Abstract
Animals may assess the quality of other individuals by using information that different ornaments may provide. The European Roller (Coracias garrulus) is a socially monogamous species in which males and females display highly conspicuous plumage colouration. According to the mutual selection hypothesis, we predicted that, in this species, plumage coloration could signal individual quality in both sexes because both female and male rollers invest a considerable amount of time caring for their offspring. We used spectrophotometric measurements to investigate the information content of multiple plumage colour traits. We found that the roller is actually a sexually dimorphic and dichromatic species. Different plumage colours from different origins were correlated within individual. Head and back brightness correlated with body condition in both sexes, and in males, head brightness correlated with the number of fledglings in successful nests, while head green-yellow saturation correlated with parental provisioning. Meanwhile, in females, back brightness was related to the number of fledglings in successful nests and to parental provisioning rate. In addition, there was a positive assortative mating in relation to weight, body condition, head green-yellow saturation and back brightness. Finally, we found a positive correlation between parent and offspring coloration. Altogether, these results suggest that multiple colour traits may act as quality indicators in the roller and that they may be used by the two sexes to assess potential mate quality.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Sexual selection theory proposes that ornamental traits, such as plumage colour or elaborate visual and vocal displays, reliably signal individual quality (Darwin 1871; Andersson 1994). Because elaborate ornamental traits may be costly to produce and maintain, their expression may be quality dependent and only high quality individuals could achieve maximum expression of such traits (Andersson 1994; Hamilton and Zuk 1982; Kodric-Brown and Brown 1984; Zahavi 1975; Zahavi and Zahavi 1997). These sexual selected ornaments might thus provide honest signals of individual phenotypic or genetic quality.
Until the last decade, most sexual selection studies focussed on male secondary sexual traits of sexually dimorphic species in which the male is the most ornamented sex. Several studies demonstrated that females prefer to mate with well-ornamented males, as well as fitness benefits of making this choice (review in Andersson 1994). Comparatively fewer studies have investigated the functional role of female ornaments (review in Amundsen 2000; Amundsen and Pärn 2006; Kraaijeveld et al. 2007). In some monomorphic species, however, both males and females are similarly ornamented, often to a high degree. Female ornaments have been proposed as resulting from genetic correlations with male ornaments and thereby to be non-functional (Lande 1980). Developmental constraints may also lead to non-adaptive female ornamentation (Emlen et al. 2005). On the other hand, a growing body of evidence suggests that female ornaments can also signal either the same or a different aspect of quality as male ornaments (review in Amundsen 2000; Amundsen and Pärn 2006; Kraaijeveld et al. 2007). The mutual selection hypothesis proposes that mutual ornamentation is the result of mutual mate choice (Huxley 1914; West-Eberhard 1979; Johnstone et al. 1996; Johnstone 1997; Trivers 1972; Servedio and Lande 2006), which can lead to assortative mating if males and females have similar preferences. Theoretical models predict that mutual selection is likely to occur when both sexes benefit from mating with high-quality individuals of the other sex because both sexes provide a substantial amount of parental care to the offspring (Johnstone et al. 1996; Kokko and Johnstone 2002; Trivers 1972) or because their reproductive success is limited by the other sex’s reproductive or parental abilities (Grafen 1990; Heywood 1989; Hoelzer 1989).
In birds, plumage coloration is one of the most widespread and conspicuous ornament, and it has been the focus of several tests of the honest signalling theory (Andersson 1994; Hill 2006). Plumage coloration can result from three different mechanisms, which can act alone or in conjunction (carotenoid and melanin pigments or feather microstructure; Hill and McGraw 2006a). Colour production may have different associated costs depending on its origin. Therefore, plumage coloration might convey different information to potential partners and/or competitors (Hill 2006). The informative content of pigment-based plumage colorations has been profusely studied in many avian taxa. Evidence suggests that carotenoid-based plumage colorations are strongly condition-dependent (Hill 1990; von Schantz et al. 1999; McGraw and Hill 2000; reviewed in Hill and McGraw 2006b). For instance, McGraw and Hill (2000) demonstrated that males parasitised by intestinal coccidians showed less saturated carotenoid-based plumage than unparasitised males. Melanin-based plumage colorations, on the other hand, have long been considered to be under genetic control and independent from environmental conditions (e.g. Badyaev and Hill 2000; Bize et al. 2006; Roulin and Dijkstra 2003), although some studies have shown that melanic colours can also be determined by nutritional (Veiga and Puerta 1996) or rearing conditions (Fargallo et al. 2007). In addition, a recent meta-analysis has shown that carotenoid- and melanin-based plumages do not differ actually in their ability to act as honest signals of the quality of their bearers (Griffith et al. 2006). Behavioural ecologists became interested in the function of structural plumage colours only recently. There is a growing evidence that the full expression of such colorations requires good condition during moult (McGraw et al. 2002; Siefferman and Hill 2005a), suggesting that structural plumage ornaments may signal different aspects of individual quality (e.g. Doucet 2002; Keyser and Hill 1999; Keyser and Hill 2000; Doucet and Montgomerie 2003; Hill et al. 2005; Siefferman and Hill 2005b; Solís et al. 2008; Avilés et al. 2008).
