Abstract
Recent theoretical and empirical studies have shown that male dominance is often at odds with female mate preference and that indirect (genetic) benefits of mate choice may not be related to male dominance. We tested whether female preference corresponded to male dominance and whether mating with dominant males conveyed benefits to offspring fitness in a small freshwater fish, the African annual killifish Nothobranchius korthausae (Cyprinodontiformes), a species without parental care. The experimental design used controlled for the effect of male age, possibility of sperm and egg depletion, and accounted for a potential that females express their preference through maternal effects by manipulation of egg mass during ovulation. By sequentially mating females with males of known dominance, we found that female N. korthausae showed no mate preference in terms of egg numbers deposited with respect to male dominance or body size and no congruent mate preference to specific males was detected. However, males sired offspring with consistently higher hatching success and the effect was repeatable across individual females. Thus, some males provided females with indirect benefits related to additive genetic quality (“good genes”) and expressed via increased hatching rate, but this benefit was not related to male dominance status or body size.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Sexual selection theory considers that females prefer to mate with dominant males (Andersson 1994; Candolin 1999). This effect is because males successful in intrasexual competition can provide better resources, such as superior breeding territories, nesting sites, parental care, or nuptial gifts, collectively termed direct benefits of mate choice. It is also generally assumed that two key aspects of sexual selection, male–male competition for dominance (intrasexual selection) and female mate choice (intersexual selection), are mutually reinforcing and that males successful in intrasexual competition are preferred by females. For example, female three-spined sticklebacks (Gasterosteus aculeatus) prefer to mate with males possessing bright red coloration in some populations (Milinski and Bakker 1990). Male redness appears to serve as a signal of genetic quality through heritable resistance to parasites (Barber et al. 2001) and indicates success in intrasexual competition (De Fraipont et al. 1993). Variation in redness among males may increase under male–male competition, which facilitates female discrimination (Candolin 2001) and reinforces the synergy between intrasexual and intersexual selection in the three-spined stickleback.
Dominant males may also impose direct costs on females as they may trade off their dominance status against parental effort (Qvarnström 1997), be sperm depleted (Warner et al. 1995; Preston et al. 2001), or increase the risk of female injury due to coercive mating (Watters 2005). Those potential costs are often overwhelmed by direct benefits that females may obtain from dominant males (Alatalo et al. 1986; Smith et al. 2002; Engqvist 2007). However, there are many mating systems where males only contribute sperm during reproduction and cannot provide any direct benefits to females. In these cases, females still make sophisticated choices among males (Houde 1997), apparently in order to choose males with superior genetic quality that will be passed on to their offspring (termed indirect benefits of mate choice; Neff and Pitcher 2005).
Indirect benefit of mating with attractive males is commonly accepted aspect of sexual selection (Møller and Alatalo 1999; Kokko et al. 2006; Head et al. 2005), but its link to male dominance is ambiguous (Jacob et al. 2007). Indeed, there is growing evidence from various mating systems that female choice often targets traits unrelated to male dominance (reviewed in Qvarnström and Forsgren 1998) and females often do not prefer dominant over subordinate males (Kangas and Lindström 2001; Spence and Smith 2006) or even actively avoid mating with dominant males (Ophir and Galef 2003; Watters 2005). Under such scenario, dominant males may enjoy high reproductive success in nature solely as a consequence of control over breeding sites (Alatalo et al. 1986; Warner 1987) or mating coercion rather than female preference (Clutton-Brock and Parker 1995; Arnqvist and Lowe 2005). Hence, apparent female preference for dominant males may actually represent harassment avoidance when females are often constrained from their choice by intrusion of dominant males (Kokko 2005; Reichard et al. 2007).
