techniques for studying avian vocal duets. J Field Ornithol 81(2):115–129. doi:10.1111/j.1557-9263.2010.00268.x
Elias DO, Mason AC, Hoy RR (2004) The effect of substrate on the efficacy of seismic courtship signal transmission in the jumping spider Habronattus dossenus (Araneae: Salticidae). J Exp Biol 207(23):4105–4110
Elias DO, Land BR, Mason AC, Hoy RR (2006a) Measuring and quantifying dynamic visual signals in jumping spiders. Comp Physiol A Neuroethol Sens Neural Behav Physiol 192(8):785–797. doi:10.1007/s00359-006-0116-7
Elias DO, Lee N, Hebets EA, Mason AC (2006b) Seismic signal production in a wolf spider: parallel versus serial multi-component signals. J Exp Biol 209(6):1074–1084. doi:10.1242/jeb.02104
Elias DO, Mason AC, Hebets EA (2010) A signal-substrate match in the substrate-borne component of a multimodal courtship display. Curr Zool 56(3):370–378
Elias DO, Maddison WP, Peckmezian C, Girard MB, Mason AC (2012) Orchestrating the score: complex multimodal courtship in the Habronattus coecatus group of Habronattus jumping spiders (Araneae: Slaticidae). Biol J Linnean Soc 105:522–547
Endler JA (1992) Signals, signal conditions, and the direction of evolution. Am Nat 139:S125–S153
Endler JA, Basolo AL (1998) Sensory ecology, receiver biases and sexual selection. Trends Ecol Evol 13(10):415–420
Felsenstein J (1981) Evolutionary trees from DNA sequences: a miximum likelihood approach. J Mol Evol 17:368–376
Fowler-Finn KD (2009) Exploring the maintenance of and selection on two distinct male morphs in a Schizocosa wolf spider. University of Nebraska, Lincoln
Framenau VW, Hebets EA (2007) A review of leg ornamentation in male wolf spiders, with the description of a new species from Australia, Artoria schizocoides (Araneae, Lycosidae). J Arachnol 35(1):89–101
Gerhardt HC (1982) Sound pattern-recognition in some North-American treefrogs (Anura, Hylidae)—implications for mate choice. Am Zool 22(3):581–595
Gerhardt HC, Huber F (2002) Acoustic communication in insects and anurans. University of Chicago Press, Chicago
Gibson JS, Uetz GW (2008) Seismic communication and mate choice in wolf spiders: components of male seismic signals and mating success. Anim Behav 75:1253–1262
Gibson JS, Uetz GW (2012) Effect of rearing environment and food availability on seismic signalling in male wolf spiders (Araneae: Lycosidae). Anim Behav 84(1):85–92. doi:10.1016/j.anbehav.2012.04.010
Gordon SD, Uetz GW (2011) Multimodal communication of wolf spiders on different substrates: evidence for behavioural plasticity. Anim Behav 81:367–375
Grant PR, Grant BR (1997) Genetics and the origin of bird species. Proc Natl Acad Sci U S A 94(15):7768–7775. doi:10.1073/pnas.94.15.7768
Hasegawa M, Kishino K, Yano T (1985) Dating the human-ape splitting by a molecular clock of mitochondrial DNA. J Mol Evol 22:160–174
Hebets E (2003) Subadult experience influences adult mate choice in an arthropod: exposed female wolf spiders prefer males of a familiar phenotype. Proc Natl Acad Sci U S A 100(23):13390–13395
Hebets EA (2005) Attention-altering signal interactions in the multimodal courtship display of the wolf spider Schizocosa uetzi. Behav Ecol 16(1):75–82. doi:10.1093/beheco/arh133
Hebets EA (2007) Subadult female experience does not influence species recognition in the wolf spider Schizocosa uetzi Stratton 1997. J Arachnol 35(1):1–10
Hebets EA (2008) Seismic signal dominance in the multimodal courtship display of the wolf spider Schizocosa stridulans Stratton 1991. Behav Ecol 19(6):1250–1257
Hebets EA (2011) Current status and future directions of research in complex signaling. Current Zoology 57:i–v
Hebets EA, Papaj DR (2005) Complex signal function: developing a framework of testable hypotheses. Behav Ecol Sociobiol 57(3):197–214
Hebets EA, Uetz GW (1999) Female responses to isolated signals from multimodal male courtship displays in the wolf spider genus Schizocosa (Araneae: Lycosidae). Anim Behav 57:865–872
