Abstract
Social structure emerges from the patterning of interactions between individuals and plays a critical role in shaping some of the main characteristics of animal populations. The topological features of social structure, such as the extent to which individuals interact in clusters, can influence many biologically important factors, including the persistence of cooperation, and the rate of spread of disease. Yet, the extent to which social structure topology fluctuates over relatively short periods of time in relation to social, demographic, or environmental events remains unclear. Here, we use social network analysis to examine seasonal changes in the topology of social structures that emerge from socio-positive associations in adult female rhesus macaques (Macaca mulatta). Behavioral data for two different association types (grooming and spatial proximity) were collected for females in two free-ranging groups during two seasons: the mating and birth seasons. Stronger dyadic bonds resulted in social structures that were more tightly connected (i.e., of greater density) in the mating season compared to the birth season. Social structures were also more centralized around a subset of individuals and more clustered in the mating season than those in the birth season, although the latter differences were mostly driven by differences in density alone. Our results suggest a degree of temporal variation in the topological features of social structure in this population. Such variation may feed back on interactions, hence affecting the behaviors of individuals, and may therefore be important to take into account in studies of animal behavior.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Social structure emerges from the patterning of interactions among the individuals in a society (Hinde 1976). Social structure is often described using categories based on group composition (e.g., multi-male and multi-female) or based on types of interactions, including sexual (e.g., monogamous and polygamous), affiliative (e.g., kin- and sex-biased), and agonistic (e.g., egalitarian and despotic) interactions. These categories are often then used as the basis of empirical hypothesis testing (e.g., Thierry 1990; Lyons et al. 1992; Hemelrijk 1999; Lehmann and Boesch 2009; Madden et al. 2009).
Social structure can also be characterized in a manner that extends beyond categories based on dyadic interactions, to the topology of the social network as a whole (Clutton-Brock and Sheldon 2010). Social structure topology includes information, such as the density of connections—both direct and indirect—among all members of a society, and the extent to which individuals fall into cliques or clusters. Social structure topology is important, as it can play a critical role in shaping some of the emergent characteristics of animal populations, such as the persistence of cooperative behaviors, and the rate at which both disease and information can spread (Watts and Strogatz 1998; Keeling and Eames 2005; Voelkl and Noë 2008; Fehl et al. 2011).
Quantifying social structure topology is a major computational challenge, requiring an interconnected web of multiparty interactions to be summarized in a meaningful manner (Hinde 1976; Lusseau et al. 2006; Kasper and Voelkl 2009). To cope with this challenge, many researchers adopt social network analysis, an analytical tool of increasing importance in the study of animal behavior (Wey et al. 2008; Brent et al. 2011), which uses complex algorithms to visualize and quantify social structure topology (Croft et al. 2008). Social network-based studies have examined, for example, whether topologies differ between species (Maryanski 1987; Sundaresan et al. 2007; Kasper and Voelkl 2009; Sueur et al. 2011) or between groups of the same species (Hanby 1980; Godfrey et al. 2009; Madden et al. 2009; Crofoot et al. 2011) and have demonstrated that social structures often exhibit certain fundamental topological properties (e.g., small-world properties; Lusseau 2003; Croft et al. 2005; Sundaresan et al. 2007). What is less clear, however, is the extent to which the topology for a given group’s social structure is static or, alternatively, the extent to which it fluctuates over time and/or in response to particular events (Sih et al. 2009). Experimental manipulations, including the actual or simulated removal of individuals (Hanby 1980; Chapais et al. 1995; Flack et al. 2006; Williams and Lusseau 2006; Lehmann and Dunbar 2009), adjustments to sex ratios (Darden et al. 2009; Jacoby et al. 2010), and alterations in the amount of time individuals have been housed together (Corner et al. 2003) have been found to result in changes to social structure topology. Similar changes in naturalistic settings have been demonstrated in relation to changes in ecological factors, such as food abundance (Henkel et al. 2010; Foster et al. 2012), to changes over time (Sade 1965; Ramos-Fernández et al. 2009), as well as to changes in demographic factors, such as the birth of infants (Seyfarth 1977; Hanby 1980), or large changes in group size (Lehmann and Boesch 2009). Changes in social structure topology in relation to large changes in group size may not be surprising, as this type of demographic shift considerably alters the number of possible dyadic partnerships, which is likely to be translated directly into changes in social structure topology. In addition, resource availability can vary along with group size, which may consequently influence competition levels. Because dyadic social relationships are assumed to be more valuable during periods of greater competition (Barrett et al. 1999), social structure topology (as well as other features of social structure) is likely to change in relation to large changes in group size (Lehmann and Boesch 2009).
Changes to social structure topology may also occur in relation to reproductive seasonality. Wild male and female Tasmanian devil (Sarcophilus harrisii) social structures were more tightly connected, more clustered, and had a greater number of connections that ran through a single individual in the mating season compared to those in the non-mating season (Hamede et al. 2009). Similar differences may exist between the mating and birth season social structures of adult male Barbary macaques (Macaca sylvanus) (Henkel et al. 2010), although this interpretation of these results is based solely upon inspection of the graphs used to visualize social structure topology (i.e., the sociograms), as quantitative measures of social structure were not reported.
