Abstract
A conflict over male production arises in social insects where workers are able to lay unfertilized male eggs. This happens because each female (queen or worker) is most closely related to her own sons and is thus predicted to reproduce. The conflict is modulated by worker policing where workers prevent each other from reproducing by aggression or egg cannibalism. In this study, we show that in the ant Formica fusca, worker policing occurs by egg cannibalism rather than by overt aggression among workers. Furthermore, we show that, contrary to bees, wasps and other ant species, egg discrimination in F. fusca is not based only on a universal queen signature chemical and that nest mate recognition of eggs occurs.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cooperative societies are characterized by evolutionary conflicts between individual fitness interests and collective group benefits (Maynard-Smith and Szathmary 1995). In extant societies, a balance between group benefits and individual interests prevails, whereas societies where selfish interests have overruled group benefits have dissolved under evolutionary time (Keller 1999). Kin selection theory gives a powerful framework for predicting both the evolution of extreme cooperation as well as the emergence of conflicts between members of the same society (Hamilton 1964; Trivers and Hare 1976; Frank 1998). Recent theoretical advances have shown that the expression and resolution of such conflicts are dictated by the fitness return functions for individual selfishness on the one hand and collective benefits on the other (Frank 2003; Foster 2004).
In social insects, workers largely forgo personal reproduction to the benefit of the group (Bourke 1988; Bourke and Franks 1995; Crozier and Pamilo 1996). Nevertheless, a wealth of conflicts between colony members occur, one of which is the conflict over male parentage in species where workers, although unable to mate, are able to lay haploid male eggs (Bourke and Franks 1995; Sundström and Boomsma 2001; Ratnieks et al. 2006). However, mutual worker policing, i.e. selective egg cannibalism or directed aggression towards fertile workers should evolve when colony-level costs of worker reproduction are high, or workers are more related to sons of the queen than to sons of the other workers (Woyciechowski and Lomnicki 1987; Ratnieks 1988; Frank 1995, 1996, 2003; Wenseleers et al. 2004). Ultimately, efficient policing and low individual fitness returns may favour self policing (Ratnieks 1988; Wenseleers et al. 2004). Conversely, in species where colony orphaning is common, enhanced fertility may be a bonus. This may favour reduced policing in orphaned, compared to queen right colonies, all else being equal (Wenseleers et al. 2004).
In queen right colonies, worker policing by egg removal has been demonstrated in bees, wasps and ants (reviewed in Ratnieks and Wenseleers 2005), but in orphaned colonies, policing of worker laid eggs is relaxed (Miller and Ratnieks 2001; Iwanishi et al. 2003). Policing by aggression towards fertile workers has also been observed in bees, wasps and ants, both under queen right and orphaned conditions (Landolt et al. 1977; Visscher and Dukas 1995; Liebig et al. 1999; Monnin and Ratnieks 2001). However, nest mate recognition by workers may deteriorate after orphaning (Vienne et al. 1998; Vander Meer and Alonso 2002; Boulay et al. 2003). These studies, together, indicate that the presence of a queen affects the behaviour of workers towards both nest mates and non-nest mates, and that studies of recognition behaviour must pay attention on the social context.
In the bees, wasps and ants studied to date, egg policing is apparently mediated by a chemical signature applied to the eggs by the queen (Ratnieks 1995; Martin et al. 2002; Endler et al. 2004), whereas policing by aggression is mediated by changes in the cuticular hydrocarbon profile of fertile workers (Monnin and Peeters 1999; Liebig et al. 1999, 2000). Interestingly, despite considerable hostility towards alien conspecific adults, queen-laid eggs are accepted across colony, and even subspecies boundaries in bees, wasps and the two ant species studied to date (Ratnieks and Visscher 1989; Foster and Ratnieks 2001; Pirk et al. 2003; Martin et al. 2002; Endler et al. 2004; D’Ettorre et al. 2004). This suggests that the queen signature is similar across colonies, and nest mate recognition does not apply to eggs. To date, no studies have demonstrated differential treatment of con-colonial and hetero-colonial queen-laid eggs, but the species studied only represent a small sample of social insect species. This begs the question whether the presence of uniform, queen-derived chemical markers that allows indiscriminate within-species acceptance of queen-laid eggs is the rule among social insects.
