Abstract
Chimpanzees produce acoustically distinct calls when encountering food. Previous research on a number of species has indicated that food-associated calls are relatively widespread in animal communication, and the production of these calls can be influenced by both ecological and social factors. Here, we investigate the factors influencing the production of food-associated calls in wild chimpanzees and examine whether male chimpanzees produce food-associated calls selectively in the presence of important social partners. Male chimpanzees form stable long-term social relationships with each other, and these social bonds are vital in enabling a range of cooperative activities, such as group hunting and territory defence. Our data show that males were significantly more likely to produce food-associated calls if an important social partner was nearby, regardless of the size of the audience or the presence of oestrus females. Call production was also mediated by the size of the food patch and by whether or not the food could be monopolised. The presence of important social partners explained most of the variation in male calling behaviour, indicating that food-associated calls are socially directed and serve a bonding function.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
A wide variety of avian and mammalian species give specific calls in the feeding context. Such calls usually attract other conspecifics to the food source and as such can act as recruitment calls (Wilkinson and Boughman 1998). In dolphins, the low frequency ‘bray’ call is associated with feeding on salmonoids, but this call is produced primarily to aid prey capture, with the recruitment of others being a mere by-product (Janik 2000). In contrast, in Carolina chickadees, individuals manipulate the acoustic structure of their calls according to whether another individual has joined them to feed, and experiments showed that such ‘recruitment calls’ attracted more individuals to a feeding site than calls given after other individuals have joined the caller (Mahurin and Freeberg 2009).
Previous research across a variety of species has revealed that production of food-associated calls can be mediated by ecological variables, such as the quantity (Caine et al. 1995), divisibility (Hauser et al. 1993), type (Bugnyar et al. 2001) and quality of the food (Benz et al. 1992; Elowson et al. 1991; Roush and Snowdon 2000; Gros-Louis 2003). In addition, social factors can also have an effect on call production, as shown by various audience effects that have been observed in this context. For instance, male chickens call more in the presence of females (Marler et al. 1986; Evans and Marler 1994) and wild tufted capuchins alter the latency to call as a function of the proximity of other group members (Di Bitetti 2005). Captive brown capuchins call more for larger audiences containing kin (Pollick et al. 2005). Pinyon jays are sensitive to the presence of an audience and tend to call more when audiences contain their long-term mate (Dahlin et al. 2005). Comparable effects have been observed in a number of species in the context of alarm calling (e.g. Cheney and Seyfarth 1985; Dunford 1977; Gyger et al. 1986; Ridley et al. 2007; Wich and Sterck 2003).
Chimpanzee food-associated calls grade acoustically from soft grunts to high-pitched squeaks, typically described under the umbrella term ‘rough grunts’ (Marler and Tenaza 1977, p. 987). Wild chimpanzees, especially males, often produce ‘rough grunts’ at the base of a feeding tree, whilst climbing up, or during the first few minutes of feeding. These calls are intraparty calls (Goodall 1986) that are generally only audible to close-by animals (Marler and Tenaza 1977, p. 990) within 100 m or closer. This is in contrast to the high-amplitude pant hoot calls that males often produce when finding food (Notman and Rendall 2005), which appears to function in announcing food to distant individuals in other parties. Rather than simply announcing the presence of food to individuals in the vicinity, ‘rough grunts’ provide listeners with information regarding food quality. Research with captive chimpanzees has shown that ‘rough grunts’ vary in their acoustic structure depending on a caller's assessment of food quality (Slocombe and Zuberbühler 2006). Listeners are able to attend to this acoustic variation and adjust their foraging behaviour accordingly, suggesting that the call variants are meaningful to them (Slocombe and Zuberbühler 2005). In the wild, obtaining information about the quality of food is likely to be highly beneficial for listeners. First, it is likely to maximise the foraging efficiency of listeners, including the caller's own kin and allies, by preventing initially unsuccessful foragers from abandoning the patch prematurely (Valone 1996). In chimpanzees, rough grunts will help listeners in taking energetically costly decisions as to whether or not to climb a tree. In addition, given the variation in fruit quality within a tree (Houle et al. 2007), calls could inform listeners about the quality of different areas of a feeding patch, thus allowing them to maximise their foraging success.
