Abstract
Small mammal populations often exhibit large-scale spatial synchrony, which is purportedly caused by stochastic weather-related environmental perturbations, predation or dispersal. To elucidate the relative synchronizing effects of environmental perturbations from those of dispersal movements of small mammalian prey or their predators, we investigated the spatial dynamics of Microtus vole populations in two differently structured landscapes which experience similar patterns of weather and climatic conditions. Vole and predator abundances were monitored for three years on 28 agricultural field sites arranged into two 120-km-long transect lines in western Finland. Sites on one transect were interconnected by continuous agricultural farmland (continuous landscape), while sites on the other were isolated from one another to a varying degree by mainly forests (fragmented landscape). Vole populations exhibited large-scale (>120 km) spatial synchrony in fluctuations, which did not differ in degree between the landscapes or decline with increasing distance between trapping sites. However, spatial variation in vole population growth rates was higher in the fragmented than in the continuous landscape. Although vole-eating predators were more numerous in the continuous agricultural landscape than in the fragmented, our results suggest that predators do not exert a great influence on the degree of spatial synchrony of vole population fluctuations, but they may contribute to bringing out-of-phase prey patches towards a regional density level. The spatial dynamics of vole populations were similar in both fragmented and continuous landscapes despite inter-landscape differences in both predator abundance and possibilities of vole dispersal. This implies that the primary source of synchronization lies in a common weather-related environment.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Spatial synchrony in population fluctuations has been documented in a diverse group of taxa, accompanied by an equally diverse range in the geographic extent of synchrony (reviewed by, e.g. Liebhold et al. 2004). Large-scale patterns of spatial synchrony, i.e. covering hundreds of square kilometres, have most often been associated with species exhibiting cyclic population dynamics, including lepidoptera (e.g. Myers 1998; Williams and Liebhold 2000; Klemola et al. 2006), game birds (e.g. Ranta et al. 1995a; Cattadori et al. 1999) and mammals (e.g. Steen et al. 1996; Bjørnstad et al. 1999a; Huitu et al. 2003; Stenseth et al. 2004a). Small rodents, for example voles, at northern latitudes demonstrate synchronous fluctuations across distances of some tens to several hundreds of kilometres (Steen et al. 1996; Bjørnstad et al. 1999a; Huitu et al. 2003; Sundell et al. 2002).
Spatial synchronization of population fluctuations has been suggested to occur through three possible, mutually nonexclusive mechanisms: (1) stochastic, spatially correlated, climate-related environmental perturbations (i.e. Moran effect; Moran 1953; Ranta et al. 1995b, 1999; Grenfell et al. 1998; Koenig 2002; Stenseth et al. 1999, 2004a), (2) predation by mobile enemies (Ydenberg 1987; Heikkilä et al. 1994; Norrdahl and Korpimäki 1996; Ims and Andreassen 2000; Korpimäki et al. 2002; Sundell et al. 2002), or (3) dispersal movements of the focal organisms themselves (Sutcliffe et al. 1996; Blasius et al. 1999; Paradis et al. 1999; Schwartz et al. 2002; Ranta et al. 2006). It has remained challenging to tease apart the synchronizing effects of dispersal (of either prey or predator) and environmental stochasticity on spatial population dynamics (e.g. Paradis et al. 1999; Ranta et al. 1999, 2006; Kendall et al. 2000; Ripa 2000; Abbott 2007). Some studies have managed to control for dispersal as a synchronizing factor, either by measuring synchrony among isolated island populations (Heikkilä et al. 1994; Grenfell et al. 1998) or by experimental confinement (Holyoak and Lawler 1996; Holyoak 2000; Ims and Andreassen 2000; Huitu et al. 2005). Unfortunately, neither approach is practically applicable to all species and spatial scales at which synchrony is observed.
An alternative approach for elucidating the relative effects of environmental stochasticity and dispersal on spatial synchrony is to carry out studies in areas with similar environmental weather conditions but naturally varying degrees of restriction on the dispersal movements of both prey and predator. For most animal species, landscape fragmentation provides this setting, as movement rates of animals between habitat patches decrease with increasing fragmentation (Diffendorfer et al. 1995; Ranta et al. 1995b; Wolff et al. 1997; Debinski and Holt 2000). Increasing fragmentation is thereby expected to decrease the degree of synchrony among local populations if synchrony is maintained by the movements of prey or predator (e.g. Petty et al. 2000; Bellamy et al. 2003; Huitu et al. 2003).
Recent evidence suggests that the large-scale spatial synchrony observed among populations of northern voles is not influenced to any great degree by the dispersal of voles themselves (Ims and Andreassen 2000, 2005; Sundell et al. 2002; Huitu et al. 2005). However, landscape fragmentation might also influence the temporal and spatial patterns of vole dynamics through negative effects on predator movements (e.g. Huffaker 1958; Bernstein et al. 1991; With et al. 2002; Ryall and Fahrig 2006). Increasing limitation on predator movements in a landscape may, in fact, inhibit the generation of predator–prey population cycles altogether (De Roos et al. 1991).