Here, we explored changes to social structure topology in relation to reproductive season in two groups of free-ranging adult female rhesus macaques (Macaca mulatta). Rhesus macaques are seasonal breeders (Hadidian and Bernstein 1979), and substantial differences exist between the mating season, when females engage in sexual interactions with males, and birth season, when females give birth and care for new infants, which may translate into considerable changes in the topology of their social structures. Unlike males, adult female rhesus macaques are philopatric and form stable linear dominance hierarchies (Chikazawa et al. 1979; Hadidian and Bernstein 1979). As such, adult females are considered to form the social core of rhesus macaque groups (Chikazawa et al. 1979) and frequently engage in interactions with one another, such as grooming, which may be associated with benefits, such as coalitionary support (Seyfarth 1976). We examined social structure topology in two different groups—one small and one large—in order to determine whether changes with reproductive season were consistent, despite differences in factors such as group size and year of data collection. Upon detection of changes to the topological features of social structure in the mating and birth seasons, we set out to explore the differences in the patterning of interactions among individuals that might underlie these differences. We focused on three non-mutually exclusive social factors: (1) differing levels of female competition between seasons, (2) sexual interactions with males in the mating season, and (3) the presence of infants in the birth season.
Methods
Subjects and study site
We studied free-ranging rhesus macaques (M. mulatta) from two groups (“V” and “F”) living on Cayo Santiago island, Puerto Rico. These animals are provisioned daily with commercial feed and browsed on natural vegetation with water supplied ad libitum from rain water collection troughs. Population control is undertaken once yearly, involving the removal of mostly juveniles. Animals are not handled outside the removal period, and there is no regular medical intervention. Group V was studied from April to November 2007, and group F, from September 2010 to August 2011. At the time of study, group V contained 21–22 adult females, 15–20 adult males, and roughly 55 juveniles and infants, while group F contained 58 adult females, 29 adult males, and roughly 164 juveniles and infants. All adults were individually recognized, and the age and maternal kin relationships of all individuals were known. Subjects of this study were 21 adult females in group V and 58 adult females in group F (total n = 79), ranging from 6 to 25 years of age. The lowest ranking adult female in group V died from an injury during the middle of data collection on this group (June 25). As the social interactions of this female were impeded severely by this injury, she was not included as a subject. During this study, the mating seasons took place between March and August, with the majority of births occurring between September and February.
Data collection
We collected behavioral data during 10-min continuous focal animal samples using a Psion handheld computer in conjunction with Noldus Observer software (version 5.0). We recorded the occurrence of all affiliative social interactions along with the identity of the focal female’s social partner. We recorded the identities of all adults in spatial proximity (0–2 m) to the focal female, as well as her activity (rest, feed, travel, or social) at 5-min intervals during focal animal samples. We collected a total of 333.8 h of behavioral data in group V and 354.5 h in group F.
Association data
We described and compared the social structures that emerged from two socio-positive associations: grooming and spatial proximity. These association types were selected as they occur frequently, are easily recorded, and have been proposed to be indicative of the social bonds between nonhuman primates (Cords 1997; Barrett and Henzi 2002). We recorded only grooming bouts that were 5 s or longer. We counted a new bout of grooming if the identity of one of the individuals involved changed, if a pause of >30 s occurred, or if the direction of grooming changed. We did not observe any triadic grooming interactions.
In order to increase the independence of proximity and grooming data, we considered individuals in proximity to each other if they were not in physical contact. Moreover, we included only proximity data collected, while focal animals were resting or socializing, as travelling individuals may have little control over who is nearby during group travel, and nonsocial factors (e.g., use of narrow passable terrain) may be involved, while proximity among feeding females (“co-feeding”) may be of different biological significance, such as social tolerance (Silk et al. 2006).
Quantitative measures of social structure topology
We generated social networks containing all study subjects for the mating and birth seasons using subsets of data taken from continuous periods of behavioral sampling. We selected data subsets such that they contained a similar number of focal animal samples (or scans in the case of the proximity network) for individual females within each group for each season in order to reduce spurious differences in network parameters that can be brought about by large differences in the amount of observation time between individual members of the same social network (Croft et al. 2008) (group V, 14.5–15.2 h of focal data/female; group F, 2.0–3.0 h/female). We took mating season data from the heights of mating activity (group V, May 3–June 8; group F, March 3–July 29) and birth season data from the date of the first infant birth until the end of data collection for that year for each group (group V, November 30, 2007; group F, December 9, 2010). For both seasons, we avoided periods during which there were major group events that may result in changes to social networks, such as instability at the top of the male hierarchy (e.g., Engh et al. 2006; Wittig et al. 2008).
We constructed networks using “weighted” data, with connections representing either the amount of time a pair engaged in grooming (seconds of grooming per total number of hours the pair were observed) or the number of scans a pair were recorded in spatial proximity to one another (relative to the number of scan samples collected for each member of a pair). We used weighted data, as these afford greater resolution to social interactions than do binary data (presence/absence of interactions) and are less prone to sampling errors or biases (Altmann 1974; Lusseau et al. 2008). We did not filter the association networks; all ties between dyads were considered to be of potential biological importance, regardless of weight. In each season, social partners were found in spatial proximity to one another between four and 40 times and were observed grooming between one and 11 times.