Interestingly, males do not always call when finding food and it has been suggested that this is mediated by the amount and divisibility of the food (Hauser et al. 1993; Hauser and Wrangham 1987). Since rough grunts reliably attract conspecifics (Goodall 1986; Slocombe and Zuberbühler 2005), calling is likely to increase feeding competition, suggesting that these costs need to be offset somehow. In smaller bodied species, the benefits to the caller may be in terms of reduced predation risk (e.g. Elgar 1986), but this is unlikely to be important for chimpanzees. It is currently unclear in what ways chimpanzee callers benefit from such a seemingly altruistic behaviour in order to make this an evolutionary stable strategy.
Given the complexity of chimpanzee social behaviour, one possible benefit of producing food-associated calls is the strengthening of affiliative relationships with important social partners. Male chimpanzees can form long-term social relationships with unrelated individuals that remain stable over years (Mitani 2009). These social bonds are crucial to allow a range of cooperative activities, such as group hunting and territory defence (Mitani et al. 2000). There is strong evidence that grooming is the primary mechanism through which primates express affiliation and maintain social bonds (Dunbar 1996). In chimpanzees, grooming can occupy up to 25% of daily time budgets (Goodall 1986). High and equitable grooming rates between two partners are related to strong social bonds (Fedurek and Dunbar 2009) and other social phenomena, such as increased coalitionary support during intra-group aggression (Hemelrijk and Anneke 1991) and increased food sharing (de Waal 1997). Although the evidence for reciprocal interchange of different services in chimpanzees is mixed (Gomes and Boesch 2009; Gilby 2006; Mitani and Watts 2001; Schino and Aureli 2009), it is possible that food call production may be a ‘currency’ that is exchanged amongst individuals for other services.
In this study, we investigated the social and ecological variables influencing the production of food calls in wild chimpanzees. In terms of social variables, we examined whether these calls were produced preferentially when an audience was present and whether the composition of the audience mediated call production. First, we tested the hypothesis that males were prepared to incur the increased costs of feeding competition if important social partners were in the vicinity to benefit from them. We examined whether the presence of long-term and short-term grooming partners affected call production, and we explored the possibility that food calls were produced preferentially for individuals who provided grooming prior to feeding, as part of an interchange of services. Second, we tested the hypothesis that calls were produced to attract potential mates (Marler et al. 1986) by examining if the presence of oestrus females affected call production. In terms of ecological variables, we hypothesised that calls were more likely to be produced when individuals were feeding on large rather than small food patches, in line with previous research (Caine et al. 1995). Finally, we investigated whether chimpanzees called less to monopolisable food (Hauser et al. 1993), where the costs of increased feeding competition were elevated, particularly for subordinate individuals.
Methods
Study site
Data were collected by KS, LT and PS on the Sonso chimpanzee community of Budongo Forest, Uganda, (Reynolds 2005) for 12 months between January 2004 and March 2006. Budongo Forest covers an area of 428 km2 of moist, semi-deciduous tropical forest, between 1°35′ and 1°55′N and 31°08′ and 31°42′E. The study site is located at an altitude of 1,100 m and has an annual rainfall of about 1,600 mm. There is a dry season between December and February in between two rainy seasons (Newton-Fisher 1999). Habituation of the Sonso community to humans began in 1990 and provisioning has never been used. At the end of the data collection period in 2006, the community consisted of 72 individuals; 8 adult males, 21 adult females, 8 sub-adult males, 5 sub-adult females, 18 juveniles and 12 infants. Adults were defined as individuals above 15 years of age, and sub-adults were defined as those between the ages of 10 and 15, who were regularly seen without their mothers (Reynolds 2005).
Data collection
Focal data relating to feeding and grooming (see below) were collected on nine male chimpanzees; six adults (18–45 years) and three sub-adults (13–15 years), all of whom could be followed without difficulties. Data were collected over 125 full days (0730–1600 h), but continuous observation was not always possible, resulting in a total of 613.42 h of observation. Direct observation time only included periods of direct visual contact with the focal animal, which resulted in a mean of 316 min (SD = 119) direct focal observation each day.