Hitherto, studies of bird male and female ornamentations have mainly focussed on single conspicuous ornaments. Many species, however, possess multiple ornaments that may or may not be simultaneously displayed by both sexes and that may convey exclusive, overlapping or unreliable information on diverse aspects of condition (Møller and Pomiankowski 1993). Studies of multiple ornaments have focussed on males of promiscuous and polygynous avian species because these groups include some of the most extravagantly ornamented species (Zuk et al. 1990, 1993; Ligon and Zwartjes 1995; Omland 1996; Andersson et al. 2002). Comparatively less attention has been given to multiple colour ornamentation in females (see review in Kraaijeveld et al. 2007).
The general aim of our study is to assess the information content of multiple plumage-coloured traits in the European Roller (Coracias garrulus L.). The Roller is a socially monogamous Coraciiform species with biparental care (Cramp and Simmons 1988). Both sexes incubate the eggs, brood and feed the young, even though the female takes a larger share. Females and males appear monochromatic to humans and are similar in size (Avilés 2006). Typical individuals exhibit highly conspicuous structural violet and turquoise colorations in scapulars and rump and head and belly, respectively. Furthermore, they show a melanin-based chestnut back plumage (see “Materials and methods”). Both structural and melanin-based plumage patches are conspicuously displayed during the characteristic ‘rolling’ flight of the species. We used spectrophotometric measurements to account for ultraviolet (UV) information hidden to humans with the aim to investigate in breeding rollers: (1) whether the species is sexually dichromatic, (2) the relationship among the various colour traits in both males and females (Jawor and Breitwisch 2004), (3) whether breeding pairs show assortative mating in relation to components of coloration as would be expected for species in which the two sexes provided substantial care to the offspring, (4) whether coloured traits correlate with individual quality (i.e. body condition, reproductive success and/or reproductive investment) in the two sexes and (5) whether the expression of coloured traits is correlated between parents and offspring, which would suggest that coloured traits are transmitted either genetically or by maternal or parental effects or are the result of a common environmental effect affecting parents and their offspring colorations.
Materials and methods
Study system
The study was conducted during the 2005–2007 breeding seasons in an unwooded area of Cáceres province in western Spain (39°27′ N, 6°20′ E) where rollers breed in nest boxes placed on the poles of electric lines (Avilés et al. 1999; 2000). The area is characterised by the predominance of dry pastures, which are used by livestock.
The European Roller is a migratory secondary hole-nesting bird (Cramp and Simmons 1988). They mostly arrive to breeding grounds in April and begin to lay in early May (Avilés et al. 1999). The mean clutch size in the study area is 4.23 eggs (range, 1–7; Avilés et al. 1999). Incubation usually begins after the laying of the third egg and takes around 20 days. Due to the incubation pattern, chicks hatch asynchronously. Chick rearing takes 24 days, and both sexes participate in incubation and feeding tasks (authors, unpublished data).
Field data collection
During the three study years, nest boxes were monitored weekly from mid-April to fledging to determine laying dates, clutch sizes and fledging success. Adult rollers were captured at the nest with an automatic trap at the beginning of the nestling period. We captured eight females and one male in 2005, eight females and seven males in 2006 and 29 females and 28 males in 2007. Adults were ringed and measured at capture. We calculated body condition for each individual as the residuals from the linear regression between body weight and tarsus length. Upon capture, a drop of blood was extracted by brachial venipuncture and stored in ethanol for later sexing (see Parejo et al. 2007 for the detailed protocol) by molecular methods as described by Fridolfsson and Ellegren (1999). Additionally, we plucked three to five feathers from the same location of the following body regions: head (turquoise), scapulars (violet) and back (chestnut). In 2007, to investigate plumage colour transmission, we also collected feathers from fledglings with the same protocol as in adults. Chick feathers were collected from all chicks of a brood when the older chick was 19 days old. At this moment, fly feathers are not fully developed, thus minimising the risk of early nest abandonment.
Parental investment
In 2006 and 2007, we made behavioural observations at nests in order to estimate parental investment. Observations were made in the morning (0730–1200 hours) and extended 1 h after the moment we saw at least one adult roller approaching the nest. Two observations were made on each nest, one during incubation (around the 13th day after the onset of incubation; range, 8–18), which allowed us to calculate the time spent by parents incubating at nests per hour and the other in the middle of the chick-rearing period (around the 15th day after hatching; range, 10–20) from which we calculated the rate of visits that parents made to feed their offspring in a per hour basis. As identification of parents was not always possible due to their fast entrance in nests, we calculated all variables for both parents together.
Determination of the presence of carotenoid pigments in the different plumage patches
We investigated the presence of carotenoid pigments in feathers by following the method described in McGraw et al. (2005). Briefly, 3–5 mg of coloured parts were cut from feathers of the three body regions (see above) from different individuals and introduced in a glass vial. To extract pigments, 1 ml of acidified pyridine was added to each vial, and then the tubes containing tissues and pyridine were capped tightly and heated in a 95°C water bath for 4 h. After this time, tubes were allowed to cool at room temperature. Pigments were present in the feathers whenever the heated pyridine solution was colourful after the treatment, as occurred for back feathers. As pigments may be carotenoids or other different ones, we proceeded with a follow-up analysis to confirm the presence of carotenoids. In this last test, 2 ml of distilled water were added to the coloured acidified pyridine solution, and then the mixture was capped and homogenised by inversion of the tube. Next, 1 ml of hexane/tert-butyl methyl ether (1:1) was added, the tube capped and the mixture shaken vigorously for 2 min. Finally, the solution was centrifuged at 3,000 rpm for 5 min to see whether the colour was retained in the upper (carotenoids) or bottom (non-carotenoids pigments) phases of the solution.