We used egg and worker transfers to record nest mate recognition and worker policing in queen right and artificially orphaned colonies of the ant F. fusca. In this species, worker reproduction is suppressed in the presence of a queen (Helanterä and Sundström 2005), suggesting worker policing. Specifically, we tested whether workers act aggressively towards fertile workers, as predicted, if worker policing is mediated by worker–worker aggression. We also tested whether workers selectively destroy eggs laid by nest mate and non-nest mate queens and workers. We expect nest mate worker-laid eggs to be destroyed if worker policing is mediated by egg eating. Furthermore, if the mechanism of egg recognition involves nest mate recognition, we expect eggs laid by non-nest mate queens and workers to be destroyed. Because sons of workers are reared in orphaned colonies of the species (Helanterä and Sundström 2005), we also predict weaker discrimination in orphaned colonies, both with respect to the parentage of eggs and the colony of origin.
Materials and methods
Study colonies
Our study population is located in south-western Finland on the Hanko Peninsula. Entire colonies were excavated in 2001 and 2002 (“Experiment I”, ten colonies each year), and 2003 and 2005 (“Experiment II”, seven and nine colonies, respectively). Details of collection procedures, population and colony kin structure and worker reproduction are given in Hannonen et al. (2004), Helanterä (2004) and Helanterä and Sundström (2005). Excavated colonies were brought into the laboratory, and queens and workers were separated before setting up the laboratory nests. To avoid pseudoreplication, each field colony was used for only one replicate per treatment even if multiple queens were found. The number of queens in colonies of F. fusca vary from one to about 20 (Hannonen et al. 2004), but as previous data showed no effects of queen number and colony kin structure on worker reproduction, we included both monogynous and polygynous colonies in our study.
Experiment I: interactions among workers
The aim of this experiment was to study whether workers behave differently towards nest mate versus non-nest mate workers on the one hand and fertile versus infertile nest mate workers on the other. The experimental colonies were constructed by splitting each field colony into two parts. One part comprised the observation nests, which were established in small plastic jars (about 10 cm diameter) with a plaster bottom. The observation nests contained a queen and about 70 workers, all marked with a dot from a paint marker. The remaining part formed the orphaned source nests with unmarked workers. Orphaning induces ovary development and subsequent egg laying within a week in workers of F. fusca (personal observations). The source nests were established in larger nest boxes as described in Helanterä and Sundström (2005) to mimic natural conditions. Observations began once egg laying had started in both the source and the observation fragment to ensure the presence of fertile workers in the orphaned source fragments.
The experiments were carried out in two treatments: In “nest mate”, an unmarked focal worker was transferred from a source nest fragment that originated from the same field colony as the observation nest; in “non-nest mate”, the unmarked focal worker originated from a different field colony. During transfer, we took great care not to agitate the focal worker or the resident workers in the recipient nest. Nests were observed for 20 min, and aggressive actions [biting of legs or antennae and spraying acid (Le Moli and Parmigiani 1981; Le Moli and Mori 1986)] towards the focal worker were recorded, along with antennations received (antenna–antenna contacts and antennal contacts with abdomen). After each observation period, the focal worker was removed, dissected, and its ovary development scored from 0–3 (empty ovaries = 0, ovaries with undifferentiated ova or ova less than 20% of the size of an egg = 1, ovaries with ova 20–90% the size of an egg = 2, ovaries with ova >90% of the size of an egg = 3). Observations were replicated on ten colonies per treatment, each encompassing ten (nest mate treatment) or five (non-nest mate treatment) workers.