Sonso chimpanzees occasionally feed on Raphia farinifera, a monopolisable food source (Reynolds et al. 2009; see more details below). We collected ad libitum data on any individual feeding on this species during the main study period and during an additional period between February and March 2009. If multiple feeding bouts occurred, then we only considered the first feeding bout from each individual. A bout was defined as the period from when the individual first retrieved pith from the tree and consumed it to the point where the individual moved more than 5 m from the tree or stopped retrieving and consuming pith.
Identification of important social partners
We used grooming interactions to identify important social partners of each focal male. Recent studies have highlighted the importance of bi-directional grooming for social bonding in chimpanzees (Fedurek and Dunbar 2009). However, bi-directional grooming was not common in our community, accounting for only 33% of interactions (compared to 75% in captivity: Fedurek and Dunbar 2009). Thus, we considered all individuals whom the focal individual groomed as potential candidates for being an important social partner. For every grooming event involving the focal animal, we recorded the duration of grooming, the identity of all participants and their role (producer or recipient). We then determined the three individuals that received the most grooming bouts from the focal animal (‘long-term grooming recipients’) over the following periods of 3–4 months duration: January to March 2004, February to April 2005, May to August 2005 or January to March 2006. If two recipients were equal in terms of grooming bout numbers, we then used the total grooming duration as a subsidiary ranking criterion. We chose to identify the top three groomers, as most grooming effort was usually dedicated to three individuals (see Table 1). We also determined all individuals that had received grooming from the focal animal on the same day (‘short-term grooming recipients’). This second measure was not so accurate because focal individuals could often not be followed for full days (mean 316 min), suggesting that some grooming interactions will have been missed. Nevertheless, it was relevant as a measure to capture the daily changes in social affiliation. Using the same methodology, we also identified the top three grooming providers to the focal over the same time periods (‘long-term’ grooming providers). In order to examine short-term reciprocal interchange of grooming for grunting, we also identified all individuals who had provided grooming to the focal on the same day prior to each of the feeding events (‘short-term grooming providers’).
Feeding events
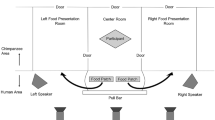
For every feeding event that involved a focal individual, we recorded the tree species, time of arrival within 30 m of the tree (for all individuals), start and end time of feeding (for all individuals), time and type of calls produced and the identity of the callers. We only analysed complete feeding events for the focal animal, i.e. observations from the time of arrival to the cessation of feeding. We also excluded all events where calls could not be assigned to an individual, a relatively common occurrence if the visibility was poor. Finally, we only considered feeding events of the focal animal that lasted for at least 4 min, as feeds of 3 min or less were more than one standard deviation away from the average feeding duration (mean = 24.54 min; SD = 21.20).
If a feeding event passed these criteria, we checked whether or not (a) the focal animal produced rough grunts, (b) long- or short-term grooming recipients were present in the focal individual's party (a party was defined as all individuals within a 30-m radius of the focal individual, Newton-Fisher 2004), (c) maximally swollen oestrous females were present in the party. Degree of swelling was ranked on a scale from 0 to 4, with 4 being maximally swollen (Townsend et al. 2008), and (d) long-term or short-term grooming providers were present in the party .We also recorded party size, which consisted of the number of individuals in the focal's party. Finally, we assessed the patch size by calculating the total number of ‘chimp minutes’ (= the cumulative time spent feeding by all individuals present; following White and Wrangham 1988). It was not uncommon that some individuals were already feeding before the focal animal arrived or stayed after it departed, so this measure was sometimes an underestimate of the patch size.
Non-monopolisable and monopolisable foods
Most of the feeding events took place in large trees where fruits and other food items were divisible and dispersed. We classified these feeding sources as ‘non-monopolisable’ as food was distributed in a way that multiple individuals could feed without reducing each other's foraging efficiency. A notable exception was the palm tree species R. farinifera (‘Raphia’), which is visited by chimpanzees to consume decaying pith from inside the trunk, a valuable source of sodium (Reynolds et al. 2009). The pith is accessible by creating small holes in the trunk, and as a result, only a small number of individuals can feed simultaneously at this resource. We thus refer to Raphia as a ‘monopolisable’ food source. Feeding competition at Raphia trees is usually high, with lower-ranking individuals often blocked from this resource by adult males.