The aim of this study is to determine the effects of landscape structure on the patterns and degree of spatial synchrony in the fluctuations of agricultural farmland-inhabiting Microtus vole populations. Previous work has indicated that the collective effect of landscape fragmentation and more continental weather conditions is negatively associated with the degree of spatial synchrony among Microtus vole populations (Huitu et al. 2003). To elucidate the relative contributive effects of these two factors on the spatial dynamics of vole populations, we monitored vole dynamics for three years in an area which exhibits uniform weather conditions but contrasting degrees of landscape fragmentation. During this time, predator abundances were also monitored. We predicted that if the degree of spatial synchrony in vole population fluctuations is influenced by the movements of voles or their predators, a more fragmented landscape would be associated with reduced spatial synchrony.
Materials and methods
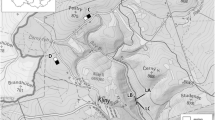
The study was conducted in western Finland in a ca. 120 × 60 km area centred around 63°N, 23°E (Fig. 1). The landscape of the area is largely agricultural, with a municipality-wise mean percentage of farmland approaching 20–30% (Huitu et al. 2003). Overall, the landscape is a mosaic of agricultural and forest patches of varying size and degree of interconnectivity. Long uniform expanses of agricultural farmland can be found bordering larger river systems, whereas farmland patches further from large water bodies are smaller and more isolated from one another. The area is climatically uniform and exhibits relatively mild winters with little snow, at least when considering its location at latitude 63°N (Solantie et al. 1996; Solantie 2000; see also Huitu et al. 2003; Fig. 1). Snow covers the ground for ca. 125–150 days from the beginning of November to mid-April, with a mean maximum depth of ca. 30 cm (data from the Kauhava municipality; FMI 1994).
Map of the study area in western Finland. On the left-hand map, dark grey shading indicates sea, light grey shading agricultural field areas, and white all remaining types of land cover, including mainly forest, but also peatland bogs, lakes and urban areas. The line-connected filled symbols indicate trapping sites on the continuous agricultural landscape transect, and open symbols sites on the fragmented landscape transect. Contours with adjoined numerical figures on the right-hand map indicate climatic zones represented by the 1971–2000 mean annual number of days with snow cover [contours reproduced from: http://www.fmi.fi/saa/tilastot_10.html (Finnish Meteorological Institute)]. Map reproduced with permission from the National Land Survey of Finland (permit no. 596/MML/07)
The two most numerous agriculture-affiliated small rodent species in the area are the closely related field vole (Microtus agrestis) and the sibling vole (M. rossiaemeridionalis). The two species similarly inhabit primarily fields and meadows in agricultural surroundings and are only rarely encountered in forests or bogs in our study area (Norrdahl and Korpimäki 1993, 2005; see also Hansson 1994). Both species exhibit well-documented three-year population cycles (Korpimäki et al. 2005). Together, these rodents support a substantial number of both avian and mustelid predators, e.g. the Eurasian kestrel (Falco tinnunculus), the common buzzard (Buteo buteo), the short-eared owl (Asio flammeus), the long-eared owl (A. otus), Tengmalm’s owl (Aegolius funereus), the least weasel (Mustela nivalis) and the stoat (M. erminea). Predator numbers are strongly determined by the abundance of their rodent prey (Korpimäki and Norrdahl 1991; Korpimäki et al. 1991; Norrdahl and Korpimäki 2002).
To quantify fluctuations in the abundances of these voles and their predators, we determined two transects running in a roughly north–south direction through the study area using a landscape map (Fig. 1). One transect was set in an entirely continuous agricultural tract of the area (hereafter called continuous transect/landscape) and the setting for the other (hereafter called fragmented transect/landscape) was such that all trapping sites were isolated from their neighbouring sites by variable amounts of forests, rivers and peatland bogs to act as dispersal barriers or hindrances for voles (Fig. 1). Also predators, particularly small mustelids, may experience non-agricultural matrix habitats as hindrances for movement (Klemola et al. 1999). Both transects were divided into a northern and a southern half to accommodate trapping schedule restraints (see below). Seven trapping areas per transect half were initially selected on a 1:200,000 scale landscape map in a symmetric configuration which produces an even distribution of pairwise distances between trapping sites while reducing site redundancy (see Koenig 1999) (Fig. 1). On each transect half, trapping areas were located ca. 5–15 km apart, with increasing inter-area distances towards the middle of the transect half (see Fig. 1).
All trapping sites were ultimately selected in situ within each map-designated area as fields or meadows containing open ditches old enough to have been inhabited by voles for several years (judged from the appearance of runways on ditch banks). Vole trapping was carried out at the same sites twice a year, every May and October, for three years. At one site in autumn 2002, trapping had to be carried out in a habitat-wise similar neighboring meadow ca. 50 m away from the original ditches due to chemical grass control measures by a local farmer. Due to the change in trapping ditches, the population growth rate value for October 2002–May 2003 was excluded from that series for analyses.