Hebets EA, Uetz GW (2000) Leg ornamentation and the efficacy of courtship display in four species of wolf spider (Araneae: Lycosidae). Behav Ecol Sociobiol 47(4):280–286
Hebets EA, Vink CJ (2007) Experience leads to preference: experienced females prefer brush-legged males in a population of syntopic wolf spiders. Behav Ecol 18:1010–1020
Hebets EA, Stratton GE, Miller GL (1996) Habitat and courtship behavior of the wolf spider Schizocosa retrorsa (Banks) (Araneae, Lycosidae). J Arachnol 24(2):141–147
Hebets EA, Elias DO, Mason AC, Miller GL, Stratton GE (2008a) Substrate-dependent signalling success in the wolf spider, Schizocosa retrorsa. Anim Behav 75:605–615
Hebets EA, Wesson J, Shamble PS (2008b) Diet influences mate choice selectivity in adult female wolf spiders. Anim Behav 76:355–363
Hebets EA, Stafstrom JA, Rodriguez RL, Wilgers DJ (2011) Enigmatic ornamentation eases male reliance on courtship performance for mating success. Anim Behav 81:963–972
Johnstone RA (1996) Multiple displays in animal communication: ‘backup signals’ and ‘multiple messages’. Philos Trans R Soc Lond Ser B-Biol Sci 351(1337):329–338
Kembel S, Cowan P, Helmus M, Cornwell W, Morlon H, Ackerly D, Blomberg S, Webb C (2010) Picante: R tools for integrating phylogenies and ecology. Bioinformatics 26:1463–1464
Kraaijeveld K, Kraaijeveld-Smit FJL, Maan ME (2011) Sexual selection and speciation: the comparative evidence revisited. Biol Rev 86(2):367–377. doi:10.1111/j.1469-185X.2010.00150.x
McClintock WJ, Uetz GW (1996) Female choice and pre-existing bias: visual cues during courtship in two Schizocosa wolf spiders (Araneae: Lycosidae). Anim Behav 52:167–181
Miller GL, Stratton GE, Miller PR, Hebets E (1998) Geographical variation in male courtship behaviour and sexual isolation in wolf spiders of the genus Schizocosa. Anim Behav 56:937–951
Møller AP, Pomiankowski A (1993) Why have birds got multiple sexual ornaments. Behav Ecol Sociobiol 32(3):167–176
Nakagawa S, Cuthill IC (2007) Effect size, confidence interval and statistical significance: a practical guide for biologists. Biol Rev 82(4):591–605. doi:10.1111/j.1469-185X.2007.00027.x
Nylander JAA (2008) MrModeltest 2.3. In. Department of Systematic Zoology, Uppsala University, Uppsala
Page R (1996) TREEVIEW: an application to display phylogenetic trees on personal computers. Comp Appl Biol Sci 12:357–358
Paradis E, Claude J, Strimmer K (2004) APE: analyses of phylogenetics and evolution in R language. Bioinformatics 20:289–290
Partan S, Marler P (1999) Behavior—communication goes multimodal. Science 283(5406):1272–1273
Persons MH, Uetz GW (2005) Sexual cannibalism and mate choice decisions in wolf spiders: influence of male size and secondary sexual characters. Anim Behav 69:83–94
Posada D, Buckley TR (2004) Model selection and model averaging in phylogenetics: advantages of Akaike information criterion and Bayesian approaches over likelihood ratio tests. Syst Biol 53:793–808
Rambaut A, Drummond AJ (2009) Tracerv1.5. In, 1.5 edn
Ritchie MG (2007) Sexual selection and speciation. Annu Rev Ecol Evol Syst 38:79–102
Rodriguez RL, Boughman JW, Gray DA, Hebets EA, Hobel G, Symes LB (2013) Diversification under sexual selection: the relative importance of preference divergence versus preference strength (in review)
Rodriguez RL, Ramaswamy K, Cocroft RB (2006) Evidence that female preferences have shaped male signal evolution in a clade of specialized plant-feeding insects. P Roy Soc B-Biol Sci 273(1601):2585–2593. doi:10.1098/rspb.2006.3635
Ronquist F, Huelsenbeck JP (2003) MrBayes3: Bayesian phylogenetic inference under mixed models. Bioinformatics 19:1572–1574
Rosenthal M, Hebets E (2012) Resource heterogeneity interacts with courtship rate to influence mating success in the wolf spier S. floridana. Anim Behav 84:1341–1346