Aggression was quantified as the proportion of aggressive interactions of all interactions recorded. The significance of the main effects treatment, ovary status, observation nest (nested within treatments because different nests were used in the two treatments) and time since the start of the experiment, on aggression were tested by permutation analyses of a general linear model of the data. This was necessary because unequal variances and sample sizes prevented the use of F-tests of significance. We randomly permuted the aggression scores among observation nests and ovary classes 10,000 times, constructed a general linear model for each permuted data set and calculated the sum of squares for each factor and their interactions. The p values for the effects of the factors were then obtained from the proportion of permuted data sets that gave a sum of squares greater than or equal to that of the original data.
Experiment II: actions towards eggs
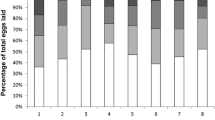
The aim of this experiment was to test whether workers discriminate against (1) eggs laid by their nest mate workers, (2) eggs laid by non-nest mate queens and/or (3) behave differently with respect to 1 and 2 in queen right and orphaned nests. Also, for this experiment, colonies were split into queen right (single-queen) and orphaned fragments. Both fragments were placed in observation nests as above, but with about 100 unmarked workers. The experiments started upon onset of egg laying in both fragments of a pair. Eggs were transferred individually from source nests into the observation nests, and each egg was observed individually. The eggs were carefully transferred with forceps to a 5 × 5-mm piece of paper then checked under a microscope to make sure they were intact and fertile (as opposed to trophic, Gösswald 1989). The paper was then transferred into the observation nest without agitating the workers. The fate of each egg (i.e. destroyed or not) was monitored for 20 min or until the egg was destroyed or placed on the egg pile. If the observer lost track of the egg during observation, the procedure was repeated with a new egg. Observations were carried out on 16 queen right and 14 orphaned discriminator colonies, with eggs from four different sources (treatments; Fig. 1). Each observation nest was used in more than one treatment, and the order of assays was randomized for each colony.
Types of observation nests, egg sources and predictions on policing and nest mate recognition in “Experiment II”. In the control treatments (queen right: treatment 1; orphaned: treatment 2), eggs were taken from and re-introduced into the observation nest box
The effect of the type of discriminator colony (orphaned or queen right) and treatment (egg source) on the counts of eggs destroyed and accepted per colony were fitted into a generalized linear model with binomial error structure. We used the counts of the two types of eggs (destroyed/accepted) per nest and treatment as a binomially distributed response variable. This allowed us to maintain the nest (instead of the egg) as the unit of replication, although several eggs were used per colony and treatment (mean = 2.9 eggs, SD = 1.2). The significance of the pairwise differences between treatments was assessed by first generating a random distribution of the data by permuting samples randomly 10,000 times within each pair and then obtaining a p value for the pairwise difference from the proportion of permuted data sets that gave a difference larger than or equal to that of the original data. The effect of time since the start of the experiment was analysed in a separate model, with time and treatment as explanatory variables and egg fate (destroyed/accepted) as a binomially distributed response variable. In this analysis, each individual egg had to be used as a replicate because we were interested in the effects of time on egg acceptance within nests and treatments over the time interval of the study.
Our hypotheses a priori define the comparisons that are relevant for testing the predictions and, therefore, we only carried out the comparisons indicated by our hypotheses. Thus, to test whether worker policing by egg eating occurs in queen right colonies, we compared the fate of eggs laid by nest mate queens versus workers (treatments 1 and 2 in queen right colonies). The reciprocal comparison in orphaned colonies tests whether orphaned colonies start discriminating against eggs laid by a nest mate queen in favour of worker-laid eggs. To test whether both queen- and worker-laid eggs are subject to nest mate discrimination, we compared the fate of control eggs (treatment 1 in queen right, treatment 2 in orphaned) to those laid by alien queens and workers (treatments 3 and 4 in both queen right and orphaned colonies). All statistical analyses were carried out in S-PLUS 6.1©.