It has proven difficult to separate indirect effects of female choice from direct effects that dominant males may convey (Jacob et al. 2007), although this is a critical aspect of testing indirect benefits of male dominance for female fitness. Here, we tested whether female preference corresponded to male dominance and whether mating with dominant males conveyed benefits to offspring fitness in a small freshwater fish, the African annual killifish Nothobranchius korthausae (Cyprinodontiformes). In this species, no parental care exists and males contribute only their genes to the offspring (Wildekamp 2004). We sequentially mated females to males of known dominance and measured egg production (a measure of female preference) and hatching success (fitness benefits) of experimental crosses. Our approach controlled for the effect of male age, possibility of sperm and egg depletion, and accounted for the potential that females express their preference through maternal effects by manipulation of egg mass during ovulation. We used a sequential rather than dichotomous experimental design, because sequential encounter of mates is more likely to reflect the natural situation (Pitcher et al. 2003; Spence and Smith 2006) and allows expression of cryptic female choice (Eberhard 1996), including allocation of energy resources to eggs (Kolm 2001). Our previous experiments established that preference based on the number of spawned eggs is concordant with dichotomous mate choice trials and therefore a valid measure of female preference (M. Reichard, M. Polačik, J. Bryja, unpublished data). We predicted that females would lay more eggs with specific males and that preferred males would confer greater fitness on offspring manifested by a higher hatching rate. We further predicted that female preference would not be related to male dominance status.
The genus Nothobranchius comprises small sexually dichromatic fish that inhabit isolated savannah pools in eastern Africa. Their life cycle is annual; all adults die during the dry season and populations persist in the form of eggs deposited on the substrate. The fish hatch at the onset of the rainy season, mature within 6 weeks, and reproduce daily once reaching sexual maturity (Valdesalici and Cellerino 2003; Wildekamp 2004; Reichard et al. 2009). Mating is promiscuous and no pair bonds are formed (Haas 1976a). Males possess bright nuptial coloration. Females lay five to 50 eggs each day directly into the sediment in separate clutches of a single egg (Haas 1976b). N. korthausae has one of the longest life spans in the genus and its reproductive phase may be as long as 9 months (Huber 2000).
Materials and methods
Experimental procedures
Experimental fish originated from two populations of N. korthausae that were collected in 2001 (Kwachepa population) and 2002 (Mafia population), respectively, and were bred in captivity for at least four generations prior to the experiment. Attempts were made specifically to obtain outbred populations. At least eight fish were founders of the captive population from Kwachepa (code TZL 01-53; GPS 07°31′ S, 39°07′ E), a continental population, and at least seven fish founded the captive population from Mafia island (code TAN 02-5; GPS 07°48′ S, 39°49′ E), off the Tanzanian coast. Nothobranchius fishes live in small temporary pools and their life history makes them especially prone to strong bottleneck effects even under natural conditions (Wildekamp 2004; Genade et al. 2005). The stock fish were housed in mixed-sex 40-l glass aquaria with 14:10 light/dark cycle at a temperature of 25°C and fed with a mixture of live or frozen Chironomus and Chaoborus larvae. In the experiment, 25 males and 25 females (mean ± standard deviation body size = 29.6 ± 2.3 and 26.9 ± 2.2, respectively) of the Kwachepa population and 20 males and 20 females (30.1 ± 2.3 and 26.8 ± 1.2, respectively) of the Mafia population were used. Each of nine experimental groups contained five males and five females, always from the same population. No fish were used in more than one group.
Prior to the experiment, males were ranked for dominance. Five randomly chosen males were isolated in a 20-l aquarium in a dark room for 10 h. This period allowed males to settle and interact, but avoided fighting. After 10 h, a female in spawning condition was placed in the aquarium and fish were exposed to dim lighting. Nothobranchius males compete aggressively with each other and are in constant readiness to spawn (Haas 1976a, b). One of the males quickly established dominance, evident by his clasping the female by pressing her against the bottom (mating behavior) and chasing other males (territorial behavior). This male was removed and ranked as the most dominant male. The procedure was repeated to identify the second, third, fourth, and fifth ranked males. In three cases, only the first two males attempted to mate with the female. Ranking of the remaining three males was based on their aggressive interactions only, but even in those cases male ranking was unambiguous.