One trapping session lasted eight days, with one-half (northern or southern) of each transect trapped alternately for two days each. Altogether 50 mouse snap traps were set in four lines in two or four separate ditches, depending on site characteristics. Each line was located 15–30 m from the next, and consisted of four trap stations located 15 m apart. Each station was set with three traps (two stations had four traps) along separate vole runways at 1–2 m intervals. Due to the expectance of high vole numbers in autumn 2002, we increased the total number of traps per site to 60 to reduce risk of trap saturation (Hansson 1975). During this session, the traps were set in a configuration of three traps per station with five stations along each of four lines. Traps were baited with pieces of mixed-grain bread and collected after two days with no check after the first day. Vole indices are expressed as pooled numbers of field and sibling voles trapped per 100 trap nights (site-specific number of traps set × number of nights they were set). Pooling of the Microtus species was justified on the basis of strong interspecific synchrony and habitat overlap (Huitu et al. 2004; Korpimäki et al. 2005).
We calculated indices of predator abundance at each site and trapping session from observations of vole-eating avian (Eurasian kestrels, common buzzards, rough-legged buzzards Buteo lagopus, hen harriers Circus cyaneus, short-eared owls, long-eared owls and great grey shrikes Lanius excubitor) and mammalian (least weasels, stoats and cats Felis catus) predators. During the setting of the vole traps, and for 30 min prior to their removal, one person surveyed the surroundings of the each site for predators at an approximate radius of 500 m, using binoculars and a spotting scope. Because all the trapping and survey areas were on open agricultural farmland, the visibility of predators was similar in continuous and fragmented landscapes. The point survey method has earlier been used to estimate the number of hunting avian predators in open country (Fuller 1981), which is also closely correlated with their breeding densities (Norrdahl and Korpimäki 1995).
Small mustelid abundance was also monitored at the sites using track stations (see King and Edgar 1977). Briefly, a 7 × 50 cm acrylic glass strip, equipped in the middle with an ink pad and at each end with pieces of brown packaging paper (see King and Edgar 1977 for the chemical treatment of ink pad and papers), was inserted in a 55-cm-long, 10-cm-diameter black drainage pipe and placed in a vole runway or a similar passage at the end of a trapping ditch. Four track stations were placed at each site for the duration of the vole trapping. Tracks of mustelid predators in a track station were regarded as one observation. Although same individual predators may have been observed both on the day of trap setting and during the actual observation period two days later (e.g. nearby nesting raptors), all observations were summed for a given site to provide an index of predator abundance.
Statistical analyses
As landscape type is used in this study as a two-level class variable, and because differences in the degree of connectivity between agricultural field sites are highly obvious (Fig. 1), we chose not to quantify the degree of connectivity between the two landscapes quantitatively. Characteristics of Microtus vole population dynamics were quantified for each trapping site over the three-year study period with mean trap indices, coefficients of variation (CV; i.e. the ratio of the standard deviation to the mean), and S-indices [standard deviation of log10-transformed data; (Lewontin 1966)], which are commonly used measures of relative density variability in fluctuating populations (Hansson and Henttonen 1985; Turchin 2003). We tested whether the overall signature of vole dynamics differed between the continuous and fragmented landscapes with linear mixed models (PROC MIXED, SAS® v. 9.1 statistical software), with the three indices as dependent variables, landscape type as a fixed explanatory variable and transect half (north or south) as a random block factor to control for variation in e.g. the effects of weather on trapping success. The denominator degrees of freedom for the analyses were computed with the Satterthwaite method (Littell et al. 1996).
The degree and extent of spatial synchrony among the vole populations were assessed for both transects separately by calculating Pearson correlation coefficients between series of population growth rates [ln(n t+1/n t ), where n t is vole trapping index at time t]. Correlation coefficients were calculated for all possible pairwise combinations of sites, separately for both landscapes. By using population growth rates, we focus specifically on synchrony in population change rather than on abundance (Bjørnstad et al. 1999b). Prior to analysis, all values of zero were replaced by the minimum trap index value, which corresponds to one vole trapped during the session (Turchin 2003).
Due to the fact that spatially nearby sites are statistically nonindependent (e.g. Ranta et al. 1995b; Bjørnstad et al. 1999b; Koenig 1999), we executed a bootstrap procedure to estimate 95% confidence limits for the mean level of synchrony in each landscape, and for the coefficient of correlation between pairwise synchrony and distance between sites. As advocated by Lillegård et al. (2005), we drew 1,000 bootstrap replicates of the population growth rate series by resampling (with replacement) time points instead of sites. Thus, each bootstrap replicate randomly generated a new time series of population growth rates for each site from the original growth rate values. We then calculated the mean of all pairwise correlation coefficients between the sites for each bootstrap replicate, as well as a mean correlation coefficient for pairwise correlations with inter-site distance. The resulting distributions of 1,000 correlation coefficient values were used to determine the 95% confidence limits for the observed level of synchrony (obtained from the original data) and the correlation with inter-site distance. The confidence limit values equal the 2.5 and 97.5% percentiles of the generated distributions of correlation coefficients (Manly 1997). The mean level of spatial synchrony, as well as the correlation between synchrony and inter-site distance, was deemed significant if the bootstrap-generated confidence intervals did not contain zero.