As Raphia consumption is not common (0.7% feeding time; Bates 2005), we collected ad libitum data from any male individual feeding on this resource, provided the complete feeding event could be documented. We recorded the behaviour of 13 males (six adults and seven sub-adults) with each individual contributing between one and five events. We therefore predicted that, despite this being a highly desired resource, focal animals should be less willing to produce food grunts when accessing Raphia pith than other non-monopolisable resources. In addition, this effect should be especially strong for lower-ranking individuals who are most likely to be displaced while feeding on Raphia.
To address the alternative explanation that grunt production in response to Raphia was affected by the negligible contribution this tree species makes to the chimpanzees' overall diet, rather than its divisibility, we compared the chimpanzees' behaviour at Raphia trees with their behaviour at Antiaris (Antiaris toxicaria) trees. Antiaris contributes a comparably low amount to the community's total diet (0.5% feeding time, Bates 2005), but it produces a large amount of dispersed small fruits and thus is a non-monopolisable food source. Behaviour at Antiaris trees was extracted from the focal data.
Statistical analyses
We conducted an overall analysis to assess the relative influence of the different ecological and social variables on call production. We performed generalized linear mixed effects models (GLMMs) with a quasi-binomial error structure and a logit link function, using statistics programme R 2.8.1 (R Development Core Team 2009). In these analyses, we controlled for repeated sampling from the same individual with ‘individual’ fitted as a random factor (Crawley 2002) by conducting random intercepts models using the packages LME4 (Bates 2007) and MASS (Venables and Ripley 2002). We assessed the contributions of the following five variables: presence of long-term grooming recipients, presence of short-term grooming recipients, presence of maximally swollen oestrus females, party size and patch size. Non-significant factors were omitted from the final model (Crawley 2002).
To test whether the presence of an audience and important social partners affected call production, we calculated the proportion of feeding events in which a focal animal produced rough grunts in the presence or absence of other group members, a grooming recipient or a grooming provider. In order to be considered representative, a minimum of four feeding events were required for both conditions, otherwise the individual was removed from the analyses. Due to the resulting small sample sizes, we ran a non-parametric Wilcoxon signed-rank test to establish whether, as a group, the chimpanzees grunted in a higher proportion of events when a grooming partner was present rather than absent. In line with recommendations by Mundry and Fischer (1998), exact rather than asymptotic p values are reported.
Inter-observer reliability
KS trained LT and PS in data collection procedures, and LT also provided further training of PS in the field. To determine inter-observer reliability between subsequent pairs of observers (KS–LT; LT–PS) both observers focussed on the same individual and recorded data without consulting each other. A Cronbach's alpha test of inter-observer reliability yielded good scores (KS–LT, 0.86; LT–PS, 0.89), indicating that data were being collected according to the same criteria.
Results
We analysed 367 complete feeding events from nine focal males and found that rough grunts were produced by the focal individual in 205 cases (56% of events). The nine males contributed differently to the final data set (mean feeding events/male = 40, SD = 22, range = 10–74; Table 1). We therefore statistically controlled for multiple contributions by each individual in the final data set. The basic grooming and rough grunting patterns of the nine focal individuals are shown in Table 1.
Overall, both ecological and social variables affected whether food grunts were produced during a feeding bout, but social variables explained greater proportions of the variance in the model. Grunt production was significantly increased by the presence of important social partners (long-term grooming recipients, GLMM F (1 ,8) = 12.75, p < 0.001; short-term grooming recipients, GLMM F (1,8) = 51.42, p < 0.001). Grunt production was also significantly affected by the patch size (GLMM F (1, 8) = 10.21, p = 0.0014). Feeding events where grunts were produced were associated with larger patch sizes (mean chimp minutes = 216.89, SD = 384.77) than feeding events where no grunts were produced (mean chimp minutes = 88.81, SD = 125.57). The presence of maximally swollen oestrous females and party size, however, did not significantly affect grunt production (oestrous females, GLMM (F (1,8) = 0.02, p = 0.81; party size, GLMM F (1,8) = 0.86, p = 0.35). All interactions were non-significant and were thus omitted in the final model.