The condition of all experimental females was standardized prior to the start of the experiment by allowing them to spawn with randomly chosen males, which were not subsequently used in the experiment, to eliminate any effect of the initial number of ovulated eggs. Our pilot study revealed that an interval of 45 h between successive spawning bouts was sufficient for females to fully recover egg mass and 45 h was used as the length of the recovery/ovulation phase between standardization and the first experimental trial as well as between consecutive experimental trials.
The experiment consisted of successive pairings of each experimental male with each experimental female. One male and one female from each group were randomly chosen to represent a control. On the first day of the experiment, each female was allowed to spawn with one male. During subsequent experimental trials within each group, experimental females were assigned to a different experimental male each time, whereas control males and females were always paired together. Therefore, four experimental and one control spawning took place on each day of the experiment, with a total of 16 (4 males × 4 females) experimental and four control pairings for each of the nine groups.
After ranking males, the experiment started by placing each male with one female in a 20-l aquarium according to a randomly predetermined order following a Latin square design. Given that female fish may quickly modulate number and mass of the eggs they ovulate according to the perceived attractiveness of their partner (Eaton and Farley 1974; Côte and Hunte 1989; Kolm and Olsson 2003; Spence and Smith 2006), each pair was initially separated by a perforated transparent Plexiglas slide that allowed visual and olfactory communication, but prevented spawning, for 45 h. Therefore, our experimental design did not exclude the effect of prespawning egg mass modulation, though egg mass was not estimated given our interest in hatching rate that required unaltered egg development (see below). During the prespawning period, fish were fed twice each day with the amount of food they consumed within 15 min. A pilot study showed that this amount of food approached an ad libitum feeding regime, while ensuring that water quality did not deteriorate.
After 45 h, each pair was carefully moved to a 2-l plastic container with a substrate of fine sand and the fish were allowed to spawn for 3 h. Fish typically started to spawn within less than 5 min and spawning behavior ceased entirely within 3 h. After spawning, the four experimental males were transferred to an aquarium with another female following the Latin square design, whereas the control male was released into the original aquarium with the same female. The next trial always started after the recovery/ovulation phase (45 h) at the same time of day. After all four trials were completed, body size (from tip of the snout to the margin of caudal peduncle) of all fish was measured to the nearest 0.5 mm.
Spawned eggs were washed out of spawning containers and individually counted. Egg washing was repeated until no eggs were found in the container over two consecutive washings. The eggs from each pair were placed into a coconut fiber substrate and stored in plastic bags in darkness at room temperature (23–26°C) for 6 weeks (Wildekamp 2004). After 6 weeks, the substrate was wetted with dechlorinated tap water and all hatched fish were counted after 12 h. A previous study indicated that repeated wetting of N. korthausae eggs did not produce significant numbers of additionally hatched fish and more than 80% of developing embryos hatched after the first wetting (M. Reichard, M. Polačik, J. Bryja, unpublished data).
Data analysis
Egg production was defined as the total number of eggs laid during the 3-h test by a particular pair of fish. Hatching rate was calculated as proportion of eggs that successfully hatched when the substrate was watered after 6 weeks. Therefore, hatching rate was independent of egg production. Fertilization was not scored, and so failure of the egg to hatch could be the result of lack of fertilization, mortality during the embryo development, and inability of developed embryos to leave the egg envelope. Pairings that produced less than five eggs were not included in the hatching rate analysis. Two females died during the experiment and were excluded from analyses. For one group of experimental fish, hatching success was not estimated due to logistical constraints. Data on egg numbers and hatching rates were standardized within each group to account for any possible seasonal or parental age effects on egg production and embryonic development. Hatching rate data (proportions) were arcsine square-root transformed before standardization to meet assumptions of normality.