Colour measurements
For colour measurements, feathers were carefully placed on black paper in a fashion that mimicked the way the feathers naturally lay on the bird. Spectral data was always recorded by the same person (N.S.) in total darkness with an Ocean Optics DH 2000 spectroradiometer. Plumage reflectance was quantified in the range 300–700 nm with a deuterium and a halogen light source using a bifurcated micron fibre optic probe at a 45° angle from the feather surface and illuminating an area of 1 mm2. Using the spectra acquisition software package, OOIBase, we sequentially recorded ten spectra relative to a standard white reference (WS-2) and then averaged the spectra to reduce electrical noise from the collection array within the spectrometer. This process was repeated three times, the probe lifted and replaced on the feather sample between each scan. We then averaged the three spectra for each body region and individual.
Colour variables
We summarised reflectance data using the three standard descriptors of reflectance spectra: brightness, chroma and hue. Brightness or the total amount of light reflected by the feather was calculated as the summed reflectance from 300 to 700 nm (e.g. Siefferman and Hill 2005b). Chroma, a measure of spectral purity, was the ratio of the total reflectance in the range of interest and the total reflectance of the entire spectrum (300–700 nm; Siefferman and Hill 2003). If the spectra showed a bimodal pattern (i.e. head; Fig. 1), two measures of chroma were calculated, each one corresponding to each peak of reflectance (Chroma UV-blue: 300–475 nm; Chroma green-yellow, 475–625 nm). Hue referred to the wavelength at which the maximum peak of reflectance is reached. As for the chroma, when the spectra showed a bimodal pattern, one hue value for each of the two peaks was calculated. The spectra of the chestnut back was invariably truncated at 700 nm (Fig. 1); therefore, we used yellow-red chroma and total brightness to summarise chestnut back spectra. Colour variables were then entered into a principal components analysis (PCA) for each body region separately (e.g. Doucet and Montgomerie 2003, Siefferman and Hill 2005b). In Table 1, component loadings for each PCA are summarised.
PC scores originated from the three PCAs were then used to define inter-individual differences in coloration: Regarding the head, individuals with a high-positive PC1 colour score displayed an overall less saturated UV-blue plumage coloration and high saturated green-yellow plumage coloration in the head and showed a main peak of maximum reflectance at a higher wavelength than individuals with negative PC1 colour scores (Table 1). In addition, individuals with high-positive PC2 scores for the head showed a brighter head and a secondary peak of maximum reflectance at higher wavelengths in the heads than individuals with negative PC2 colour scores (Table 1). Concerning the scapulars, individuals with a high-positive PC1 colour score showed brighter and more saturated UV-blue scapulars and a hue displaced towards shorter wavelengths than individuals with negative PC1 colour scores (Table 1). Regarding the back, individuals with high PC1 colour scores exhibited more brilliant and less saturated yellow-red plumage coloration in the back than individuals with negative PC1 colour scores (Table 1).
Statistical analysis
Analyses were performed using SAS statistical software (SAS 2001 Institute, Cary, NC, USA). Sexual differences in size and coloration were tested by running linear mixed models (MIXED SAS procedure) with identity link function and normal distribution, in which sex and study year were entered as a fixed and a random factor, respectively. When investigating sexual differences in coloration, we also introduced in analyses the body condition as a covariable and the interaction term between this variable and the sex to study the relationship between plumage coloration and body condition in the two sexes. Relationships between different plumage measures of an individual were investigated separately for males and females by Pearson correlations (CORR SAS procedure).
Assortative mating was tested by performing a Pearson correlation matrix between mate colour attributes. Since an exploratory analysis showed that the year was non-significant, we did not perform linear mixed models with year as a random factor.
Relationships between plumage coloration and reproductive variables or measures of parental investment were analysed separately for males and females by means of linear mixed models, except when reproductive success (nesting success versus nesting failure) was the dependent variable for which we used a generalised linear mixed model (GLIMMIX procedure in SAS) with logistic link function and binomial distribution. In these analyses, reproductive variables (laying dates, clutch size, reproductive success and number of fledglings in successful nests) and measures of parental investment (time spent incubating per hour and number of feeding visits per hour) were entered as dependent variables, and all colour variables were entered as covariables. Multicollinearity due to correlation of color of different traits was considered unimportant in these analyses because correlations were largely below 0.7 (Table 3), which is the threshold value over which collinearity should be corrected for (Green 1979). In all models, the year was entered as a random factor, and no interaction terms were considered due to low sample sizes.