Rowe C (1999) Receiver psychology and the evolution of multicomponent signals. Anim Behav 58:921–931
Rundus AS, Santer RD, Hebets EA (2010) Multimodal courtship efficacy of Schizocosa retrorsa wolf spiders: implications of an additional signal modality. Behav Ecol 21(4):701–707. doi:10.1093/beheco/arq042
Rundus AS, Sullivan-Beckers L, Wilgers DJ, Hebets EA (2011) Females are choosier in the dark: environment-dependent reliance on courtship components and its impact on fitness. Evolution 65(1):268–282. doi:10.1111/j.1558-5646.2010.01125.x
Scheffer SJ, Uetz GW, Stratton GE (1996) Sexual selection, male morphology, and the efficacy of courtship signalling in two wolf spiders (Araneae: Lycosidae). Behav Ecol Sociobiol 38(1):17–23
Servedio MR (2012) The relationship between sexual selection and speciation. Curr Zool 58(3):413–415
Shamble PS, Wilgers DJ, Swoboda KA, Hebets EA (2009) Courtship effort is a better predictor of mating success than ornamentation for male wolf spiders. Behav Ecol 20(6):1242–1251. doi:10.1093/beheco/arp116
Shaw KL, Parsons YM (2002) Divergence of mate recognition behavior and its consequences for genetic architectures of speciation. Am Nat 159:S61–S75. doi:10.1086/338373
Stafstrom JA, Hebets EA (2013) Female mate choice for multimodal courtship and the importance of the signaling background for selection on male ornamentation. Current Zoology 59(2):200–209
Stratton GE (1991) A new species of wolf spider, Schizocosa stridulans (Araneae, Lycosidae). J Arachnol 19(1):29–39
Stratton GE (1997) A new species of Schizocosa from the southeastern USA (Araneae, Lycosidae). J Arachnol 25(1):84–92
Stratton GE (2005) Evolution of ornamentation and courtship behavior in Schizocosa: insights from a phylogeny based on morphology (Araneae, Lycosidae). J Arachnol 33:347–376
Stratton GE, Uetz GW (1981) Acoustic communication and reproductive isolation in 2 species of wolf spiders. Science 214(4520):575–577
Stratton GE, Uetz GW (1983) Communication via substratum-coupled stridulation and reproductive isolation in wolf spiders (Araneae, Lycosidae). Anim Behav 31(FEB):164–172
Sullivan-Beckers L, Hebets EA (2011) Modality-specific experience with female feedback increases the efficacy of courtship signalling in male wolf spiders. Anim Behav 82(5):1051–1057. doi:10.1016/j.anbehav.2011.07.040
Swofford DL (2002) PAUP*: phylogenetic analysis using parsimony (*and other methods). In, 4.0b10 edn. Sinauer Associates, Sunderland
Taylor PW, Roberts JA, Uetz GW (2005) Flexibility in the multi-modal courtship of a wolf spider, Schizocosa ocreata. J Ethol 23(1):71–75
Taylor PW, Roberts JA, Uetz GW (2006) Mating in the absence of visual cues by Schizocosa ocreata (Hentz 1844) wolf spiders (Araneae, Lycosidae). J Arachnol 34(3):501–505
Tietjen WJ (1979) Is the sex pheromone of Lycosa rabida (Araneae, Lycosidae) deposited on a substratum? J Arachnol 6:207–212
Tobias JA, Aben J, Brumfield RT, Derryberry EP, Halfwerk W, Slabbekoorn H, Seddon N (2010) Song divergence by sensory drive in Amazonian birds. Evolution 64(10):2820–2839. doi:10.1111/j.1558-5646.2010.01067.x
Uetz GW, Dondale CD (1979) New wolf spider in the genus Schizocosa (Araneae, Lycosidae) from Illinois. J Arachnol 7(1):86–88
Uetz GW, Norton S (2007) Preference for male traits in female wolf spiders varies with the choice of available males, female age, and reproductive state. Behav Ecol Sociobiol 61:631–641
Uetz GW, Roberts JA (2002) Multisensory cues and multimodal communication in spiders: insights from video/audio playback studies. Brain Behav Evol 59(4):222–230
Uetz GW, Papke R, Kilinc B (2002) Influence of feeding regime on body size, body condition and a male secondary sexual character in Schizocosa ocreata wolf spiders (Araneae, Lycosidae): condition-dependence in a visual signaling trait. J Arachnol 30(3):461–469