Landscape-related differences in predator abundance during the study were analyzed separately for avian and mammalian predators with repeated measures linear mixed models, with landscape and time as explanatory variables and transect half as a random block factor to control for variation in the effects of weather on predator activity. Using analyses of covariation, we also estimated the effects of landscape type and predator abundance on spatial variation in vole abundance and population growth rate. Predator abundances were expressed as the mean number of observations across all sites per landscape type per trapping session. Spatial variation in vole abundance and population growth rate were similarly measured as coefficients of variation (CV) across all sites per landscape type per trapping session. Nonsignificant interaction terms were omitted from the models. All analyses involving predators were executed with PROC MIXED, SAS®.
Results
Neither the mean (±SE) number of Microtus voles trapped per trapping site (9.6 ± 2.2 in the fragmented landscape, 8.1 ± 2.2 in the continuous landscape; F (1,25) landscape = 1.93, P = 0.18) nor the relative variability of vole abundance differed among the landscapes (CV: fragmented, 93.6 ± 6.6; continuous, 98.7 ± 6.6; F (1,26) landscape = 0.29, P = 0.59; S-index: fragmented, 0.78 ± 0.10; continuous, 0.69 ± 0.10; F (1,25) landscape = 0.77, P = 0.39). However, spatial variation in vole population growth rates was higher in the fragmented than in the continuous landscape (F (1,6) landscape = 20.20, P < 0.01; see Fig. 2 for population trajectories) and also associated with predator abundances (see details below).
Population growth rates of Microtus voles fluctuated synchronously within both fragmented and continuous landscapes. The degree of synchrony did not differ among the landscapes, as judged from overlapping confidence limits (fragmented landscape: mean pairwise correlation 0.45, bootstrapped 95% confidence limits 0.32–0.55; continuous landscape, mean 0.57, 95% c.l. 0.12–0.79; Fig. 3). The degree of spatial synchrony did not decline notably with increasing distance between sites in either landscape (fragmented, correlation between synchrony and distance 0.02, bootstrapped 95% confidence limits −0.04 to +0.18; continuous, correlation −0.10, 95% c.l. −0.17 to +0.10; Fig. 3).
The relationship between the degree of synchrony between vole population fluctuations among trapping sites and inter-site distance. Each symbol represents a Pearson correlation coefficient between the population growth rate time series of two trapping sites. Correlation coefficients were calculated for all possible pairwise combinations of sites, separately for both landscapes. Filled symbols and open symbols indicate pairwise correlations in the continuous and the fragmented landscapes, respectively
Both avian (F (1,155) landscape = 5.86, P = 0.02; F (5,155) time = 3.57, P < 0.01; F (5,155) landscape × time = 0.28, P = 0.92) and mammalian (F (1,155) landscape = 4.01, P = 0.047; F (5,155) time = 1.87, P = 0.10; F (5,155) landscape × time = 0.89, P = 0.49) predators were more abundant in the continuous landscape than in the fragmented landscape (Fig. 4). As mentioned, spatial variation in vole abundance tended to be negatively associated with the abundance of avian predators (parameter estimate ± SE = −59.8 ± 26.7; F (1,8) av. pred. = 5.03, P = 0.055; F (1,8) mamm. pred. = 0.06, P = 0.82; F (1,8) landscape = 2.49, P = 0.15; Fig. 5a, b). Spatial variation in vole population growth rates was higher in the fragmented landscape and positively associated with mammalian predator abundance; the same tended to apply also to avian predators (parameter estimate ± SE = 5.0 ± 2.2; F (1,6) av. pred. = 5.29, P = 0.061; parameter estimate ± SE = 17.8 ± 2.4; F (1,6) mamm. pred. = 56.81, P < 0.001; F (1,6) landscape = 20.20, P < 0.01; Fig. 5c, d).
Relationships between the trapping session-specific mean predator abundance at trapping sites and spatial variation in Microtus vole population fluctuations, measured as coefficients of variation (CV) of vole trapping indices or population growth rates. Filled symbols indicate trapping sessions in the continuous agricultural landscape and open symbols those in the fragmented landscape
Discussion
We monitored the temporal and spatial dynamics of Microtus vole population fluctuations for three years in both fragmented and continuous agricultural landscapes in western Finland, in an area exhibiting uniform climatic conditions. The temporal dynamics of vole populations proved to be similar in both landscapes and well-synchronized spatially. Neither the mean degree of spatial synchrony nor its relation to distance between trapping sites differed quantitatively between the landscapes. However, spatial variation in vole population growth rates was higher in the fragmented than in the continuous landscape. Predators were found to be more numerous in the continuous landscape. Spatial variation in vole abundance tended to be negatively associated with the abundance of avian predators, while spatial variation in vole population growth rates was clearly positively associated with the abundance of mammalian predators, and weakly so with the abundance of avian predators.