Finally, to study the relationship between parent and offspring phenotypes, linear mixed models were run in which the colour attribute of the chick was entered as the dependent variable and colour attributes of the same trait of its parents as covariables. The nest was introduced as a random effect to account for the fact that chicks from the same nest are not independent. We performed backward model selection using P = 0.05 as the threshold value for elimination. Final models only contained significant effects.
Results
Origin of roller coloration
In the roller, only feathers of the back presented pigments, and head and scapulars feathers were structural plumage. In the back, as the bottom phase (pyridine–water phase) of the solution retained the colour of feathers, we concluded that these feathers did not contain carotenoids either, although they seem to contain some other pigment such as phaeomelanin (McGraw et al. 2005). Therefore, we can reasonably assume that the colour traits of rollers that we study here have two different origins: the head and scapulars with a structurally based coloration and the back with a melanin-based coloration.
Reflectance patterns of the three plumage parts
The reflectance patterns of roller head, scapulars and back are shown in Fig. 1. The head showed a bimodal pattern with a main peak in the green-yellow region (475–625 nm, peak around 555 nm) and a secondary peak in the UV-blue region (300–475 nm, peak around 385–390 nm). The scapular region reflected the most strongly in the UV-blue region, with a peak in the blue one (400–475 nm, peak around 440 nm). Finally, the back’s reflectance spectrum increased continuously from the yellow region (from 550 nm) onwards.
Sexual dimorphism and dichromatism
Some of the morphological and plumage attributes varied between the two sexes (Fig. 1, Table 2). Rollers were sexually dimorphic in wing length (Table 2), with males (mean ± SE, n = 20.08 ± 1.32 cm, 36) showing longer wings than females (mean ± SE, n = 19.62 ± 0.94 cm, 43). Furthermore, rollers were sexually dichromatic in structural colorations since head and scapular coloration varied between sexes (Table 2). More specifically, males displayed purer UV-bluish heads than females (i.e. lower PC1 scores for the head than females) and purer UV-bluish and brighter scapulars than females (i.e. higher PC1 scores for the scapulars than female; Fig. 1). Melanin-based backs, however, were sexually monochromatic (Table 2), and no year effect was found. Because of these sexual differences in coloration, all the following analyses were done in males and females separately.
Relationships between plumage measurements
Some of the measurements of plumage coloration were correlated despite the fact that some of these traits have different origins (i.e. pigment versus structural; Table 3). Females that exhibited more green-yellowish heads (i.e. high PC1 scores for the head) displayed less brilliant and purer yellow-red backs (i.e. low PC1 scores for the back; Table 3). On the other hand, males that exhibited more green-yellowish heads (i.e. high PC1 scores for the head) exhibited less brilliant and pure UV-blue scapulars (i.e. low PC1 scores for the scapulars; Table 3). Additionally, males with brighter heads (i.e. high PC2 scores for the head) showed brighter and less pure yellow-red backs (i.e. high PC1 scores for the back; Table 3).
Plumage attributes and body condition
Body condition was positively correlated to plumage brightness in the head (head PC2) (regression coefficient = 0.03) and also positively related to plumage brightness and negatively to yellow-red saturation in the back (back PC1; regression coefficient = 0.03) in the two sexes (Table 2). All other relationships between colour attributes and body condition were non-significant. Furthermore, we did not find any significant year effect in these analyses (Table 2).
Assortative mating
We did not find assortative mating according to tarsus (Pearson correlation coefficient, r = −0.02, P = 0.90, N = 27) and wing length (Pearson correlation coefficient, r = 0.30, P = 0.12, N = 27) but found significant positive assortative mating in weight (Pearson correlation coefficient, r = 0.42, P = 0.03, N = 26, Fig. 2a) and body condition (Pearson correlation coefficient, r = 0.50, P = 0.008, N = 27, Fig. 2b). These two positive relationships were maintained after the removal of outliers (Fig. 2a,b; weight, r = 0.60, P = 0.002, N = 25; body condition, r = 0.42, P = 0.04, N = 25). We further found assortative mating in the degree of green-yellowish colour in the head (Pearson correlation coefficient, r = 0.42, P = 0.03, N = 27, Fig. 2c), as well as in the total brightness of the back (Pearson correlation coefficient, r = 0.41, P = 0.035, N = 27, Fig. 2d). After leaving out outliers (Fig. 2c), we found random mating according to the degree of green-yellowish colour in the head (r = 0.20, P = 0.33, N = 25). Assortative mating was not significant for the remaining colour traits (P > 0.05, N = 27 in all cases).
Plumage attributes and reproductive success and investment
Breeding success correlated positively with female back brightness, dullness and yellow-red colour purity (back PC1) once controlled for laying date (Table 4, regression coefficient = −0.41). Additionally, parental provisioning was correlated in the same way with this maternal trait (Table 4, regression coefficient = −0.83). On the other hand, breeding success was correlated to male head brightness (head PC2; Table 4, regression coefficient = 0.98). Moreover, parental provisioning was higher in nests of males with pure green-yellow heads (head PC1; Table 4, regression coefficient = 0.44). Except for the relationships between clutch size, reproductive success and success of successful nests with laying date, none of the other relationships were significant (Table 4).