We used three standard and complementary network parameters to quantify social structure topology: density, network centralization, and mean clustering coefficient. Density represents the level of dyadic connection in a population and is calculated by dividing the total sum of all tie weights by the number of possible ties, where ties represent the presence of a social association among pairs of individuals, and the weight of ties represents the frequency at which social association occurs (Hanneman and Riddle 2005). Network centralization or “global centrality” measures the extent to which interactions are focused around a few individuals in a group (Scott 2000). Network centralization is a complementary measure to density, with both referring to different aspects of the compactness of a social group; density measures the extent to which a social group is connected, while network centralization measures the extent to which connections are organized around particular individuals (Wasserman and Faust 1994). Like indices of sociality (Silk et al. 2006) and grooming diversity (Wittig et al. 2008), network centralization describes the differentiation of social relationships within a social group; although unlike these individual-based measures that refer to the relative prominence of individuals, network centralization measures the unevenness of social partnership distribution in the social group overall. Network centralization is calculated by determining the average difference between the relative centrality of the most central node and that of other nodes, with values ranging from 0 to 100. Network centralization values approaching zero represent networks in which all individuals are equally involved in all partnerships, while higher values represent networks in which only one or a small number of individuals are involved in all partnerships (Wasserman and Faust 1994).
Finally, mean clustering coefficient represents the extent to which a network is clustered—that is, the extent to which social interactions occur in cliques. For individuals, clustering coefficient is measured as the proportion of a given individual’s social partners who also are partners with each other (Croft et al. 2008). Values range from 0 to 1, with zero indicating that none of an individual’s social partners are partners with each other, and one indicating that all of an individual’s social partners are also partners with one another. Mean clustering coefficient is calculated as the mean of all individual-based clustering coefficients.
We calculated network metrics using R (http://www.r-project.org). We used the “tnet” package (Opsahl 2009) to calculate mean clustering coefficient and the “sna” package (Butts 2010) to calculate density and network centralization.
Statistical analyses
To compare densities between the mating and birth seasons, we used the “compare-densities” bootstrapping test in UCINET (Borgatti et al. 2002). To compare network centralization and mean clustering coefficient values, we used a similar bootstrapping technique as for the density comparison but used the tnet package in R instead of UCINET, which is only able to compare densities. We first randomized our association matrices by shuffling the tie strengths among dyads using the “link reshuffle” procedure in tnet, which reshuffles the topology of a network while retaining its degree distribution (Opsahl 2009). We then calculated the mean clustering coefficient and network centralization for each randomized network (1,000 iterations) and used 95 % confidence intervals to compare values between seasons (Lusseau et al. 2008).
The extent to which a network is centralized and clustered can be driven not only by exogenous factors but also by the density of the network alone. We therefore determined whether the network centralization and mean clustering coefficient values of our observed networks differed significantly from the values we would expect for a network of that density. To do so, we generated 1,000 random networks with the same number of nodes, same probability of a tie between nodes, and same range of tie weights. Network centralization and mean clustering coefficient values were determined to differ from values expected based on the density of the network alone if they were greater or less than 2 SD from the mean of the randomly generated values.
In order to determine whether female competition levels in our study differed between the mating and birth seasons, we compared the rate at which individual females were involved in agonistic interactions using a related sample permutation test. In order to explore the impact of sexual interactions with males upon female–female interactions, we used consortships to demarcate periods of intense sexual activity. Consortships in rhesus macaques are common during the mating season, with consorting pairs staying together for up to 11 days (Hill 1987). We used related sample permutation tests to determine whether individual females groomed or were in proximity to other females more or less frequently when they were in consort with males compared to times during the mating season when they were not in consort. We calculated these values for females with an adequate amount of data recorded during consortships (≥40 min of focal observation, n = 9 females in group V, mean 94.4 min/female; nb, data in group F were unavailable).
In order to determine whether the presence of infants impacted upon female interactions, we used related sample permutation tests to compare the frequency of participation in social interactions in the mating and birth seasons for females that gave birth to infants during our study (n = 44). We also tested whether there was a difference in the rate of social interaction for these females directly after compared to before the birth of their infants (frequencies of interaction were calculated from 60 min of focal data collected directly before and 60 min of focal data directly after the birth of infants). We performed related sample permutation tests (10,000 iterations) using PSAM software (Mundry 1999). All tests were two tailed with a significance threshold of α < 0.05.
Results
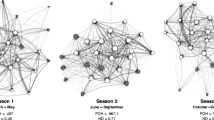
Associations based on spatial proximity resulted in social structure topologies that were fully connected in the mating and birth seasons. That is, all individuals could be directly or indirectly connected to all other individuals (Fig. 1). Social structure topologies based on grooming interactions were not fully connected in either season, with the exception of the group V mating season network (Fig. 2). The number of females that were “unconnected” (i.e., that did not have any grooming partners) was greater for the birth season than that for the mating season in both groups. In group V, one out of 21 females did not have any grooming partners in the birth season compared to zero females in the mating season, while, in group F, nine out of 58 females did not have any grooming partners in the birth season, compared to four females in the mating season (Fig. 2).
Social structures for two groups of female rhesus macaques based on spatial proximity during the mating and birth seasons (spring-embedded sociograms generated in UCINET; Borgatti et al. 2002). Symbols represent individual females. Line thickness represents the number of times pairs of females were observed in proximity (<2 m) to one other relative to the amount of time they were observed, with thicker lines representing more frequent proximity
Social structure for two groups of female rhesus macaques based on grooming interactions during the mating and birth seasons (spring-embedded sociograms generated in UCINET; Borgatti et al. 2002). Symbols represent individual females. Line thickness represents the amount of time pairs of females spent grooming one another (in seconds/hour a pair was observed), with thicker lines representing more frequent grooming
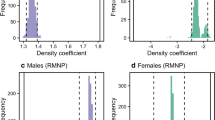
Network densities were greater during the mating season compared to those during the birth season for all networks (Fig. 3). These differences were statistically significant, apart from the group V grooming network (Fig. 3d), where a trend was seen (group V, groom t = −1.81, p = 0.063; proximity t = 3.83, p < 0.001; group F, groom t = 2.25, p = 0.001; proximity t = −5.71, p = 0.001).