The Moran effect and dispersal are considered the two most plausible mechanisms for the generation of spatial synchrony (reviewed by, e.g. Bjørnstad et al. 1999b; Koenig 1999; Liebhold et al. 2004; Ranta et al. 2006). However, in the case of vole populations, the role of dispersal as a synchronizing mechanism appears to be minor and restricted to relatively small spatial scales (Steen et al. 1996; Bjørnstad et al. 1999a; Sherratt et al. 2000; Ims and Andreassen 2005; Huitu et al. 2005). Both mechanisms have a tendency to produce identical patterns of synchrony, namely that synchrony declines with increasing distances between sites (e.g. Steen et al. 1996; Sutcliffe et al. 1996; Paradis et al. 1999; Ranta et al. 1999, 2006). We did not find such a relationship in either landscape type, despite inter-site distances approaching 120 km (Fig. 3).
It is well documented that increasing habitat fragmentation reduces inter-patch movements in small mammals (e.g. Diffendorfer et al. 1995; Wolff et al. 1997; Debinski and Holt 2000), which thereby translates into a decrease in the synchronizing effect of dispersal. The fact that we did not observe a negative relationship between synchrony and inter-site distance, even among populations in the fragmented landscape (Fig. 3; see Bellamy et al. 2003), can be regarded as evidence against vole dispersal acting as a major synchronizing mechanism in our study system.
However, predators may also exhibit reduced rates of mobility between prey patches that are separated by suboptimal habitat (Huffaker 1958; Bernstein et al. 1991, 1999; With et al. 2002; Ryall and Fahrig 2006). Assuming that the synchronizing effect of predators relies on an efficient spatial response to prey densities (Ydenberg 1987; Korpimäki and Norrdahl 1991), increasing landscape fragmentation will reduce this effect. Although we found that the abundance of predators was higher in the continuous landscape than in the fragmented (Fig. 4), this was not reflected as inter-landscape differences in the degree of spatial synchrony among vole populations. This, in turn, suggests that predators are not the primary source of synchronization among vole populations at the spatial scale examined. This is supported by the fact that the temporal dynamics of vole fluctuations were essentially the same in both landscapes and thus appeared not to be influenced by inter-landscape differences in predator activity (see De Roos et al. 1991).
We did find that increasing avian predator abundance tended to be negatively associated with spatial variation in vole numbers (Fig. 5a) and positively with spatial variation in vole population growth rates (Fig. 5c). Similar findings were presented by Norrdahl and Korpimäki (1996), who showed that an experimental reduction of vole-eating avian predators led to increased variation in vole densities between areas. Vole-eating avian predators have been shown to be capable of efficiently locating and utilising hot spots where prey densities are higher than in the landscape on average (Viitala et al. 1995; Ims and Andreassen 2000).
Similarly, mammalian predator abundance was positively associated with spatial variation in the growth rates of vole populations (Fig. 5d; see also Fig. 2 for population trajectories). This may also be indicative of hot spot predation; selective predation in prey patches with densities above the landscape average results in vole populations declining with negative growth rates while below-average density patches increase, in the absence of predation, towards the landscape average. Although predator abundance does appear to be dynamically associated with the spatial dynamics of their vole prey, causalities are hard to infer from our data. However, our results do appear to corroborate earlier experimental findings that both avian and mammalian predators are involved in reducing spatial variation in vole abundance (Norrdahl and Korpimäki 1996; Ims and Andreassen 2000; Korpimäki et al. 2002).
Seeing that opportunities for vole dispersal and predator abundance differed between the landscapes while climatic conditions or the degree of vole population synchrony did not, climate-related environmental conditions appear by exclusion to be the most plausible common denominator to account for similarities in patterns of spatial synchrony. In a previous study, Microtus vole dynamics were found to be spatially better synchronized in an agricultural landscape exhibiting relatively mild winters than in a forested landscape with more continental climatic conditions, including more snow (Huitu et al. 2003). It was suggested that the observed difference was primarily due to differences in landscape structure, although possible effects of varying climatic conditions could not be entirely ruled out (Huitu et al. 2003).
Even subtle differences in climatic conditions between adjacent areas may produce distinct differences in the spatial dynamics of animal populations (Rueness et al. 2003; Stenseth et al. 2004b; Klemola et al. 2006). For example, Stenseth and coworkers (2004b) demonstrated that the spatial dynamics of lynx (Lynx canadensis) differ on the two sides of a nongeographic barrier in response to differences in the physical properties of snow cover. Similarly, varying levels of snow thickness may have differing effects on the dynamics of vole populations, for example through the effectiveness of their generalist predators (Hansson and Henttonen 1985; Hanski et al. 1991). In areas with relatively little snow, sporadic warm spells during winter may also result in ice formation on the ground, which restricts access to food resources and reduces vole survival (Aars and Ims 2002; Korslund and Steen 2006), while in areas with a more stable snow cover these effects may not occur. Such effects may have contributed to the inter-areal differences observed in vole population synchrony as reported in Huitu et al. (2003).