Given the importance of social factors in the model, we examined these audience effects in more detail. Firstly, we explored whether calling was influenced by the presence of an audience. In 66 of the 367 feeding events, the focal fed alone, defined as the absence of any other individuals within 30 m. Eight focal males were observed in this context, each during 1–17 feeding events. Overall, grunts were produced in only 20% of these solitary feeding events as opposed to 63% in the other 301 ‘social’ feeding events where at least one other chimpanzee was present. Only five individuals had a minimum of four feeding events in both solitary and social conditions. These individuals all produced grunts more often when there was an audience within 30 m (see Table 2), and the effect size for this pattern of behaviour was large (Cohen's d = 4.22), although it failed to reach significance (Wilcoxon signed-rank test, z = 2.02, exact two-tailed p = 0.062), most likely due to small sample size.
In order to explore the effect the composition of the audience had on calling in more detail, the social feeding events, where at least one other individual was present, were examined in greater depth. Males produced rough grunts during a significantly higher proportion of social feeding events when an important social partner was present than absent. This was the case for both short-term and long-term grooming recipients (short-term, z = 2.02, N = 7, p = 0.047; long-term, z = 2.1, N = 8, p = 0.039; Wilcoxon signed-rank tests, two-tailed; Fig. 1).
We found that males were also more likely to call during social feeding events when long-term grooming providers were present, rather than absent (Wilcoxon signed-rank test, N = 8, z = 2.1, p = 0.039). There was substantial overlap between the identity of long-term recipients and providers, with 69% and 42% of top three recipients also being top three providers for the focal individuals (adult and sub-adult males, respectively). This indicates that although levels of simultaneous reciprocal grooming were relatively low in this community, reciprocation of grooming with important social partners did occur over longer periods of time.
We found no evidence that focal males were more likely to produce grunts during social feeding events in the presence of individuals who had provided them with grooming earlier in the day. The presence or absence of these short-term grooming providers did not influence calling behaviour in any systematic manner (Wilcoxon signed-rank test, N = 8, z = 0.169, exact two-tailed p = 0.938).
Non-monopolisable and monopolisable food sources
We were able to observe 29 complete feeding events on Raphia with rough grunts given in only seven cases (24%). In 4 of 29 cases, the focal individual fed alone, and they were silent in all of these four cases. Due to the low number of Raphia feeding events recorded for the focal individuals, it was not possible to systematically examine whether the presence or absence of important social partners affected calling behaviour at this food source. Instead, we examined whether calling patterns at this food source were comparable to a non-monopolisable source of equivalent importance.
The overall low call rates during Raphia feeding could not be explained by its relative rarity in the chimpanzees' diet. When feeding on Antiaris trees, which contributed a similar proportion to the overall diet, individuals reliably produced rough grunts (75% of feeding events; N = 12; Table 3).
Sub-adult males, who were all lower ranking than the adult males, were significantly less likely to produce calls than adult males while feeding on Raphia (Fisher's exact test; two-tailed, p = 0.029; Table 3). Only one sub-adult male was ever observed to produce calls in this context, and this was while he was co-feeding with just one adult male. In contrast, when food was non-monopolisable, sub-adult males did not differ from adult males in their willingness to produce rough grunts. When the 11 feeding events (nine Raphia and two honey events) on monopolisable food sources were removed from the focal data set, call rates did not differ between adults (58%) and sub-adults (53%; Fisher's exact test; two-tailed; p = 0.402; Table 3).
Discussion
In our study, wild chimpanzees produced rough grunts in only about half of all feeding events. Grunts were rarely given when the focal individual fed alone, indicating that they serve a communicative function rather than representing an automatic reflexive response to the food source. Social variables had the greatest influence over their decision to produce rough grunts in a feeding context. Basic social factors, such as the number of individuals in the focal individual's party, did not influence whether grunts were produced, indicating that the behaviour was not driven by something as simple as social facilitation. Instead, the composition of the audience and, more specifically, the presence of an important social partner had the greatest influence over an individual's decision to produce calls. Other social factors, such as the presence of an oestrous female, did not influence call production, indicating calls are not given to attract mating partners.