Violin plots of the bootstrapped (1,000 iterations) densities of female rhesus macaque proximity (a, b) and grooming (c, d) social structures. Network densities were determined for two social groups: group F (a, c) and group V (b, d). Violin plots are composed of frequency distributions mirrored on both sides of box plots. Plots were generated using R (http://www.r-project.org). Overlap of 95 % confidence intervals was measured for pairwise comparisons. **p < 0.05
Network centralization, indicating the extent to which social structures are centered on a small subset of individuals, was significantly greater in the mating season than that in the birth season for both grooming and proximity in both groups (Fig. 4). For grooming, differences in network centralization were not driven by differences in the densities of the social structures between the mating and birth seasons alone, as our observed values were significantly lower in both seasons than those that would be expected for networks of these densities (group V mating D real = 49.552, D random = 157.01, p < 0.001; group V birth D real = 49.484, D random = 93.978, p < 0.001; group F mating D real = 68.120, D random = 998.932, p < 0.001; group F birth D real = 40.219, D random = 453.784, p < 0.001). In contrast, for proximity, network centralization values did not differ from expected based on comparisons to randomly generated networks of the same densities (group V mating D real = 10.903, D random = 10.861, p = 0.382; group V birth D real = 10.534, D random = 10.832, p = 0.098; group F mating D real = 29.653, D random = 29.286, p = 0.274; group F birth D real = 29.085, D random = 29.280, p = 0.378).
Violin plots of the bootstrapped (1,000 iterations) network centralization scores of female rhesus macaque proximity (a, b) and grooming (c, d) social structures. Network centralization was determined for two social groups: group F (a, c) and group V (b, d). **p < 0.05. Violins and whiskers do not appear on plots due to narrow ranges of variation
Mean clustering coefficients, indicating the extent to which social structures are organized in cliques, were greater in the mating season compared to those in the birth season for proximity in group V (Fig. 5b); no such differences were seen for grooming in group V (Fig. 5d) or for grooming or proximity in group F (Fig. 5a, c). However, clustering coefficient values in group V appeared to be largely driven by the densities of these social structures alone; clustering coefficient values for grooming in group V did not differ from the values we would expect for social structures of these densities (groom mating D real = 0.309, D random = 0.203, p = 0.963; groom birth D real = 0.09, D random = 0.131, p = 0.304), although there was a trend toward suggesting that proximity in group V did differ from the values we would expect for social structures of these densities (proximity mating D real = 0.845, D random = 0.797, p = 0.050; proximity birth D real = 0.654, D random = 0.596, p = 0.051). Although not significantly different between the mating and birth seasons, group F social structures were significantly more clustered than would be expected for structures of the same densities (groom mating D real = 0.228, D random = 0.031, p = 0.001; grooming birth D real = 0.136, D random = 0.029, p = 0.012; proximity mating D real = 0.309, D random = 0.184, p < 0.001; proximity birth D real = 0.273, D random = 0.159, p < 0.001).
Violin plots of the bootstrapped (1,000 iterations) mean clustering coefficients of female rhesus macaque proximity (a, b) and grooming (c, d) social structures. Mean clustering coefficients were determined for two social groups: group F (a, c) and group V (b, d). **p < 0.05. Distributions of clustering coefficient values for individual females in the mating (m) and birth (b) seasons are presented in the inset graphs
The overall average rate of agonistic interactions among the adult female rhesus macaques in both groups was higher during the mating season (group V = 1.67 events/h, group F = 2.23 events/h) than that during the birth season (group V = 1.00, group F = 1.97 events/h). For individual females, rates of agonistic interactions were significantly greater in the mating season compared to those in the birth season in group V (PSAM-related samples test, test statistic = 0.011, p = 0.049), but not in group F (test statistic = 1.690, p = 0.802).
Of the nine females for whom sufficient data during consort were available, five did not groom other females while in consort, but all groomed other females when they were not involved in a consort. The rates of grooming and the amount of time spent in proximity to others were significantly lower when in consort compared to when not in consort for these females (groom, test statistic = 7.33, p = 0.013; proximity, test statistic = 2.30, p = 0.012). This pattern remained when the direction of grooming between females was taken into account; females gave (test statistic = 57.708, p = 0.009) and received (test statistic = 49.652, p = 0.008) significantly less grooming when in consort compared to when not in consort.
Of the females that gave birth, between 16.6 % (group V = 1/6 females) and 18.4 % (group F = 7/38 females) did not participate in grooming during the birth season. These females all participated in grooming during the mating season (Fig. 2). All females that had infants in the birth season were found in proximity to other adult females in both the mating and birth seasons (Fig. 1). Females that gave birth to infants did not participate in grooming with greater frequency in the birth season compared to the mating season (group V, test statistic = 0.01, p = 0.143; group F, test statistic = 33.64, p = 0.264). This pattern remained when the direction of grooming was taken into account; females that gave birth to infants did not give (group V, test statistic = 93.537, p = 0.505; group F, test statistic = 18.504, p = 0.337) or receive (group V, test statistic = 213.134, p = 0.136; group F, test statistic = 14.750, p = 0.414) a greater amount of grooming in the birth season compared to that in the mating season. Mothers in group V were also not more likely to be found in proximity to other females during the birth season compared to those during the mating season (test statistic = 0.05, p = 0.403). However, mothers in group F were significantly more likely to be found in proximity to another female in the mating season compared to those in the birth season (test statistic = 0.164, p = 0.0001).