To account for any effect of spawning rank on egg production or hatching rate, repeated measures analysis of variance (ANOVA)s were used to compare those effects between treatment and control females and across male dominance ranks. Nested ANOVA with experimental male and female (random factors) nested within group and spawning rank as a repeated measure was used to test for effects of particular individuals on egg production and hatching success (Spence and Smith 2006). In this analysis, a significant female effect represents a bias in the number of eggs produced by particular females (or hatching success of eggs produced by particular females), a male effect denotes a bias in the number of eggs received by particular males (and hatching success from eggs sired by particular males, respectively), and a spawning order effect indicates whether egg production or hatching success varied among days of the experiment. We further tested congruence between female preference (egg production with a particular male) and hatching success using a mixed-model ANOVA with the standardized number of eggs as a continuous variable, male identity as random factor, and hatching success as response variable. Means are given with ± 1 standard deviation unless stated otherwise.
For each analysis of variance, except mixed models, we estimated effect size in addition to conventional significance probabilities. The effect size measures the magnitude of a treatment effect independent of sample size (Nakagawa and Cuthill 2007). We used partial η 2, which represents the proportion of variance that is attributed to the particular treatment effect and is calculated as a ratio of the effect variance to the variance of that effect plus its associated error variance. Two N. korthausae populations were used, but there were no consistent differences between the two populations and data were pooled for all analyses. However, for major results, we report effect size measures for each population separately (Table 1).
Results
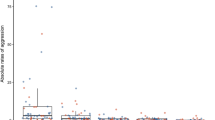
Male dominance was not significantly related to male body size (one-way ANOVA with body size standardized within groups, F 4,40 = 2.32, P = 0.074, η 2 = 0.19 0.001), though the dominant males tended to be larger than the most inferior males (Fig. 1), and there was an overall correlation between male body size and dominance (Spearman correlation, r S = 0.416, N = 45, P = 0.005).
Egg production
Treatment and control females did not differ in egg production (repeated measures ANOVA on egg numbers standardized within groups, F 4,38 = 0.90, P = 0.473, η 2 = 0.01–0.08 for particular spawning ranks). Mean egg production was 24 ± 12.0 eggs per individual treatment spawning (N = 136, range 0–58). Larger females laid more eggs (general linear model (GLM), F 1,32 = 8.86, P = 0.006, η 2 = 0.22 for treatment females). There was also a significant effect of the order of spawning on the number of eggs (spawning order effect in nested GLM, F 27,48 = 1.89, P = 0.027, η 2 = 0.28); the number of eggs increased over successive trials (Fig. 2a). Individual females differed in their egg production even after controlling for the size–fecundity relationship (female effect in nested GLM, F 25,48 = 2.14, P = 0.012, η 2 = 0.29).
There was no relationship between male dominance rank and the number of eggs a male received (repeated measures ANOVA on egg numbers standardized within groups, F 12,72 = 1.01, P = 0.451, η 2 = 0.01, 0.15, 0.12, and 0.10 for particular spawning ranks; Fig. 3a) and no relationship between male size and the number of eggs received (GLM, F 1,34 = 1.11, P = 0.230, η 2 = 0.03). Further, there was no congruence among females in the number of eggs laid with specific males, regardless of their size or dominance (male effect in nested GLM, F 27,48 = 1.03, P = 0.458, η 2 = 0.14). The effects were similar for Mafia and Kwachepa populations (Table 1).
Hatching success
The hatching success of eggs produced during treatment trials was higher than the hatching success of controls (repeated measures ANOVA on hatching success standardized within groups, F 4,24 = 3.42, P = 0.024). The univariate results revealed that this difference arose due to a lower hatching success of the last control spawning (P = 0.314 and η 2 = 0.04, P = 0.357 and η 2 = 0.03, P = 0.238 and η 2 = 0.05, and P = 0.001 and η 2 = 0.33 for the first, second, third, and fourth spawning, respectively). Mean hatching success in treatment trials was 26 ± 18.6% (N = 119, range 0–75%). Hatching success differed among rank order of spawnings (spawning order effect in nested GLM, F 24,33 = 2.01, P = 0.031, η 2 = 0.14; Fig. 2b) and among individual females (female effect in nested GLM, F 22,33 = 4.56, P < 0.001, η 2 = 0.30).