We did not find unanimous support for our main prediction, in that a greater degree of landscape fragmentation was not associated with a lower degree of spatial synchrony in the dynamics of Microtus voles, despite differences in landscape structure and in predator abundance between the landscapes. This suggests that the source of spatial synchronization of vole dynamics lies primarily in a synchronous stochastic environment, most likely related to weather conditions. However, the degree of fragmentation in this study may not have been severe enough to inhibit the movements of predators, particularly avian predators, which may also redistribute themselves every spring according to prey densities before settling to breed in their summer territories (Norrdahl and Korpimäki 2002). Predators therefore remain a potential source of synchronization, and they may indeed be accountable for decreasing spatial variation in vole dynamics in the continuous landscape. Patterns of spatial synchrony among vole population dynamics might be different in ecosystems where, e.g. islands or mountains form true year-round barriers for predator movement. In these kinds of landscapes, we may expect to see a stronger degree of asynchrony between vole populations, even at geographical scales similar to those investigated in this study (see Klemola et al 2006).
References
Aars J, Ims RA (2002) Intrinsic and climatic determinants of population demography: the winter dynamics of tundra voles. Ecology 83:3449–3456
Abbott KC (2007) Does the pattern of population synchrony through space reveal if the Moran effect is acting? Oikos 116:903–912
Bellamy PE, Rothery P, Hinsley SA (2003) Synchrony of woodland bird populations: the effect of landscape structure. Ecography 26: 338–348
Bernstein C, Kacelnik A, Krebs CJ (1991) Individual decisions and the distribution of predators in a patchy environment. II. The influence of travel costs and structure of the environment. J Anim Ecol 60: 205–225
Bernstein C, Auger P, Poggiale JC (1999) Predator migration decisions, the ideal free distribution, and predator-prey dynamics. Am Nat 153:267–281
Bjørnstad ON, Stenseth NC, Saitoh T (1999a) Synchrony and scaling in dynamics of voles and mice in northern Japan. Ecology 80:622–637
Bjørnstad ON, Ims RA, Lambin X (1999b) Spatial population dynamics: analyzing patterns and processes of population synchrony. Trends Ecol Evol 11:427–432
Blasius B, Huppert A, Stone L (1999) Complex dynamics and phase synchronization in spatially extended systems. Nature 399:354–359
Cattadori IM, Hudson PJ, Merler S, Rizzoli A (1999) Synchrony, scale and temporal dynamics of rock partridge (Alectoris graeca saxatilis) populations in the Dolomites. J Anim Ecol 68:540–549
Debinski DM, Holt RD (2000) A survey and overview of habitat fragmentation experiments. Conserv Biol 14:342–355
De Roos AM, McCauley E, Wilson WG (1991) Mobility versus density-limited predator–prey dynamics on different spatial scales. Proc R Soc Lond B 246:117–122
Diffendorfer JE, Gaines MS, Holt RD (1995) Habitat fragmentation and movements of 3 small mammals (Sigmodon, Microtus and Peromyscus). Ecology 76:827–839
FMI (1994) Monthly climate observations in Finland. Finnish Meteorological Institute, Helsinki
Fuller MR (1981) Methods of detecting and counting raptors: a review. Stud Avian Biol 6:235–246
Grenfell BT, Wilson K, Finkenstädt BF, Coulson TC, Murray S, Albon SD, Pemberton JM, Clutton-Brock TH, Crawley MJ (1998) Noise and determinism in synchronized sheep dynamics. Nature 391:674–677
Hanski I, Hansson L, Henttonen H (1991) Specialist predators, generalist predators, and the microtine rodent cycle. J Anim Ecol 60:353–367
Hansson L (1975) Comparison between small mammal sampling with small and large removal quadrats. Oikos 26:398–404
Hansson L (1994) Vertebrate distributions relative to clear-cut edges in a boreal forest landscape. Landscape Ecol 9:105–115
Hansson L, Henttonen H (1985) Gradients in density variations of small rodents: the importance of latitude and snow cover. Oecologia 67:394–402
Heikkilä J, Below A, Hanski I (1994) Synchronous dynamics of microtine rodent populations on islands in Lake Inari in northern Fennoscandia: evidence for regulation by mustelid predators. Oikos 70:245–252
Holyoak M (2000) Habitat patch arrangement and metapopulation persistence of predators and prey. Am Nat 156:378–389
Holyoak M, Lawler SP (1996) The role of dispersal in predator–prey metapopulation dynamics. J Anim Ecol 65:640–652