Parent–offspring relationships in coloration
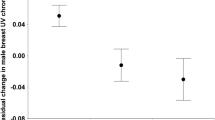
Scapular brightness and UV-blue saturation appeared positively correlated between parents (males and females) and offspring (female scapulars PC1, F 1,51 = 4.29, P = 0.04, regression coefficient = 0.38; male’s scapulars PC1, F 1,51 = 10.65, P = 0.002, regression coefficient = 0.99; nest effect, Z = 2.37, P = 0.009; Fig. 3a). However, only the relationship between male-offspring scapular brightness and UV-blue saturation remained significant after removing the two outliers (Fig. 3a; male’s scapulars PC1, F 1,51 = 9.81, P = 0.003, regression coefficient = 1.03; nest effect, Z = 2.53, P = 0.006). In addition, male back brightness was correlated with that of their chicks (male’s back PC1, F 1,79 = 4.55, P = 0.04, regression coefficient = 0.30; nest effect non-significant; Fig. 3b).
Parent–offspring regressions in plumage coloration in the a scapulars and in the b back. In a, values for offspring are mean values per nest because the random effect of the nest was significant in the analysis (see text). In b, values are given for each chick of each nest because the random effect of the nest was not significant (see text). Arrows indicate outliers that we removed to repeat the analysis
Discussion
The European Roller appears as a sexually dimorphic and dichromatic species. Males have longer wings and exhibit brighter and more saturated UV-blue heads and scapulars than females. Thus, this dichromatism mainly exists in the UV part of the spectrum so that it remains undetected to humans. Birds usually have a tetrachromatic vision (Goldsmith 1980) and can perceive ultra-violets. Such ‘hidden’ sexual dichromatism is common in passerines (Eaton 2005), and our results suggest that this also occurs in non-passerines. Furthermore, recent findings suggest that birds vary in their maximum sensitivity to the lower wavelengths and can be classified as violet or ultraviolet sensitive species (Cuthill et al. 2000). Evidence suggests that rollers are violet sensitive (Ödeen and Håstad 2003), and accordingly, we found that the hue of the head and scapulars is biased toward relatively long wavelengths (violet ~ 390 nm) within the ultraviolet region of the spectrum (see Fig. 1).
We studied the coloration of three different plumage traits that are either structural- (head and scapulars) or melanin-based (back). Despite these differences, we found that variation in coloration from different body regions is correlated within individuals (Table 3). Male and female melanic chestnut back coloration correlated with the structural head coloration. In addition, colorations with the same origin were correlated in males. However, different plumage patches did not provide redundant information. Different aspects of male head coloration predicted body condition, breeding success and food provisioning, while female head coloration only predicted its condition. On the other hand, female but not male back coloration predicted food provisioning and nest breeding success. This suggests that the various colour signals in that species are not redundant and inform on various aspects of the phenotype (Møller and Pomiankowski 1993). Interestingly, results differed in males and females. In the latter, multiple ornaments may function as exclusive indicators of quality (see also Jawor and Breitwisch 2004). But in the former, results suggest that only the head may potentially convey information on individual quality.
Interestingly, our results suggest that structural and melanin-based colorations might be condition-dependent in both sexes. Body condition was correlated with head and back brightness in females and males, and the number of fledglings in successful nests was positively related to male head brightness and to female back brightness. Moreover, female back brightness related to the number of parental provisioning rate. Thus, head and back brightness appear to be indicators of quality in males and females, respectively. This supports the condition-dependent expression of structural coloration found in other species in relation to food restriction (McGraw et al. 2002; Siefferman and Hill 2005a) and parasite load (Hill et al. 2005) for instance. Moreover, our results suggest that female traits in rollers signal phenotypic quality as in other species where males provide a significant amount of parental care and in which mate choice is probably reciprocal (Amundsen et al. 1997; Siefferman and Hill 2005a; Johnsen et al. 1996). As an alternative, however, plumage colour could well express social dominance, and for instance, it may be important in competition for territories, affecting the probability of obtaining a place to breed. This could be one factor leading to mutual ornamentation in the roller whenever both sexes experienced the same selection pressure (Kraaijeveld et al. 2007). Unfortunately, this hypothesis cannot be tested with our data set because our sample is restricted to breeders. Therefore, if plumage colour covaries with social dominance, our data are likely to be biased to more ornamented individuals (those that acquired a breeding territory), and then variability in plumage traits should be limited. Considering this, we can affirm that our data are conservative, which enhances the importance of the found patterns. Finally, it has been shown that mutual ornamentation may result from selection for sexual mimicry in females that frequently interact with courting males (Kraaijeveld et al. 2007). However, rollers usually arrive mated to their breeding territories and, once settled, only very rarely interact with arriving floaters (Parejo, own unpublished data). Nonetheless, it may still be possible that selection for concealing the sex in females was very strong in winter territories or during migration when group-living habits in rollers are common (Cramp and Simmons 1988).