Results
On average, 37% (range 0–60%) of the transferred workers in the nest mate treatment and 28% (range 0–50%) in the non-nest mate treatment had developed ovaries. The fraction of workers with developed ovaries did not differ significantly between treatments (Mann–Whitney U = 61.5, n = 20, p = 0.38). As predicted under nest mate recognition, aggression was more frequent when workers were presented with non-nest mates (mean proportion of aggressive interactions in nest mate, 0.006; SD = 0.01 and in non-nest mate, 0.1; SD = 0.07; permutation, p < 0.001). Negligible aggression towards nest mates (Fig. 2) indicates that our procedures did not cause any notable aggression. None of the other tested factors (ovary status and colony) or their interactions significantly affected aggression within either treatment (Fig. 2, all permutation p values > 0.16). This suggests that aggression towards fertile nest mate workers plays a negligible role in worker policing, and that the nests did not differ in their aggressive behaviour. The aggression level was more variable in the non-nest mate treatment than in the nest mate treatment (non-nest mate: SD = 0.035, CV = 93.5; nest mate: SD = 0.0052, CV = 35.8; test for equality of variances F = 45.2, p < 0.001).
Proportions of aggressive interactions in “Experiment I”. The scales differ for the treatments. The bars indicate means and 95%CIs across individuals. The numbers above bars indicate numbers of individuals in the ovary class
None of the control eggs (treatment 1 in queen right and treatment 2 in orphaned) were destroyed, which shows that handling of eggs did not induce discrimination. As predicted, queen right nests were more discriminating than orphaned ones (Fig. 3; GLZ-model, deviance = 6.1, residual deviance = 45.5, df = 1,109, chi-test p < 0.001), and egg fate varied according to egg source treatments (Fig. 3; GLZ-model, deviance = 19.8, residual deviance = 51.7, df = 1,110, chi-test p < 0.001). Furthermore, depending on their source, eggs were treated differently in queen right and orphaned colonies (Fig. 3; interaction between treatment and queen presence in the GLZ-model, deviance = 6.1, residual deviance = 39.4, df = 1,106, chi-test p < 0.001). Time since the start of the experiments had no effect on policing (GLZ-model using each egg as a replicate, deviance = 1.2, residual deviance = 358.4, df = 1,345, chi-test p = 0.266).
Percentages of eggs policed in “Experiment II”. The bars indicate means and 95%CIs across nests
In queen right colonies, eggs from all experimental treatments were rejected at a higher rate than in the controls (Fig. 3a). In particular, eggs laid by nest mate workers were rejected significantly more often than eggs laid by a nest mate queen (pairwise comparison of treatments 1 and 2 in queen right colonies, permutation test p = 0.0009). Notably, also eggs laid by non-nest mate queens were rejected at a higher rate than those laid by a nest mate queen (pairwise comparison of treatments 1 and 3 in queen right colonies, p < 0.001), as were eggs laid by non-nest mate workers (pairwise comparison of treatments 1 and 4 in queen right colonies, p < 0.001). All the pairwise comparisons are significant after the significance level of α = 0.05 is Bonferroni adjusted for three comparisons to α i = 0.0167.
In orphaned colonies, eggs laid by non-nest mate queens and non-nest mate workers were also rejected at a higher rate than in the corresponding control (Fig. 3b; pairwise comparison of treatments 2 and 3 in orphaned colonies p = 0.0011, and treatments 2 and 4 in orphaned colonies p = 0.016). Interestingly, some eggs laid by a nest mate queen were also rejected, but not significantly more often than in the control (comparison of treatments 1 and 2 in orphaned colonies p = 0.12). The two first comparisons are significant after the significance level of α = 0.05 is Bonferroni adjusted for three comparisons to α i = 0.0167.
Discussion
Our results show that in F. fusca, control over male parentage is mediated by selective removal of worker-laid eggs rather than by aggressive behaviour towards fertile individuals. Importantly, our results stand in contrast to previous studies on other species in that queen-laid eggs apparently are not universally accepted in this species. Thus, egg recognition cannot be based exclusively on a universal egg-marking pheromone produced by the queen, but also involves a nest mate recognition component. Interestingly, both worker policing and nest mate recognition of eggs decreased in orphaned colonies, suggesting that the presence of a queen influences the expression of several discriminatory behaviours.