Ecological variables also influenced call production, consistent with previous findings. First, the probability of calling was highest in the largest feeding patches. Second, particularly sub-adults called less in response to monopolisable food sources. The majority of food species that wild chimpanzees consume occur in large, highly divisible quantities so that the costs of attracting others to the food source with rough grunts are low. Raphia consumption is an interesting exception because this resource is monopolisable. Males still produced rough grunts to Raphia, but call rates were much lower in comparison to equivalent non-monopolisable foods. The change was most dramatic for low-ranking males, presumably because they were most likely to risk losing access to the resource by attracting higher-ranking individuals, in line with a previous captive study (Hauser et al. 1993). Overall, these calling patterns suggest that chimpanzees took the costs and benefits of their calling behaviour into account and were prepared to produce rough grunts for the benefit of important social partners, as long as the food source was non-monopolisable.
The presence of both long-term grooming providers and recipients increased the chances of a focal male producing rough grunts, indicating that consistent grooming effort in both directions is indicative of an important social relationship. We did not, however, find any evidence that grunt production was mediated by the presence of an individual who had provided grooming to the focal prior to the feeding event that day. This indicates that grunting is not a ‘service’ that is provided in exchange for grooming, at least over short time periods. Yet, as previous research indicates, the appropriate time scale for examining reciprocation is unclear (Schino and Aureli 2009), and future research will need to consider whether such trading of grunting for grooming occurs over other time periods.
Male chimpanzees dedicate much of their time and effort in maintaining and improving their social position within the group (Goodall 1986; de Waal 1982). Individuals spend considerable amounts of time grooming each other (Goodall 1986), and those with strong grooming relations tend to support each other during other activities, such as conflict, intergroup interactions, or hunting. Our data indicate that rough grunt production may represent a mechanism by which males demonstrate and reinforce their social bonds with preferred group members. As feeding is not compatible with social activities, particularly grooming, rough grunt production in the presence of important social partners may function as an affiliative social mechanism in the vocal domain that strengthens the social bonds between individuals, perhaps similar to the suggested function of gossiping in human language (Dunbar 1996). Further research is required to systematically test this hypothesis, but if rough grunting does strengthen affiliative bonds with important social partners, this may explain how males offset the costs associated with producing these calls.
The role of kinship in determining important social partners and mediating the calling behaviour for these chimpanzees is unknown. Unfortunately, the necessary genetic relatedness data were not available for these individuals and thus it was not possible to test the effect of kinship on calling behaviour. Evidence from other sites indicates that maternal relatedness can have significant effects over social behaviour in this species (Langergraber et al. 2007), so future research should test whether the presence of maternal kin influences the willingness of individuals to produce rough grunts.
The production of rough grunts in wild chimpanzees cannot be explained as a response to finding food alone. Instead, this communicative behaviour is strongly modified by the presence and composition of the nearby audience. Callers are sensitive to the identity of the individuals surrounding them, and they preferentially produce rough grunts in the presence of important social partners. Our data are consistent with the idea that these calls are directed at particular group members, rather than being indiscriminately broadcast (Slocombe and Zuberbühler 2007; Townsend et al. 2008), but further research is needed to establish whether there is a direct causal relationship between the presence of an important social partner and the production of rough grunts.