Rollers appear to mate assortatively in relation to weight, body condition, and head green-yellow saturation as well as back brightness. These results are not surprising because these two plumage characteristics seem to indicate individual phenotypic quality. Indeed, parental provisioning rate related to head green-yellow saturation in males and to back brightness in females. In addition, female back brightness correlated with the number of fledglings in successful nests. These relationships between coloration and phenotypic quality may reveal the information conveyed by each colour. Assortative mating based on plumage coloration revealing quality may result from the existence of a mutual choice based on the same ornament. Since the roller males invest in reproduction as much as females, mutual selection would be beneficial. Alternatively, assortative mating may also result from an effect of breeding date if the best individuals arrived earlier to their breeding areas. However, laying date was not significant in all the analyses of assortative mating. Finally, it may be possible that individuals mated assortatively by age and that ornamentation was age-dependent. This might lead to a positive correlation between ornaments of the members of a pair. Therefore, only experiments may allow us to conclude the indicator function of these traits. Interestingly, rollers mated assortatively by two plumage colours of different origin. Positive assortative mating based on structural and melanin-based coloration has been also shown in other birds (e.g. Amundsen et al. 1997; Andersson et al. 1998; Potti and Merino 1996; Roulin 1999).
We finally document a positive correlation between parent and offspring coloration, suggesting some kind of transmission of these traits or similar strong environmental effects on parents and their offspring coloration. This transmission may be of various kinds, from genes to maternal or paternal effects. Indeed, there is some evidence that structural colours may be condition-dependent in nestlings while other colours would be relatively more genetically determined (Johnsen et al. 2003). For instance, the transmission of the melanin-based throat patch of house sparrows, Passer domesticus, was shown to be due to environmental factors (Griffith et al. 1999). In addition, although correlative results in the field remain highly controversial, melanin-based colours can also show high ‘broad sense heritability’ (see revision in Mundy 2006). In the case of the roller, as we did not perform a cross-fostering, the mechanism of transmission cannot be known. Nevertheless, adult back brightness may be under mutual sexual selection, and its transmission from parents to offspring suggests that it may be an important signal in that species. Rollers only make a partial post-juvenile moult that involves head, body, lesser and median upper wing-covers and part of tail (Cramp and Simmons 1988). Furthermore, the first prebreeding moult is rather limited and occasionally absent (Cramp and Simmons 1988). Therefore, first breeders may still have feathers grown as nestlings.
In conclusion, we provide tentative evidence that structural and melanin-based colorations of male and female rollers might function as reliable signals of their phenotypic and parental qualities. Furthermore, the existence of assortative mating on some of these traits may suggest that they may be used in mate choice. We finally document the potential for a transmission of structural and melanin-based plumage coloration from parents to offspring. Altogether, these results give correlative support to the hypothesis that multiple coloured traits may act as quality indicators in the roller and that they may be used by the two sexes to assess potential mate quality mutually. Nonetheless, experimental studies are clearly needed to confirm these interpretations.
References
Amundsen T (2000) Why are female birds ornamented? Trends Ecol Evol 15:149–155
Amundsen T, Pärn H (2006) Female coloration: review of functional and non-functional hypothesis. In: Hill GE, McGraw KJ (eds) Bird coloration, vol. II: function and evolution. Harvard University Press, Harvard, pp 280–348
Amundsen T, Forsgren E, Hansen LTT (1997) On the function of female ornaments: male bluethroats prefer colourful females. Proc R Soc Lond B Biol Sci 264:1579–1586
Andersson M (1994) Sexual selection. Princeton University Press, New Jersey
Andersson S, Örnborg J, Andersson M (1998) Ultraviolet sexual dimorphism and assortative mating in blue tits. Proc R Soc Lond B Biol Sci 265:445–450
Andersson S, Pryke SR, Örnborg J, Lawes MJ, Andersson M (2002) Multiple receivers, multiple ornaments, and a trade-off between agonistic and epigamic signaling in a widowbird. Am Nat 160:683–691
Avilés JM (2006) Carraca europea–Coracias garrulus. In: Carrascal LM, Salvador A (eds). Enciclopedia Virtual de los Vertebrados Españoles. Museo Nacional de Ciencias Naturales. http://www.vertebradosibericos.org
Avilés JM, Sánchez JM, Sánchez A, Parejo D (1999) Breeding biology of the Roller Coracias garrulus in farming areas of the southwest Iberian Peninsula. Bird Study 46:217–223