Discriminating against eggs from a different nest box (regardless of colony status) is unlikely to be the cause for our results. First, discrimination did not increase over the 3-week study period, as would be expected if recognition cues diverge after separation. Second, separation did not induce aggression against nest mate workers, even if the orphaned workers were not housed in similar plaster boxes as the discriminators. Third, earlier results show that eggs laid by nest mate queens are not policed in queen right colonies despite nest box separation for up to 3.5 weeks (Helanterä and Sundström 2005). Fourth, studies of cuticular hydrocarbons and aggression in other ants suggest that 3 weeks is insufficient to induce intolerance between separated nest mates (Liu et al. 2001; Boulay et al. 2001; Lenoir et al. 2001).
Aggression does not seem to play a role in worker policing in F. fusca. This may be due to high costs of aggression relative to the costs of having fertile workers in the nest. As the proportion of fertile workers can be as high as 60% (see also Helanterä 2004) in queen right colonies, colony-level costs of aggression may be prohibitive. However, if the costs of worker reproduction mainly arise through decreased investment in foraging and colony defence, the cost of having fertile workers may be artificially low in our laboratory colonies, as the experimental nests were fed ad libitum and natural enemies were absent. Alternatively, there may be a high premium on having fertile workers in case of colony orphaning, as in this case, both the workers and the deceased queen(s) benefit from male production by workers (Ratnieks 1988; Bourke 1988; Franks et al. 1990). Orphaning is a likely risk for F. fusca, as colonies frequently contain only one or few queens, and it is an ecologically submissive species, which faces the risk of attacks by dominant Formica species (Savolainen 1990).
Lack of information on ovary development is unlikely to cause lack of discrimination, as workers were able to discriminate between worker-laid and queen-laid eggs and are able to respond to differences in queen fertility based on chemical cues (Hannonen et al. 2002). However, ovary development associated with laying trophic eggs (Gösswald 1989) may act to scramble cues related to ovary development. Ambiguous cues may prevent aggressive intervention among workers, owing to high costs associated with undue damage during fights. Nevertheless, genetic data show that workers of F. fusca do lay fertile (as opposed to trophic) eggs in the presence of the queen, and these eggs can represent a considerable fraction of the eggs (on average 12% of eggs of both sexes, Helanterä and Sundström 2005).
Although policing occurred, some eggs from both nest mate workers and non-nest mates were added to egg piles. This is consistent with earlier data from F. fusca where worker-laid eggs were found in egg piles of over 50% of queen right colonies. Nevertheless, worker-derived males are very rarely reared (Helanterä and Sundström 2005), which indicates that the process of elimination occurs during a longer period than the one applied here. Our observations confirm that the lack of worker-derived pupae observed earlier (Helanterä and Sundström 2005) is not exclusively due to selective removal of male larvae.