Huffaker CB (1958) Experimental studies on predation: dispersion factors and predator–prey oscillations. Hilgardia 27:343–383
Huitu O, Norrdahl K, Korpimäki E (2003) Landscape effects on temporal and spatial properties of vole population fluctuations. Oecologia 135:209–220
Huitu O, Norrdahl K, Korpimäki E (2004) Competition, predation and interspecific synchrony in cyclic small mammal communities. Ecography 27:197–206
Huitu O, Laaksonen J, Norrdahl K, Korpimäki E (2005) Spatial synchrony in vole population fluctuations—a field experiment. Oikos 109:583–593
Ims RA, Andreassen HP (2000) Spatial synchronization of vole population dynamics by predatory birds. Nature 408:194–196
Ims RA, Andreassen HP (2005) Density-dependent dispersal and spatial population dynamics. Proc R Soc Lond B 272:913–918
Kendall BE, Bjørnstad ON, Bascompte J, Keitt TH, Fagan WF (2000) Dispersal, environmental correlation, and spatial synchrony in population dynamics. Am Nat 155:628–636
King CM, Edgar RL (1977) Techniques for tracking and trapping stoats (Mustela erminea); a review and a new system. NZ J Zool 4:193–212
Klemola T, Korpimäki E, Norrdahl K, Tanhuanpää M, Koivula M (1999) Mobility and habitat utilization of small mustelids in relation to cyclically fluctuating prey abundances. Ann Zool Fenn 36:75–82
Klemola T, Huitu O, Ruohomäki K (2006) Geographically partitioned spatial synchrony among cyclic moth populations. Oikos 114:349–359
Koenig WD (1999) Spatial autocorrelation of ecological phenomena. Trends Ecol Evol 14:22–26
Koenig WD (2002) Global patterns of synchrony and the Moran effect. Ecography 25:283–288
Korpimäki E, Norrdahl K (1991) Numerical and functional responses of kestrels, short-eared owls, and long-eared owls to vole densities. Ecology 72:814–826
Korpimäki E, Norrdahl K, Rinta-Jaskari T (1991) Responses of stoats and least weasels to fluctuating vole abundances: is the low phase of the vole cycle due to mustelid predation?. Oecologia 88:552–561
Korpimäki E, Norrdahl K, Klemola T, Pettersen T, Stenseth NC (2002) Dynamic effects of predators on cyclic voles: field experimentation and model extrapolation. Proc R Soc Lond B 269:991–997
Korpimäki E, Norrdahl K, Huitu O, Klemola T (2005) Predator-induced synchrony in population oscillations of co-existing small mammal species. Proc R Soc Lond B 272:193–202
Korslund L, Steen H (2006) Small rodent winter survival: snow conditions limit access to food resources. J Anim Ecol 75:156–166
Lewontin RC (1966) On the measurement of relative variability. Syst Zool 15:141–142
Liebhold A, Koenig WD, Bjørnstad ON (2004) Spatial synchrony in population dynamics. Ann Rev Ecol Syst 35:467–490
Lillegård M, Engen S, Sæther BE (2005) Bootstrap methods for estimating spatial synchrony of fluctuating populations. Oikos 109:342–350
Littell RC, Milliken GA, Stroup WW, Wolfinger RD (1996) SAS® system for mixed models. SAS Institute Inc., Cary, NC
Manly BFJ (1997) Randomization, bootstrap and Monte Carlo methods in biology, 2nd edn. Chapman & Hall/CRC, Boca Raton, FL
Moran PAP (1953) The statistical analysis of the Canadian lynx cycle. I: Structure and prediction. Aust J Zool 1:163–173
Myers JH (1998) Synchrony in outbreaks of forest Lepidoptera: a possible example of the Moran effect. Ecology 79:1111–1117
Norrdahl K, Korpimäki E (1993) Predation and interspecific competition in two Microtus voles. Oikos 67:149–158
Norrdahl K, Korpimäki E (1995) Effects of predator removal on vertebrate prey populations: birds of prey and small mammals. Oecologia 103:241–248
Norrdahl K, Korpimäki E (1996) Do nomadic avian predators synchronize population fluctuations of small mammals? A field experiment. Oecologia 107:478–483
Norrdahl K, Korpimäki E (2002) Seasonal changes in the numerical responses of predators to cyclic vole populations. Ecography 25:428–438
Norrdahl K, Korpimäki E (2005) Survival through bottlenecks of vole cycles: refuge or chance events? Evol Ecol 19:339–361
Paradis E, Baillie SR, Sutherland WJ, Gregory RD (1999) Dispersal and spatial scale affect synchrony in spatial population dynamics. Ecol Lett 2:114–120
Petty SJ, Lambin X, Sherratt TN, Thomas CJ, Mackinnon JL, Coles CF, Davison M, Little B (2000) Spatial synchrony in field vole Microtus agrestis abundance in a coniferous forest in northern England: the role of vole-eating raptors. J Appl Ecol 37:136–147
Ranta E, Lindström J, Lindén H (1995a) Synchrony in tetraonid population dynamics. J Anim Ecol 64:767–776