Avilés JM, Sánchez JM, Parejo D (2000) Nest-site selection and breeding success in the Roller (Coracias garrulus) in the Southwest of the Iberian península. Journal für Ornithologie 141:345–350
Avilés JM, Solís E, Valencia J, de la Cruz C, Sorci G (2008) Female and male plumage brightness correlate with nesting failure in azure-winged magpies. J Avian Biol 39:257–261
Badyaev AV, Hill GE (2000) Evolution of sexual dichromatism: contribution of carotenoid-versus melanin-based coloration. Biol J Linn Soc 69:153–172
Bize P, Gasparini J, Klopfenstein A, Altwegg R, Roulin A (2006) Melanin-based coloration is a nondirectionally selected sex-specific signal of offspring development in the Alpine swift. Evolution 60:2370–2380
Cramp S, Simmons KEL (1988) The birds of the western Palearctic, vol. V. Oxford University Press, Oxford
Cuthill IC, Partridge JC, Bennett ATD, Church SC, Hart NS, Hunt S (2000) Ultraviolet vision in birds. Adv Stud Behav 29:159–214
Darwin C (1871) The descent of man and selection in relation to sex. London, Murray
Doucet SM (2002) Structural plumage coloration, male body size, and condition in the blue-black grassquit. Condor 104:30–38
Doucet SM, Montgomerie R (2003) Multiple sexual ornaments in satin bowerbirds: ultraviolet plumage and bowers signal different aspects of male quality. Behav Ecol 14:503–509
Eaton MD (2005) Human vision fails to distinguish widespread sexual dichromatism among sexually “monochromatic” birds. Proc Natl Acad Sci USA 102:10942–10946
Emlen DJ, Hunt J, Simmons LW (2005) Evolution of sexual dimorphism and male dimorphism in the expression of beetle horns: phylogenetic evidence for modularity, evolutionary lability, and constraint. Am Nat 166:S42–S68
Fargallo JA, Laaksonen T, Korpimäki E, Wakamatsu K (2007) A melanin-based trait reflects environmental growth conditions of nestling male Eurasian kestrels. Evol Ecol 21:157–171
Fridolfsson AK, Ellegren H (1999) A simple and universal method for molecular sexing of non-ratite birds. J Avian Biol 30:116–121
Grafen A (1990) Sexual selection unhandicapped by the Fisher process. J Theor Biol 144:473–516
Goldsmith TH (1980) Hummingbirds see near ultraviolet light. Science 207:786–788
Green R (1979) Sampling design and statistical methods for environmental biologists. Wiley, New York
Griffith SC, Owens IPF, Burke T (1999) Environmental determination of sexually selected trait. Nature 400:358–360
Griffith SC, Parker TH, Olson VA (2006) Melanin- versus carotenoid-based sexual signals: is the difference really so black and red? Anim Behav 71:749–763
Hamilton WD, Zuk M (1982) Heritable true fitness and bright birds: a role for parasites? Science 218:384–387
Heywood JS (1989) Sexual selection by the handicap mechanism. Evolution 43:1387–1397
Hill GE (1990) Female house finches prefer colourful males: sexual selection for a condition-dependent trait. Anim Behav 40:563–572
Hill GE (2006) Environmental regulation of ornamental coloration. In: Hill GE, McGraw KJ (eds) Bird coloration, vol. 1: Mechanism and measurements. Harvard University Press, Harvard, pp 507–560
Hill GE, Doucet SM, Buchholz R (2005) The effect of coccidial infection on iridescent plumage coloration in wild turkeys. Anim Behav 69:387–394
Hill GE, McGraw KJ (2006a) Preface. In: Hill GE, McGraw KJ (eds) Bird coloration, vol. 1: Mechanism and measurements. Harvard University Press, Harvard, pp vii–viii
Hill GE, McGraw KJ (2006b) Bird coloration, vol. 1: Mechanism and measurements. Harvard University Press, Harvard
Hoelzer GA (1989) The good parent process of sexual selection. Anim Behav 38:1067–1078
Huxley JS (1914) The courtship habits of the great crested grebe (Podiceps cristatus); with an addition to the theory of sexual selection. Proc R Soc Lond B Biol Sci 35:491–562
Jawor JM, Breitwisch R (2004) Multiple ornaments in male northern cardinals, Cardinalis cardinalis, as indicators of condition. Ethology 110:113–126
Johnsen TS, Hengeveld JD, Blank JL, Yasukawa K, Nolan V (1996) Epaulet brightness and condition in female redwinged blackbirds. Auk 113:356–362
Johnsen A, Delhey K, Andersson S, Kempenaers B (2003) Plumage colour in nestling blue tits: sexual dichromatism, condition dependence and genetic effects. Proc R Soc Lond B Biol Sci 270:1263–1270
Johnstone RA (1997) The tactics of mutual mate choice and competitive search. Behav Ecol Sociobiol 40:51–59
Johnstone RA, Reynolds JD, Deutsch JC (1996) Mutual mate choice and sex differences in choosiness. Evolution 50:1382–1391
Keyser AJ, Hill GE (1999) Condition-dependent variation in the blue-ultraviolet coloration of a structurally based plumage ornament. Proc R Soc Lond B Biol Sci 266:771–777
Keyser A, Hill GE (2000) Structurally based plumage coloration is an honest signal of quality in male blue grosbeaks. Behav Ecol 11:202–209
Kodric-Brown A, Brown JH (1984) Truth in advertising: the kinds of traits favored by sexual selection. Am Nat 124:309–323
Kokko H, Johnstone RA (2002) Why is mutual mate choice not the norm? Operational sex ratios, sex roles and the evolution of sexually dimorphic and monomorphic signalling. Proc R Soc Lond B Biol Sci 357:319–330
Kraaijeveld K, Kraaijeveld-Smit FJL, Komdeur J (2007) The evolution of mutual ornamentation. Anim Behav 74:657–677