There were no significant differences in the frequency with which females that gave birth during the study period participated in grooming directly before compared to directly after the birth of their infants (group V, test statistic = 0.01, p = 0.286; group F, test statistic = 0.469, p = 0.641). Nor were there differences when the direction of grooming was taken into account; there were no differences in the amount of grooming females gave (group V, test statistic = 0.008, p = 0.298; group F, test statistic = 0.069, p = 0.750) or received (group V, test statistic = 0.003, p = 0.999; group F, test statistic = 0.172, p = 0.243) directly before compared to directly after the birth of their infants. Group V mothers were in proximity to other females more often after birth compared to before birth (test statistic = 0.21, p = 0.036), but this difference was not observed in group F (test statistic = 0.025, p = 0.322).
Discussion
This study has demonstrated that changes to the topological features of social structure occur in relation to reproductive seasonality. In particular, we have shown that considerable changes in social structure topologies that emerge from patterns of grooming and spatial proximity occur between the mating and birth seasons in two groups of free-ranging adult female rhesus macaques. These differences were consistent despite differences in the size of the two groups, as well as the year in which data were collected. Most notably, social structures were more densely connected in the mating season compared to those in the birth season, which subsequently drove some of the observed differences in the extent to which these structures were clustered and centralized. Independent of differences in density, social structures that emerged from grooming interactions were also more centered on a subset of individuals in the mating compared to those in the birth season.
Social structure topology emerges from the patterning of social interactions among individuals (Hinde 1976) and can, in turn, feed back on individuals to shape their relationships by influencing dominance interactions (Hemelrijk et al. 1999), cooperative behaviors (Croft et al. 2006; Ryder et al. 2008; Fehl et al. 2011), and individual recognition (Darden et al. 2009). Social structure topology can also impact upon the transmission of information (McComb et al. 2001; Flack and Krakauer 2006; Morrell et al. 2008) and cultural traditions (Whiten et al. 2005). Yet, whether social structure topology fluctuates within a social group over time and/or in response to certain events, particularly those that occur naturally, is not clear and should be examined before the potentially important consequences of such fluctuations are explored. Although the results of our study must be replicated in other species in a greater variety of conditions before broad generalizations can be drawn, they nevertheless complement previous evidence from naturalistic settings (e.g., Hamede et al. 2009; Lehmann and Boesch 2009; Foster et al. 2012) that suggest that social structure topology is not a static entity. Yet, whether the changes observed in this and other studies are substantial enough to result in downstream changes to features, such as information flow, remains an open question, and one that ultimately must be confirmed using experimental or modelling-based paradigms or with longer-term observational data. If downstream consequences are confirmed, an implication of these findings may be that fluctuations in social structure topology should sometimes be taken into account in studies that use short-term datasets to evaluate the costs and benefits of social behaviors, whose expression may vary along with structural changes.
Changes to social structure topology were relatively consistent between the mating and birth seasons for the two types of affiliative association examined in our study. Social structures based both on grooming and spatial proximity were more tightly connected (greater density) in the mating season compared to those in the birth season. Similar differences in social structure topology have been found between reproductive seasons in other species. The more tightly connected association networks of male and female Tasmanian devils found in the mating season (Hamede et al. 2009) may be expected, as males and females must come into contact in order to mate. Intra-sexual differences in social structure topology in relation to reproductive seasonality, on the other hand, may be less intuitive. Nevertheless, pairs of male Barbary macaques have tighter affiliative connections with other males in the mating season (Henkel et al. 2010), possibly resulting in changes to social structure topology similar to those we have observed in the current study.
Differences in social structure topology in relation to reproductive season for members of one sex could be brought about by changes to interactions among members of that sex (e.g., females’ interactions with females could differ from one season to the next) and/or be brought about by changes to interactions among males and females (e.g., females’ interactions with males could impact upon females’ interactions with other females). Interactions that are not purely dyadic (e.g., interactions involving mothers, infants, and other females) as well as other external factors, such as the activities of other closely neighboring groups, and changes to the environment may also contribute. The consistent differences between social structure topologies in the mating and birth seasons found in the current study may be explained by some of the substantial changes to the social lives of adult female rhesus macaques that occur along with reproductive season. For instance, tighter connections in the mating season compared to those in the birth season may result from the impact of consortships with males on female–female relationships. Females in this study who were in consort with males groomed other females less frequently and spent less time in proximity to other females, compared to times when they were not in consort. Similar results were found for grooming in a previous study that examined the impact of consortships on rhesus macaque interactions (Wallen and Tannenbaum 1997). Female rhesus macaques may therefore be temporarily less available to their regular female social partners when they are in consort with a male. In wild baboons, females have been shown to compensate for the permanent loss of close social partners by broadening and strengthening their social networks (Engh et al. 2006). Female rhesus macaques may similarly increase the strength of their connections during the mating season to compensate for temporary shifts in the availability of some of their close social partners due to consortships, which could result in social structures that are more tightly connected in the mating season than in the birth season, when consortships are absent.