Ranta E, Kaitala V, Lindström J, Lindén H (1995b) Synchrony in population dynamics. Proc R Soc Lond B 262:113–118
Ranta E, Kaitala V, Lindström J (1999) Spatially autocorrelated disturbances and patterns in population synchrony. Proc R Soc Lond B 266:1851–1856
Ranta E, Lundberg P, Kaitala V (2006) Ecology of populations. Cambridge University Press, Cambridge
Ripa J (2000) Analysing the Moran effect and dispersal: their significance and interaction in synchronous population dynamics. Oikos 90:175–187
Rueness EK, Stenseth NC, O’Donoghue M, Boutin S, Ellegren H, Jakobsen KS (2003) Ecological and genetic spatial structuring in the Canadian lynx. Nature 425:69–72
Ryall KL, Fahrig L (2006) Response of predators to loss and fragmentation of prey habitat. Ecology 87:1086–1093
Schwartz MK, Mills LS, McKelvey KS, Ruggiero LF, Allendorf FW (2002) DNA reveals high dispersal synchronizing the population dynamics of Canada lynx. Nature 415:520–522
Sherratt TN, Lambin X, Petty SJ, Mackinnon JL, Coles CF, Thomas CJ (2000) Use of coupled oscillator models to understand synchrony and travelling waves in populations of the field vole Microtus agrestis in northern England. J Appl Ecol 37:148–158
Solantie R (2000) Snow depth on January 15th and March 15th in Finland 1919–98, and its implications for soil frost and forest ecology (Meteorological Publications no. 42). Finnish Meteorological Institute, Helsinki
Solantie R, Drebs A, Hellsten E, Saurio P (1996) Timing and duration of snow cover in Finland during 1961–1993 (Meteorological Publications no. 34). Finnish Meteorological Institute, Helsinki
Steen H, Ims RA, Sonerud GA (1996) Spatial and temporal patterns of small-rodent population dynamics at a regional scale. Ecology 77:2365–2372
Stenseth NC, Chan K-S, Tong H, Boonstra R, Boutin S, Krebs CJ, Post E, O’Donoghue M, Yoccoz NG, Forchhammer MC, Hurrell JW (1999) Common dynamic structure of Canada lynx populations within three climatic regions. Science 285:1071–1073
Stenseth NC, Ehrich D, Rueness EK, Lingjærde OC, Chan K-S, Boutin S, O’Donoghue M, Robinson DA, Viljugrein H, Jakobsen KS (2004a) The effect of climatic forcing on population synchrony and genetic structuring of the Canadian lynx. Proc Natl Acad Sci USA 101:6056–6061
Stenseth NC, Shabbar A, Chan K-S, Boutin S, Rueness EK, Ehrich D, Hurrell JW, Lingjærde OC, Jakobsen KS (2004b) Snow conditions may create an invisible barrier for lynx. Proc Natl Acad Sci USA 101:10632–10634
Sundell J, Huitu O, Henttonen H, Kaikusalo A, Korpimäki E, Pietiäinen H, Saurola P, Hanski I (2002) Large-scale spatial dynamics of vole populations in Finland revealed by the breeding success of vole-eating avian predators. J Anim Ecol 73:167–178
Sutcliffe OL, Thomas CD, Moss D (1996) Spatial synchrony and asynchrony in butterfly population dynamics. J Anim Ecol 65:85–95
Turchin P (2003) Complex population dynamics: a theoretical/empirical synthesis. Princeton University Press, Princeton, NJ
Viitala J, Korpimäki E, Palokangas P, Koivula M (1995) Attraction of kestrels to vole scent marks visible in ultraviolet light. Nature 373:425–427
Williams DW, Liebhold AM (2000) Spatial synchrony of spruce budworm outbreaks in eastern North America. Ecology 81:2753–2766
With KA, Pavuk DM, Worchuck JL, Oates RK, Fisher JL (2002) Threshold effects of landscape structure on biological control in agroecosystems. Ecol Appl 12:52–65
Wolff JO, Schauber EM, Edge WD (1997) The effects of habitat fragmentation on the demography and social structure of the gray-tailed vole. Conserv Biol 11:1–12
Ydenberg RC (1987) Nomadic predators and geographical synchrony in microtine population cycles. Oikos 50:270–272
Acknowledgments
We wish to thank Lauri Nikkinen for trapping assistance and Narssjön Camping, the Kauhajoki Hunting Society and family Ikola for accommodation during trapping visits. The study was financially supported by the Academy of Finland (grants no. 106036 to OH, 111195 to TK and 206140 to EK), the Graduate School in Evolutionary Ecology (grant to OH) and the Turku University Foundation (grant to OH). All aspects of this study comply with the current laws of Finland.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Hannu Ylönen.
Rights and permissions
About this article
Cite this article
Huitu, O., Laaksonen, J., Klemola, T. et al. Spatial dynamics of Microtus vole populations in continuous and fragmented agricultural landscapes. Oecologia 155, 53–61 (2008). https://doi.org/10.1007/s00442-007-0885-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00442-007-0885-x