Our results demonstrate that workers of F. fusca are capable of discriminating between eggs of different origin, both within and between colonies. Whereas eggs laid by queens are accepted across colony boundaries in the species studied to date, presumably based on a universally acceptable queen marker chemical, (Ratnieks and Visscher 1989; Ratnieks 1995; Pirk et al. 2003; Martin et al. 2002; Endler et al. 2004; D’Ettorre et al. 2004), our results strongly suggest a greater recognition precision. We have two hypothetical evolutionary scenarios to suggest why workers of F. fusca discriminate against eggs laid by non-nest mate queens. First, queens of the F. rufa group often usurp F. fusca colonies when founding colonies by temporary social parasitism (Buschinger 1986; Collingwood 1979; Czechowski et al. 2002). Given the highly sophisticated examples of chemical mimicry in parasite queens (e.g. D’Ettorre et al. 2002; Johnson et al. 2001), parasite queens may mimic the queen marker of their host. In this case, indiscriminate acceptance of eggs carrying a queen-like chemical signature makes host colonies potentially vulnerable to exploitation by parasites. Thus, we suggest that ant species targeted by social parasites may have a recognition system that also allows discrimination against eggs not laid by the nest mate queens. Furthermore, also, intruding egg laying, non-nest mate workers may generate selection for nest mate recognition of eggs. Such workers have been found in bumblebees and honeybees (Lopez-Vaamonde et al. 2004; Härtel et al. 2006), but the frequency of non-nest mate workers accepted into colonies and their ovary development need to be studied before this probability can be assessed in Formica ants. Second, in contrast to the species in which egg recognition has been studied to date, F. fusca has multi-queen colonies. Thus, workers of F. fusca do have an incentive to discriminate among eggs laid by different nest mate queens. The relatedness between workers and different queens may vary considerably (Hannonen and Sundström 2003), so there are potential inclusive fitness benefits from discrimination. Although nothing is currently known about chemical variation in eggs in this species, workers are able to discriminate among offspring of different queens (Hannonen and Sundström 2003), and nest mate queens vary in their cuticular hydrocarbon profiles (Hannonen et al. 2002). Such discrimination in favour of closely related nest mate eggs would, as a side effect, lead to discrimination against non-nest mate eggs. Furthermore, an arms race arising from the pursuit of selfish interests of colony members may select for more precise recognition (Sundström and Boomsma 2001; but see Boomsma et al. 2003).
In conclusion, our results show that queen-specific chemical signatures on eggs are not universal across colonies among social insects, but that eggs are discriminated against also according to their nest of origin. The next step is to investigate the chemical basis for this discrimination, and whether the selective pressures behind this accurate discrimination are connected to conflicts within colonies, i.e. nepotistic discrimination of eggs, or to social parasitism, i.e. defence against eggs of parasitic queens. In general, our results show that studying the mechanisms behind conflicts, not only outcomes, reveals the potential diversity of selection pressures that can modify conflict behaviour. When conflicts are studied in a diverse range of species, the better we will be able to appreciate the role of natural history of a species in conflict resolution (Beekman et al. 2003; Beekman and Ratnieks 2003).
References
Beekman M, Ratnieks FLW (2003) Power over reproduction in social Hymenoptera. Philos Trans R Soc Lond B 358:1741–1753
Beekman M, Komdeur J, Ratnieks FLW (2003) Reproductive conflicts in social animals: who has power? Trends Ecol Evol 18:277–282
Boomsma JJ, Nielsen J, Sundström L, Oldham NJ, Petersen HC, Morgan ED (2003) Kin-assessment without kin discrimination in social insects. Proc Natl Acad Sci USA 100:8799–8804