We may also expect social structure topology to be influenced by competition levels. The level of intragroup female–female aggression was greater in the mating season compared to that in the birth season in a previous study of rhesus macaque females (Wallen and Tannenbaum 1997) and in one, but not both, of the groups in the current study. In periods with higher levels of competition, female primates may attempt to increase the strength of their social bonds in order to maximize benefits, such as coalitionary support, which they may receive from social partners (Seyfarth 1976; Lehmann and Boesch 2009), which could result in more tightly connected networks in the mating season compared to in the birth season (Lehmann and Boesch 2009). Moreover, differences in competition levels may explain why grooming structures were more centralized in the mating compared to in the birth season in the current study. In their study of wild female chimpanzees, Lehmann and Boesch (2009) found that network centralization was lower when group size was larger, and competition levels were presumed higher. These authors interpret their results thus: as the value of the benefits that result from social relationships increases in periods of higher competition, so too does the number of highly valuable partners. Individuals therefore spread their social interactions more widely during periods of higher levels of competition, resulting in less centralized networks. The results of our study are in direct contrast to those of Lehmann and Boesch (2009)—female rhesus macaque social structures were more, not less, centralized in the mating season, when competition levels may have been higher.
To explain this difference, we propose, rather than an increase in the number of highly valuable partners, that rhesus macaque females that are already highly valuable (i.e., high-ranking females; Seyfarth 1976) become more so during periods of high levels of competition. This explanation is consistent with the social system of female rhesus macaques and of many other Old World monkey species in which many of the proposed benefits of social relationships are rank related (Seyfarth 1976). An increased tendency for females to direct social interactions toward the highest-ranking females during periods of high levels of competition could result in social structures that were centered on these females and more centered relative to social structures during periods of lower competition. By contrast, an increased tendency to direct social interactions toward the highest-ranking females during periods of high levels of competition may not occur to the same extent in chimpanzees, a species in which females disperse from their natal groups and form dominance hierarchies that are often stable, but not always linear (Lehmann and Boesch 2009).
Female primates are attracted to the infants of others, with attempts by females to inspect, touch, and carry the infants of others documented in many species (Hrdy 1976). As a result, we may expect social structure topologies to be more centralized in the birth season when females attempt to interact with infants and, consequently, their mothers. However, social structure topologies in the current study were consistently more centralized in the mating season, not the birth season. In addition, we found surprisingly little evidence that mothers were the focus of female social interactions; the frequency at which mothers were involved in grooming was not greater in the birth season compared to that in the mating season (when no infants were present) nor was it greater directly after compared to directly before the birth of their infants. There were no changes to these results when the direction of grooming (given or received) was taken into account. Mothers in one group were shown to spend a greater proportion of time in proximity to other adult females in the birth season compared to those in the mating season, while mothers in the other group spent a significantly greater proportion of time in proximity to other adult females directly after compared to directly before the birth of their infants.
Females of seasonally breeding species, such as rhesus macaques, may show relatively little change in their social interactions in relation to the birth of infants compared to non-seasonally breeding females in which births are widely separated and in which infants are present at times when many females do not have infants of their own (Cheney and Seyfarth 1990). In addition, female nonhuman primates with despotic relationships, such as rhesus macaques (Chikazawa et al. 1979), may harm the infants of others as a form of reproductive competition (Maestripieri 1994). For females with infants, a higher rate of proximity, but no differences in grooming, suggests that mothers with dependent infants may be attractive to other females but may avoid an increased rate of physical contact to protect their infants from harm. Regardless of whether changes to dyadic relationships in relation to the presence of infants occur for adult female rhesus macaques, those changes may perhaps not be great enough to impact on the entire social structure. Seyfarth (1977) came to a similar conclusion upon examining the impact of the birth of an infant on the social structure of a captive group of baboons.
It should also be noted that the data we have presented represent single snapshots of social structure topology. The factors that may shape social interactions and, consequentially, social structure are likely to be highly dynamic. For example, the level of female–female competition may shift on short-time scales throughout the mating season, varying along with the number of females that are fertile and engaged in mating activity. Fine-scale changes in the availability of resources, such as water in this free-ranging population, may also impact on the level of competition among individuals and, consequently, on social structure topology. Models for studying the dynamic evolution of networks are difficult to implement in studies of animal behavior due to the high resolution of data needed over short temporal periods. Although this avenue of research is beyond the scope of the current study, it may prove highly useful in future studies concerning the social structure of nonhuman societies.