No relationship was found between male dominance and hatching success (repeated measures ANOVA on hatching success standardized within groups, F 12,59 = 0.67, P = 0.771, η 2 = 0.08, 0.08, 0.06, and 0.16 for particular spawning order; Fig. 3b) or male size and hatching success (F 1,30 = 0.51, P = 0.481, η 2 = 0.02). There was no relationship between the number of eggs a male received and hatching success (mixed model ANOVA, F 1,70 = 0.69, P = 0.409). However, there was a consistent effect of specific males on hatching success among females (male effect in nested GLM, F 24,33 = 1.92, P = 0.041, η 2 = 0.13), further confirmed by a significant male effect in a mixed model ANOVA (F 35,82 = 2.18, P = 0.002). For illustrative purposes, untransformed hatching success data for individual experimental males (with dominance indicated) are shown in Fig. 4. The male effect was stronger in the Kwachepa population (Table 1).
Mean (± 1 standard error) hatching success measured as the raw proportion of eggs hatched. Males are ranked from dominant (1) to most inferior (4) within each group. Group 1 was not included in hatching rate success due to logistic constraints (no watering in two of the four runs). Note that spawning trials that produced less than five eggs were not included in the hatching success analysis
Discussion
The study showed no evidence for female mate preference in N. korthausae, measured in terms of egg number deposited with males, and no effect of male dominance or body size on the pattern of spawning. However, specific males sired offspring with consistently higher hatching success and the effect was repeatable among females. Thus, some males provided females with indirect benefits of increased hatching rate, but this benefit was not related to male dominance status or body size. Unlike other comparable studies, the present design to separate indirect benefits of mate choice did not employ an in vitro fertilization procedure. This design may have allowed females to express a preference for specific males via yolk allocation during the ovulation phase (maternal effects; Kolm 2001). Further, the approach used here controlled for the age of the fish; all fish within a group were of the same age and any difference in body size was due to differences in growth rates. This aspect of the design probably reflects the situation in nature where all Nothobranchius fish appear to hatch within a few hours of their habitat becoming flooded at the beginning of the rainy season (Wildekamp 2004). On the other hand, using natural spawning, it was impossible to directly control sperm numbers. Instead, we standardized interspawning interval, with a sufficient time for a full recovery of sperm reserves to mitigate male sperm depletion (Nakatsuru and Kramer 1982). Hence, while the experimental setup used here did not allow identification of the ontogenetic period where the greatest mortalities occurred, which may have ranged from sperm–egg incompatibility to a failure of fully developed embryos to hatch, there was little potential for sperm limitation.
The lack of a consistent bias in female egg production toward specific males may reflect either a low level of mate choosiness in female N. korthausae or an inability of females to influence the number of eggs in the experimental setting used here. However, female Nothobranchius are known to express mate choice among males within (Haas 1976a) and across (M. Reichard, M. Polačik, J. Bryja, unpublished data) populations, including egg number modulation, a characteristic means of female preference in fishes with daily spawning bouts (Eaton and Farley 1974; Côte and Hunte 1989; Spence and Smith 2006). Indeed, individual females differed in the number of eggs they produced and this variability was partly related to female body size, although other unmeasured factors also contributed to interindividual variation in fecundity. The analysis additionally indicated that egg numbers increased over the course of the experiment, with individual females producing more eggs during the fourth spawning. One possibility is that this is because females enjoyed superior conditions (no competition, no male harassment during the ovulation phase, ration approximating to ad libitum) during the experiment and these conditions had long-term consequences for energy allocation to reproduction. In summary, female N. korthausae varied the number of eggs they laid with particular males, but this variance was either not related to differential allocation to particular males or the preference of females was not consistent. It is possible that females based their choice on the nonadditive genetic quality of males (“complementary genes”) rather than additive genetic quality (“good genes”; Qvarnström and Forsgren 1998; Dziminski et al. 2008; Casalini et al. 2009) and therefore were not congruent in their mate preference. The study was not designed to test the effects of nonadditive genetic quality on female preference and fitness, because the principal aim was to investigate the effects of male dominance which, by definition, cannot be linked to nonadditive genetic benefits (Neff and Pitcher 2005) and a different approach would need to be taken to investigate the role of nonadditive genetic benefits (e.g., Pitcher and Neff 2006; Dziminski et al. 2008).