Boulay R, Hefetz A, Soroker V, Lenoir A (2001) Camponotus fellah colony integration: worker individuality necessitates frequent hydrocarbon exchanges. Anim Behav 59:1127–1133
Boulay R, Katzav-Gozansky T, Vandermeer RK, Hefezt A (2003) Colony insularity through queen control on worker social motivation in ants. Proc R Soc Lond B 270:971–977
Bourke AFG (1988) Worker reproduction in the higher eusocial Hymenoptera. Q Rev Biol 63:291–311
Bourke AFG, Franks NR (1995) Social evolution in ants. Princeton University Press, Princeton, New Jersey
Buschinger A (1986) Evolution of social parasitism in ants. Trends Ecol Evol 1:155–160
Collingwood C (1979) The Formicidae (Hymenoptera) of Fennoscandia and Denmark. Fauna Entomol Scand 8:1–174
Crozier R, Pamilo P (1996) Evolution of social insect colonies. Oxford University Press, Oxford
Czechowski W, Radchenko A, Czechowska W (2002) The ants of Poland. Museum and Institute of Zoology PAS, Warsaw
D’Ettorre P, Mondy N, Lenoir A, Errard C (2002) Blending in with the crowd: social parasites integrate into their host colonies using a flexible chemical signature. Proc R Soc Lond B 269:1911–1918
D’Ettorre P, Heinze J, Ratnieks FLW (2004) Worker policing by egg eating in the ponerine ant Pachycondyla inversa. Proc R Soc Lond B 271:1427–1434
Endler A, Liebig J, Schmitt T, Parker JE, Jones GR, Schreier P, Hölldobler B (2004) Surface hydrocarbons of queen eggs regulate worker reproduction in a social insect. Proc Natl Acad Sci USA 101:2945–2950
Foster KR (2004) Diminishing returns in social evolution: the not-so-tragic commons. J Evol Biol 17:1058–1072
Foster KR, Ratnieks FLW (2001) Paternity, reproduction and conflict in vespine wasps: a model system for testing kin selection predictions. Behav Ecol Sociobiol 50:1–8
Frank SA (1995) Mutual policing and repression of competition in the evolution of cooperation groups. Nature 377:520–522
Frank SA (1996) Policing and group cohesion when resources vary. Anim Behav 52:1163–1169
Frank SA (1998) Foundations of social evolution. Princeton University Press, Princeton, New Jersey
Frank SA (2003) Repression of competition and the evolution of cooperation. Evolution 57:693–705
Franks NR, Ireland B, Bourke AFG (1990) Conflicts, social economics and life history strategies in ants. Behav Ecol Sociobiol 27:175–181
Gösswald K (1989) Die Waldameise. AULA-Verlag, Wiesbaden
Hamilton WD (1964) The genetical evolution of social behaviour I and II. J Theor Biol 7:1–16, 17–52
Hannonen M, Sundström L (2003) Worker nepotism among polygynous ants. Nature 42:921
Hannonen M, Sledge MF, Turillazzi S, Sundström L (2002) Queen reproduction, chemical signalling and worker behaviour in polygyne colonies of the ant Formica fusca. Anim Behav 64:477–485
Hannonen M, Helanterä H, Sundström L (2004) Habitat age, breeding system and kinship in the ant Formica fusca. Mol Ecol 13:1579–1588
Helanterä H (2004) Kinship and conflicts over male production in Formica ants. PhD thesis, University of Helsinki
Helanterä H, Sundström L (2005) Worker reproduction in the ant Formica fusca. J Evol Biol 18:162–171
Härtel S, Neumann P, Raassen FS, Moritz RFA, Hepburn HR (2006) Social parasitism by Cape honeybee workers in colonies of their own subspecies (Apis mellifera capensis Esch.). Insectes Soc 53:183–193
Iwanishi S, Hasegawa E, Ohkawara K (2003) Worker oviposition and policing behaviour in the myrmicine ant Aphaenogaster smythiesi japonica Forel. Anim Behav 66:513–519
Johnson CA, Vander Meer RK, Lavine B (2001) Changes in the cuticular hydrocarbon patterns of the slave-maker queen, Polyergus breviceps after killing a Formica host queen. J Chem Ecol 27:1787–1804
Keller L (ed) (1999) Levels of selection in evolution. Princeton University Press, Princeton, New Jersey
Landolt PJ, Akre RD, Greene A (1977) Effects of colony division on Vespula atropilosa (Sladen) (Hymenoptera: Vespidae). J Kans Entomol Soc 50:135–147
Le Moli F, Mori A (1986) The aggression test as a possible taxonomic tool in the Formica rufa group. Aggress Behav 12:93–102
Le Moli F, Parmigiani S (1981) Laboratory and field observations of attack by the red wood ant Formica lugubris Zett on Formica cunicularia Latr (Hymenoptera: Formicidae). Aggress Behav 7:341–350