References
Altmann J (1974) Observational study of behavior: sampling methods. Behaviour 49:227–267
Barrett L, Henzi PS (2002) Constraints on relationship formation among female primates. Behaviour 139:263–289
Barrett L, Henzi PS, Weingrill T, Lycett JE, Hill RA (1999) Market forces predict grooming reciprocity in female baboons. Proc R Soc Lond B 266:665–670
Borgatti SP, Everett MG, Freeman LC (2002) UCINET for Windows: software for social network analysis. Analytic Technologies, Harvard, Massachusetts
Brent LJN, Lehmann J, Ramos-Fernández G (2011) Social network analysis in the study of nonhuman primates: a historical perspective. Am J Primatol 73:720–730
Butts C (2010) Tools for social network analysis. In, R package version 2.2 URL http://erzuli.ss.uci.edu/R.stuff
Chapais B, Gauthier C, Prud’homme J (1995) Dominance competition through affiliation and support in Japanese macaques: an experimental study. Int J Primatol 16:521–536
Cheney DL, Seyfarth RM (1990) How monkeys see the world: inside the mind of another species. Chicago University Press, Chicago
Chikazawa D, Gordon TP, Bean CA, Bernstein IS (1979) Mother-daughter dominance reversals in rhesus monkeys (Macaca mulatta). Primates 20:301–305
Clutton-Brock T, Sheldon BC (2010) Individuals and populations: the role of long-term, individual-based studies of animals in ecology and evolutionary biology. Trends Ecol Evol 25:562–573
Cords M (1997) Friendships, alliances, reciprocity and repair. In: Whiten A, Byrne RW (eds) Machiavellian intelligence II: extensions and evaluations. Cambridge University Press, Cambridge, pp 24–49
Corner LAL, Pfeiffer DU, Morris RS (2003) Social-network analysis of Mycobacterium bovis transmission among captive brushtail possums (Trichosurus vulpecula). Prev Vet Med 59:147–167
Crofoot MC, Rubenstein DI, Maiya AS, Berger-Wolf TY (2011) Aggression, grooming and group-level cooperation in white-faced capuchins (Cebus capucinus): insights from social networks. Am J Primatol S1:821–833
Croft DP, James R, Ward AJW, Botham MS, Mawdsley D, Krause J (2005) Assortative interactions and social networks in fish. Oecologia 143:211–219
Croft DP, James R, Thomas P, Hathaway C, Mawdsley D, Laland KN, Krause J (2006) Social structure and co-operative interactions in a wild population of guppies (Poecilia reticulata). Behav Ecol Sociobiol 59:644–650
Croft DP, James R, Krause J (2008) Exploring animal social networks. Princeton University Press, Princeton
Darden SK, James R, Ramnarine IW, Croft DP (2009) Social implications of the battle of the sexes: sexual harassement disrupts female sociality and social recognition. Proc R Soc Lond B 276:2651–2656
Engh AL, Beehner JC, Bergman TJ, Whitten PL, Hoffmeier RR, Seyfarth RM, Cheney DL (2006) Behavioural and hormonal responses to predation in female chacma baboons (Papio hamadryas ursinus). Proc R Soc Lond B 273:707–712
Fehl K, van der Post DJ, Semmann D (2011) Co-evolution of behaviour and social network structure promotes human cooperation. Ecol Lett 14:546–551
Flack JC, Krakauer DC (2006) Encoding power in communication networks. Am Nat 168:E87–E102
Flack JC, Girvan M, de Waal FBM, Krakauer DC (2006) Policing stabilizes construction of social niches in primates. Nature 439:426–429
Foster EA, Franks DW, Morrell LJ, Balcomb KC, Parsons KM, van Ginneken A, Croft DP (2012) Social network correlates of food availability in an endangered population of killer whales, Orcinus orca. Anim Behav 83:731–736
Godfrey SS, Bull CM, James R, Murray K (2009) Network structure and parasite transmission in a group living lizard, the gidgee skink, Egernia stokesii. Behav Ecol Sociobiol 63:1045–1056
Hadidian J, Bernstein IS (1979) Female reproductive cycles and birth data from an Old World monkey colony. Primates 20:429–442
Hamede RK, Bashford J, McCallum H, Jones M (2009) Contact networks in a wild Tasmanian devil (Sarcophilus harrisii) population: using social network analysis to reveal seasonal variability in social behaviour and its implications for transmission of devil facial tumour disease. Ecol Lett 12:1147–1157
Hanby JP (1980) Relationships in six groups of rhesus monkeys I: networks. Am J Phys Anthropol 52:549–564
Hanneman RA, Riddle M (2005) Introduction to social network methods. University of California, Riverside
Hemelrijk CK (1999) An individual-orientated model of the emergence of despotic and egalitarian societies. Proc R Soc Lond B 266:361–369
Hemelrijk CK, Meier C, Martin RD (1999) ‘Friendship’ for fitness in chimpanzees? Anim Behav 58:1223–1229
Henkel S, Heistermann M, Fischer J (2010) Infants as costly social tools in male Barbary macaque networks. Anim Behav 79:1199–1204
Hill DA (1987) Social relationships between adult male and female rhesus macaques. 1. Sexual Consortships. Primates 28:439–456
Hinde RA (1976) Interactions, relationships and social structure. Man 11:1–17
Hrdy SB (1976) Care and exploitation of nonhuman primate infants by conspecifics other than the mother. In: Rosenblatt JS, Hinde RA, Shaw E, Bier C (eds) Advances in the study of behavior, vol 6. Academic, New York, pp 101–158
Jacoby DMP, Busawon DS, Sims DW (2010) Sex and social networking: the influence of male presence on social structure of female shark groups. Behav Ecol 21:808–818
Kasper C, Voelkl B (2009) A social network analysis of primate groups. Primates 50:343–356
Keeling MJ, Eames KTD (2005) Networks and epidemic models. J Roy Soc Interface 2:295–307
Lehmann J, Boesch C (2009) Sociality of the dispersing sex: the nature of social bonds in West African female chimpanzees, Pan troglodytes. Anim Behav 77:377–387
Lehmann J, Dunbar RIM (2009) Network cohesion, group size and neocortex size in female-bonded Old World primates. Proc R Soc Lond B 276:4417–4422