Uetz GW, Roberts JA, Taylor PW (2009) Multimodal communication and mate choice in wolf spiders: female response to multimodal versus unimodal signals. Anim Behav 78(2):299–305. doi:10.1016/j.anbehav.2009.04.023
Uy A, Safran RD (2013) Variation in the temporal and spatial use of signals and its implications for multimodal communication. Behav Ecol Sociobiol. doi:10.1007/s00265-013-1492-y
Vaccaro R, Uetz GW, Roberts JA (2010) Courtship and mating behavior of the wolf spider Schizocosa bilineata (Araneae: Lycosidae). J Arachnol 38:452–459
Vink CJ, Paterson AM (2003) Combined molecular and morphological phylogenetic analyses of the New Zealand wolf spider genus Anoteropsis (Araneae: Lycosidae). Mol Phylogenet Evol 28:576–587
Vink CJ, Thomas SM, Paquin P, Hayashi CY, Hedin MC (2005) The effects of preservatives and temperatures on arachnid DNA. Invertebrate Systematics 19:99–104
Wilgers DJ, Hebets EA (2011) Complex courtship displays facilitate male reproductive success and plasticity in signaling across variable environments. Current Zoology 57(2):175–186
Wilgers DJ, Hebets EA (2012a) Age-related female mating decisions are condition dependent in wolf spiders. Behav Ecol Sociobiol 66(1):29–38. doi:10.1007/s00265-011-1248-5
Wilgers DJ, Hebets EA (2012b) Seismic signaling is crucial for female mate choice in a multimodal signaling wolf spider. Ethology 118(4):387–397. doi:10.1111/j.1439-0310.2012.02023.x
Acknowledgments
We would like to extend our deepest gratitude to Gail Stratton, Pat Miller, Amy Nicholas, David Reed, Dustin Wilgers, and the Unitarian Universalist Congregation of Oxford youth group for help with spider collecting. Help in spider care and maintenance from Jennifer Wesson and Alexander Vaughn was indispensable and Damian Elias kindly offered his opinion on our seismic complexity scores. Jeremy Gibson generously agreed to be the “blind” scorer of the seismic signals for all of the species included in this analysis. Stacey Smith provided much needed advice and guidance with our phylogenetic comparative methods. James Higham and two anonymous reviewers provided invaluable feedback on earlier drafts of this manuscript. This work was the culmination of useful discussions over many years with Gail Stratton, Pat Miller, and Damian Elias, as well as present and past members of the Hebets, Basolo, Shizuka and Wagner Labs. The manuscript also benefited tremendously from discussions of signal complexity with Matthew Symonds and Mark Elgar, as well as discussions of ‘Sexual Selection and Speciation’ during participation (EAH) in a National Evolutionary Synthesis Center (NESCent) working group on the topic funded by a National Science Foundation (NSF) grant (EF-0905606 to R. Safran and A. Uy). This research was funded in part by a National Science Foundation (NSF) grant (IOS—0934990 to EAH). Finally, we would like to thank the editors of BES and James Higham for the opportunity to participate in this special issue.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by J. P. Higham
This manuscript is part of the special issue Multimodal Communication—Guest Editors: James P. Higham and Eileen A. Hebets
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(PPT 357 kb)
ESM 2
(DOCX 16 kb)
Fig. 1
Bayesian consensus tree based on cytochrome c oxidase subunit 1 (COI) sequence data. Values on branches are posterior probabilities. Branch lengths are proportional to the expected number of substitutions per site (see scale bar). (JPEG 61 kb)
Rights and permissions
About this article
Cite this article
Hebets, E.A., Vink, C.J., Sullivan-Beckers, L. et al. The dominance of seismic signaling and selection for signal complexity in Schizocosa multimodal courtship displays. Behav Ecol Sociobiol 67, 1483–1498 (2013). https://doi.org/10.1007/s00265-013-1519-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00265-013-1519-4