Lande R (1980) Sexual dimorphism, sexual selection, and adaptation in polygenic characters. Evolution 34:292–305
Ligon JD, Zwartjes PW (1995) Ornate plumage of male red junglefowl does not influence mate choice by females. Anim Behav 49:117–125
McGraw KJ, Hill GE (2000) Differential effects of endoparasitism on the expression of carotenoid- and melanin-based ornamental coloration. Proc R Soc Lond B Biol Sci 267:1525–1531
McGraw KJ, Mackillop EA, Dale J, Hauber ME (2002) Different colors reveal different information: how nutritional stress affects the expression of melanin- and structurally based ornamental plumage. J Exp Biol 205:3747–3755
McGraw KJ, Hudon J, Hill GE, Parker RS (2005) A simple and inexpensive chemical test for behavioral ecologists to determine the presence of carotenoid pigments in animal tissues. Behav Ecol Sociobiol 57:391–397
Møller AP, Pomiankowski A (1993) Why have birds got multiple sexual ornaments. Behav Ecol Sociobiol 32:167–176
Mundy NI (2006) Genetic basis of color variation in wild birds. In: Hill GE, McGraw KJ (eds) Bird coloration, vol. 1: Mechanisms and measurements. Harvard University Press, Harvard, pp 469–506
Ödeen A, Håstad O (2003) Complex distribution of avian color vision systems revealed by sequencing the SWS1 opsin from total DNA. Mol Biol Evol 20:855–861
Omland KE (1996) Female mallard mating preferences for multiple male ornaments. Behav Ecol Sociobiol 39:353–36
Parejo D, Silva N, Avilés JM (2007) Within-brood size differences affect innate and acquired immunity in roller Coracias garrulus nestlings. J Avian Biol 38:717–725
Potti J, Merino S (1996) Decreased levels of blood trypanosome infection correlate with female expression of a male secondary sexual trait: Implications for sexual selection. Proc R Soc Lond B Biol Sci 263:1199–1204
Roulin A (1999) Nonrandom pairing by male barn owls (Tyto alba) with respect to a plumage trait. Behav Ecol 10:688–695
Roulin A, Dijkstra C (2003) Genetic and environmental components of variation in eumelanin and phaeomelanin sex-traits in the barn owl. Heredity 90:359–364
Servedio MR, Lande R (2006) Population genetic models of male and mutual mate choice. Evolution 60:674–685
Siefferman L, Hill GE (2003) Structural and melanin coloration indicate parental effort and reproductive success in male eastern bluebirds. Behav Ecol 14:855–861
Siefferman L, Hill GE (2005a) Evidence for sexual selection on structural plumage coloration of female eastern bluebirds. Evolution 59:1819–1828
Siefferman L, Hill GE (2005b) Blue structural coloration of male eastern bluebirds Sialia sialis predicts incubation provisioning to females. J Avian Biol 36:488–493
Solís E, Avilés JM, de la Cruz C, Valencia J, Sorci G (2008) Winter male plumage coloration correlates with breeding status in a cooperative breeding species. Behav Ecol 19:391–397
Trivers RL (1972) Parental investment and sexual selection. In: Campbell B (ed) Sexual selection and the descent of man 1871–1971. Aldine, Chicago, IL, pp 136–179
Veiga JP, Puerta M (1996) Nutritional constraints determine the expression of a sexual trait in the house sparrow, Passer domesticus. Proc R Soc Lond B Biol Sci 263:229–234
Von Schantz TV, Bensch S, Grahn M, Hasselquist D, Wittzell H (1999) Good genes, oxidative stress and condition-dependent sexual signals. Proc R Soc Lond B Biol Sci 266:1–12
West-Eberhard MJ (1979) Sexual selection, social competition, and evolution. P Am Phil Soc 123:222–234
Zahavi A (1975) Mate selection—a selection for a handicap. J Theor Biol 53:205–214
Zahavi A, Zahavi A (1997) The Handicap Principle: a missing piece of Darwin’s puzzle. Oxford University Press, Oxford
Zuk M, Thornhill R, Ligon JD, Johnson K, Austad S, Ligon S, Thornhill N, Costin C (1990) The role of male ornaments and courtship behavior in female choice of red jungle fowl. Am Nat 136:459–473
Zuk M, Ligon JD, Thornhill R (1993) Effects of experimental manipulation of male secondary sex characters on female mate preference in red jungle fowl. Anim Behav 44:999–1006
Acknowledgments
We thank all the people who collaborated in data collection either in the field (L. Derousse, M. Guillemin, M. Kauffman, M. Kriloff, C. Landsmann, V. Lartigot, X. Mandine and G. Martinerie) or in the laboratory (J. M. Gasent). We also thank I. Cuthill and one anonymous referee for constructive comments that improved our manuscript. Fieldwork was done under the permission of the Junta de Extremadura and in compliance with the Spanish laws. This research work was partially supported by a doctoral grant to NS by the European Social Fund, an I3P contract to DP funded by the European Social Fund and by the Spanish Ministerio de Educación y Ciencia-FEDER, Secretaría de Estado de Universidades e Investigación, (project ref. CGL2005-04654/BOS).
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by I. Cuthill
Rights and permissions
About this article
Cite this article
Silva, N., Avilés, J.M., Danchin, E. et al. Informative content of multiple plumage-coloured traits in female and male European Rollers. Behav Ecol Sociobiol 62, 1969–1979 (2008). https://doi.org/10.1007/s00265-008-0628-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00265-008-0628-y