References
Bates L (2005) Cognitive aspects of food location by the chimpanzees (Pan troglodytes schweinfurthii) of Budongo Forest Reserve, Uganda. Ph.D thesis: University of St Andrews
Bates D (2007) The LME4 package: linear mixed-effects models using S4 classes. R: Online
Benz JJ, Leger DW, French JA (1992) Relation between food preference and food-elicited vocalizations in golden lion tamarins (Leontopithecus-Rosalia). J Comp Psychol 106:142–149
Bugnyar T, Kijne M, Kotrschal K (2001) Food calling in ravens: are “yells” referential signals? Anim Behav 61:949–958
Caine NG, Addington RL, Windfelder TL (1995) Factors affecting the rates of food calls given by red-bellied tamarins. Anim Behav 50:53–60
Cheney DL, Seyfarth RM (1985) Vervet monkey alarm calls—manipulation through shared information. Behaviour 94:150–166
Crawley MJ (2002) Statistical computing: an introduction to data analysis using S-Plus. Wiley, Chichester
Dahlin CR, Balda RP, Slobodchikoff C (2005) Food, audience and sex effects on pinyon jay (Gymnorhinus cyanocephalus) communication. Behav Process 68:25–39
de Waal F (1982) Chimpanzee politics. Harper & Row, New York
de Waal F (1997) The chimpanzee's service economy: food for grooming. Evol Hum Behav 18:375–386
Di Bitetti MS (2005) Food-associated calls and audience effects in tufted capuchin monkeys, Cebus apella nigritus. Anim Behav 69:911–919
Dunbar R (1996) Grooming, gossip and the evolution of language. Faber and Faber, London
Dunford C (1977) Kin selection for ground squirrel alarm calls. Am Nat 111:782–785
Elgar MA (1986) House sparrows establish foraging flocks by giving chirrup calls if resources are divisible. Anim Behav 34:169–174
Elowson AM, Tannenbaum PL, Snowdon CT (1991) Food associated calls correlate with food preferences in cotton-top tamarins. Anim Behav 42:931–937
Evans CS, Marler P (1994) Food calling and audience effects in male chickens, (Gallus gallus)—their relationships to food availability, courtship and social facilitation. Anim Behav 47:1159–1170
Fedurek P, Dunbar RIM (2009) What does mutual grooming tell us about why chimpanzees groom? Ethology 115:566–575
Gilby IC (2006) Meat sharing among the Gombe chimpanzees: harassment and reciprocal exchange. Anim Behav 71:953–963
Gomes CM, Boesch C (2009) Wild chimpanzees exchange meat for sex on a long-term basis. PLoS ONE 4:e5116
Goodall J (1986) The chimpanzees of Gombe: patterns of behavior. Harvard University Press, Cambridge
Gros-Louis J (2003) The function of food-associated calls in white-faced capuchin monkeys, Cebus capucinus, from the perspective of the signaller. Anim Behav 140:565–592
Gyger M, Karakashian S, Marler P (1986) Avian alarm calling: is there an audience effect? Anim Behav 34:1570–1572
Hauser MD, Wrangham RW (1987) Manipulation of food calls in captive chimpanzees: a preliminary report. Folia Primatol 48:24–35
Hauser MD, Teixidor P, Field L, Flaherty R (1993) Food-elicited calls in chimpanzees: effects of food quantity and divisibility? Anim Behav 45:817–819
Hemelrijk CK, Anneke EK (1991) Reciprocity and interchange of grooming and ‘support’ in captive chimpanzees. Animal Behaviour 41:923–935
Houle A, Chapman CA, Vickery WL (2007) Intratree variation in fruit production and implications for primate foraging. Int J Primatol 28:1197–1217
Janik VM (2000) Food-related bray calls in wild bottlenose dolphins (Tursiops truncatus). Proc R Soc Lond B Biol Sci 267:923–927
Langergraber KE, Mitani JC, Vigilant L (2007) The limited impact of kinship on cooperation in wild chimpanzees. Proc Natl Acad Sci USA 104:7786–7790
Mahurin EJ, Freeberg TM (2009) Chick-a-dee call variation in Carolina chickadees and recruiting flockmates to food. Behav Ecol 20(1):111–116
Marler P, Tenaza R (1977) Signaling behavior of apes with special reference to vocalizations. In: Sebeok TA (ed) How animals communicate. Indiana Univ. Press, Bloomington, pp 965–1033
Marler P, Dufty A, Pickert R (1986) Vocal communication in the domestic chicken: II. Is a sender sensitive to the presence and nature of a receiver? Anim Behav 34:194–198