Lenoir A, Cuisset D, Hefetz A (2001) Effect of social isolation on hydrocarbon pattern and nestmate recognition in the ant Aphaenogaster senilis (Hymenoptera, Formicidae). Insectes Soc 48:101–109
Liebig J, Peeters C, Hölldobler B (1999) Worker policing limits the number of dominants in a ponerine ant. Proc R Soc Lond B 266:1865–1870
Liebig J, Peeters C, Oldham NJ, Markstädter C, Hölldobler B (2000) Are variations in cuticular hydrocarbons of queens and workers a reliable signal of fertility in the ant Harpegnathos saltator? Proc Natl Acad Sci USA 97:4124–4131
Liu ZB, Yamane S, Wang QC, Yamamoto H (2001) Nestmate recognition and temporal modulation in the patterns of cuticular hydrocarbons in natural colonies of Japanese carpenter ant Camponotus japonicus (Hymenoptera, Formicidae). J Ethol 16:57–65
Lopez-Vaamonde C, Koning JW, Brown RM, Jordan WC, Bourke AFG (2004) Social parasitism by male-producing reproductive workers in a eusocial insect. Nature 430:557–560
Martin SJ, Beekman M, Wossler TC, Ratnieks FLW (2002) Parasitic Cape honeybee workers, Apis mellifera capensis, evade policing. Nature 415:163–165
Maynard-Smith J, Szathmary E (1995) The major transitions in evolution. Oxford University Press, Oxford
Miller DG, Ratnieks FLW (2001) The timing of worker reproduction and breakdown of policing behaviour in queenless honey bee (Apis mellifera L) societies. Insectes Soc 48:178–184
Monnin T, Peeters C (1999) Dominance hierarchy and reproductive conflicts among subordinates in a monogynous queenless ant. Behav Ecol 10:323–332
Monnin T, Ratnieks FLW (2001) Policing in queenless ponerine ants. Behav Ecol Sociobiol 50:97–108
Pirk CWW, Neumann P, Ratnieks FLW (2003) Cape honeybees, Apis mellifera capensis, police worker-laid eggs despite the absence of relatedness benefits. Behav Ecol 14:347–352
Ratnieks FLW (1988) Reproductive harmony via mutual policing by workers in eusocial Hymenoptera. Am Nat 132:217–236
Ratnieks FLW (1995) Evidence for a queen-produced egg-marking pheromone and its use in worker policing in the honey-bee. J Apic Res 34:31–37
Ratnieks FLW, Visscher PK (1989) Worker policing in the honeybee. Nature 342:796–797
Ratnieks FLW, Wenseleers T (2005) Policing in insect societies. Science 307:54–56
Ratnieks FLW, Foster KR, Wenseleers T (2006) Conflict resolution in insect societies. Annu Rev Entomol 51:581–608
Savolainen R (1990) Colony success of the submissive ant Formica fusca within territories of the dominant Formica polyctena. Ecol Entomol 15:79–85
Sundström L, Boomsma JJ (2001) Conflicts and alliances in insect families. Heredity 86:515–521
Trivers RL, Hare H (1976) Haploidiploidy and the evolution of social insects. Science 191:249–263
Vander Meer RK, Alonso LE (2002) Queen primer pheromone affects conspecific fire ant (Solenopsis invicta) aggression. Behav Ecol Sociobiol 51:122–130
Vienne V, Errard C, Lenoir A (1998) Influence of the queen on worker behaviour in ants. Ethology 104:431–446
Visscher PK, Dukas R (1995) Honey bees recognize development of nestmates’ ovaries. Anim Behav 49:542–544
Wenseleers T, Hart A, Helanterä H, Ratnieks FLW (2004) Worker reproduction and policing in insect societies: an ESS analysis. J Evol Biol 17:1035–1047
Woyciechowski M, Lomnicki A (1987) Multiple mating of queens and the sterility of workers among eusocial Hymenoptera. J Theor Biol 128:317–327
Acknowledgement
We thank the anonymous referees of a previous version of the manuscript for constructive comments. The work was funded by The Academy of Finland (grants number 42725, 206505 and 213821), the Graduate School for Evolutionary Ecology and the Finnish Cultural Foundation. The experiments comply with the current laws of the country in which they were performed.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by L. Keller
Rights and permissions
About this article
Cite this article
Helanterä, H., Sundström, L. Worker policing and nest mate recognition in the ant Formica fusca . Behav Ecol Sociobiol 61, 1143–1149 (2007). https://doi.org/10.1007/s00265-006-0327-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00265-006-0327-5