Lusseau D (2003) The emergent properties of a dolphin social network. Proc R Soc Lond B 270:S186–S188
Lusseau D, Wilson B, Hammond PS, Grellier K, Durban JW, Parsons KM, Barton TR, Thompson PM (2006) Quantifying the influence of sociality on population structure in bottlenose dolphins. J Anim Ecol 75:14–24
Lusseau D, Whitehead H, Gero S (2008) Incorporating uncertainty into the study of animal social networks. Anim Behav 75:1809–1815
Lyons DM, Mendoza SP, Mason WA (1992) Sexual segregation in squirrel monkeys (Saimiri sciureus): a transactional analysis of adult social dynamics. J Comp Psychol 106:323–330
Madden JR, Drewe JA, Pearce GP, Clutton-Brock TH (2009) The social network structure of a wild meerkat population: 2. Intragroup interactions. Behav Ecol Sociobiol 64:81–95
Maestripieri D (1994) Influence of infants on female social relationships in monkeys. Folia Primatol 63:192–202
Maryanski AR (1987) African ape social structure: is there strength in weak ties? Social Net 9:191–215
McComb K, Moss C, Durant SM, Baker L, Sayialel S (2001) Matriarchs as repositories of social knowledge in African elephants. Science 292:491–494
Morrell LJ, Croft DP, Dyer JRG, Chapman BB, Kelley JL, Laland KN, Krause J (2008) Association patterns and foraging behaviour in natural and artificial guppy shoals. Anim Behav 76:855–864
Mundry R (1999) Testing related samples with missing values: a permutation approach. Anim Behav 58:1143–1153
Opsahl T (2009) Structure and evolution of weighted networks. University of London, London
Ramos-Fernández G, Boyer G, Aureli F, Vick LG (2009) Association networks in spider monkeys (Ateles geoffroyi). Behav Ecol Sociobiol 63:999–1013
Ryder TB, McDonald DB, Blake JG, Parker PG, Loiselle BA (2008) Social networks in the lek-mating wire-tailed manakin (Pipra filicauda). Proc R Soc Lond B 275:1367–1374
Sade DS (1965) Some aspects of parent-offspring and sibling relations in a group of rhesus monkeys, with a discussion of grooming. Am J Phys Anthropol 23:1–17
Scott J (2000) Social network analysis: a handbook. Sage Publications, London
Seyfarth RM (1976) Social relationships among adult female baboons. Anim Behav 24:917–938
Seyfarth RM (1977) A model of social grooming among adult female monkeys. J Theor Biol 65:671–698
Sih A, Hanser SF, McHugh KA (2009) Social network theory: new insights and issues for behavioral ecologists. Behav Ecol Sociobiol 63:975–988
Silk JB, Alberts SC, Altmann J (2006) Social relationships among adult female baboons (Papio cynocephalus) II. Variation in the quality and stability of social bonds. Behav Ecol Sociobiol 61:197–204
Sueur C, Petit O, De Marco A, Jacobs AT, Watanabe K, Thierry B (2011) A comparative network analysis of social style in macaques. Anim Behav 82:845–852
Sundaresan SR, Fischhoff IR, Dushoff J, Rubenstein DI (2007) Network metrics reveal differences in social organization between two fission-fusion species, Grevy’s zebra and onager. Oecologia 151:140–149
Thierry B (1990) Feedback loop between kinship and dominance: the macaque model. J Theor Biol 145:511–522
Voelkl B, Noë R (2008) The influence of social structure on the propagation of social information in artificial primate groups: a graph-based simulation approach. J Theor Biol 252:77–86
Wallen K, Tannenbaum PL (1997) Hormonal modulation of sexual behavior and affiliation in rhesus monkeys. Ann NY Acad Sci 807:185–202
Wasserman S, Faust K (1994) Social network analysis: methods and applications. Cambridge University Press, Cambridge
Watts DJ, Strogatz SH (1998) Collective dynamics of ‘small-world’ networks. Nature 393:440–442
Wey T, Blumstein DT, Shen W, Jordan F (2008) Social network analysis of animal behaviour: a promising tool for the study of sociality. Anim Behav 75:333–344
Whiten A, Horner V, de Waal FBM (2005) Conformity to cultural norms of tool use in chimpanzees. Nature 437:737–740
Williams R, Lusseau D (2006) A killer whale social network is vulnerable to targeted removals. Biol Lett 2:497–500
Wittig RA, Crockford C, Lehmann J, Whitten PL, Seyfarth RM, Cheney DL (2008) Focused grooming networks and stress alleviation in wild female baboons. Horm Behav 54:170–177
Acknowledgments
We thank the Caribbean Primate Research Center (CPRC) for the permission to undertake research on Cayo Santiago, along with Bonn Aure and Jacqueline Buhl who assisted in the data collection. We also thank Roger Mundry for his permission to use the PSAM software and Robert Seyfarth; Dan Blumstein, an anonymous reviewer; and associate editor Joan Silk for their helpful comments on earlier versions of the manuscript. This project was funded by fellowships awarded to LJNB by the Natural Sciences and Engineering Research Council of Canada, the University of Roehampton, and the Duke Center for Interdisciplinary Decision Sciences. Additional funds were provided by the International Primatological Society, the Leakey Trust, and NIMH grant no. R01-089484-01. The CPRC is supported by a grant no. 8-P40 OD012217-25 from the National Center for Research Resources (NCRR) and the Office of Research Infrastructure Programs (ORIP) of the National Institutes of Health.
Ethical standards
This study was approved by the IACUC of the University of Puerto Rico and by the Ethics Committee of the University of Roehampton.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by J. B. Silk
Rights and permissions
About this article
Cite this article
Brent, L.J.N., MacLarnon, A., Platt, M.L. et al. Seasonal changes in the structure of rhesus macaque social networks. Behav Ecol Sociobiol 67, 349–359 (2013). https://doi.org/10.1007/s00265-012-1455-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00265-012-1455-8