Mitani JC (2009) Male chimpanzees form enduring and equitable social bonds. Anim Behav 77:633–640
Mitani JC, Watts DP (2001) Why do chimpanzees hunt and share meat? Anim Behav 61:915–924
Mitani JC, Merriwether DA, Zhang C (2000) Male affiliation, cooperation and kinship in wild chimpanzees. Anim Behav 59:885–893
Mundry R, Fischer J (1998) Use of statistical programs for nonparametric tests of small samples often leads to incorrect p values: examples from animal behaviour. Anim Behav 56:256–259
Newton-Fisher NE (1999) The diet of chimpanzees in the Budongo Forest Reserve, Uganda. Afr J Ecol 37:344–354
Newton-Fisher NE (2004) Hierarchy and status in Budongo chimpanzees. Primates 45:81–87
Notman H, Rendall D (2005) Contextual variation in chimpanzee pant hoots and its implications for referential communication. Anim Behav 70:177–190
Pollick AS, Gouzoules H, De Waal FBM (2005) Audience effects on food calls in captive brown capuchin monkeys, Cebus apella. Anim Behav 70:1273–1281
Reynolds V (2005) The chimpanzees of Budongo forest: ecology, behaviour and conservation. Oxford University Press, Oxford
Reynolds V, Lloyd AW, Babweteera F, English CJ (2009) Decaying Raphia farinifera palm trees provide a source of sodium for wild chimpanzees in the Budongo Forest, Uganda. PLoS ONE 4(7):e6194
Ridley AR, Child MF, Bell MBV (2007) Interspecific audience effects on the alarm-calling behaviour of a kleptoparasitic bird. Biol Lett 3:589–591
Roush RS, Snowdon CT (2000) Quality, quantity, distribution and audience effects on food calling in cotton-top tamarins. Ethology 106:673–690
Schino G, Aureli F (2009) Reciprocal altruism in primates: partner choice, cognition, and emotions. Adv Study Behav 39:45–69
Slocombe KE, Zuberbühler K (2005) Functionally referential communication in a chimpanzee. Curr Biol 15:1779–1784
Slocombe KE, Zuberbühler K (2006) Food-associated calls in chimpanzees: responses to food types or food preferences? Anim Behav 72:989–999
Slocombe KE, Zuberbühler K (2007) Chimpanzees modify recruitment screams as a function of audience composition. Proc Natl Acad Sci 104(43):17228–17233
Townsend SW, Deschner T, Zuberbühler K (2008) Female chimpanzees use copulation calls flexibly to prevent social competition. PLoS ONE 3:e2431
Valone TJ (1996) Food-associated calls as public information about patch quality. Oikos 77:153–157
Venables WM, Ripley BD (2002) Modern applied statistics with S. Springer, New York
White FJ, Wrangham RW (1988) Feeding competition and patch size in the chimpanzee species Pan paniscus and Pan troglodytes. Behaviour 105:148–164
Wich SA, Sterck EHM (2003) Possible audience effect in Thomas Langurs (Primates; Presbytis thomasi): an experimental study on male loud calls in response to a tiger model. Am J Primatol 60:155–159
Wilkinson GS, Boughman JW (1998) Social calls coordinate foraging in greater spear-nosed bats. Anim Behav 55:337–350
Acknowledgements
We thank the Ugandan Wildlife Authority, the Uganda National Council for Science and Technology and the President's Office for permission to work in the forest. We are grateful to Thibaud Gruber and Monday Gideon for collecting additional Raphia feeding data, to Bonnie Fullard for data entry and analysis, and to Roger Mundry for statistical advice. This study was funded by the BBSRC, the Leverhulme Trust, and the Wissenschaftskolleg zu Berlin. We are grateful to the Royal Zoological Society of Scotland for providing core funding for the Budongo Conservation Field Station (BCFS) and to the field assistants of BCFS for their help with data collection.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by D. Watts
Rights and permissions
About this article
Cite this article
Slocombe, K.E., Kaller, T., Turman, L. et al. Production of food-associated calls in wild male chimpanzees is dependent on the composition of the audience. Behav Ecol Sociobiol 64, 1959–1966 (2010). https://doi.org/10.1007/s00265-010-1006-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00265-010-1006-0