Notably, individual males differed in the hatching success of the progeny they fathered, but the difference was not related to their dominance status or body size. This result suggests the existence of indirect benefits that certain males confer in the form of additive genetic quality (“good genes”) and which are expressed as higher embryo survival. Higher fitness pay offs through additive genetic quality from mating with particular males has been shown in several studies that have controlled for the effect of direct benefits though the use of in vitro fertilizations (Welch et al. 1998; Barber et al. 2001; Wedekind et al. 2001). However, those studies related fitness consequences to mating with males with the most elaborate courtship signals (breeding coloration, mating calls) that may not be linked to male dominance (Candolin 2004; Reichard et al. 2005), and other studies failed to report such a relationship (Kortet et al. 2004; Rudolfsen et al. 2005; Pitcher and Neff 2006). The only two previous studies that explicitly related indirect benefits to male dominance produced results congruent with data from the present study. There was significant variance in male effects on embryo and juvenile survival, but the effect was not related to male dominance, body size, body mass, or age in the brown trout (Salmo trutta) following in vitro fertilization (Jacob et al. 2007). In the rose bitterling (Rhodeus ocellatus), offspring survival was also significantly higher with particular males. Notably, offspring survival was the highest when eggs of a particular female were fertilized by the sperm of a male that the female preferred in a preceding mate choice trial, but there was no relationship between offspring survival and male dominance status (Casalini et al. 2009). Collectively, those results show that females may gain indirect benefits from mating with a particular male. Further, females of some species may be capable of distinguishing those males who confer them. While sexual displays appears to be honest signals of “good genes” in some species (Welch et al. 1998; Barber et al. 2001; Wedekind et al. 2001) implying additive genetic benefits, particular male–female combinations, perhaps arising from mate compatibility (“compatible genes”), may play a more significant role in other species (Dziminski et al. 2008; Casalini et al. 2009), suggesting the role of nonadditive genetic benefits.
The results here do not imply that dominant males do not enjoy higher reproductive success in nature, but show that increased reproductive success by dominant males may not necessarily be beneficial to females. At present, field data are not available to demonstrate a role of direct benefits that dominant Nothobranchius males might confer on females, and the absence of direct benefits of mating with dominant males is often difficult to demonstrate in nature (Andersson 1994; Jacob et al. 2007). Given the mating system of Nothobranchius, there is the possibility of intrasexual competition for superior oviposition sites that may be located in certain parts of natural pools or sites within a pool where predation risk for the spawning pair is reduced. Further studies in the field are needed to understand the dynamics of the mating system in Nothobranchius.
In conclusion, the current study showed that female N. korthausae may receive indirect benefits by mating with particular males through higher embryo survival, but male quality was not linked to male dominance status or body size. This result underlies an emerging view that dominance in males may restrain, rather than augment, female choice for indirect benefits (Reichard et al. 2007) and dominant males may enjoy high reproductive success due to their control of breeding resources and mating coercion rather than through female preference for dominance (Watters 2005; Reichard et al. 2005; Jacob et al. 2007; Casalini et al. 2009). Currently known examples include species with nonresource-based (Watters 2005; Spence and Smith 2006; Jacob et al. 2007; present study) and resource-based (Kangas and Lindström 2001; Reichard et al. 2005; Casalini et al. 2009) mating systems. A comparative study of the role of male dominance on female choice and indirect benefits of male dominance across a range of mating systems would be instructive in uncovering the ultimate nature of the effects of male dominance on indirect benefits of mate choice. Fishes, with their diverse mating systems within relatively small clades (Mank and Avise 2006), are an ideal group to tackle this question (Amundsen 2003).
References
Alatalo RV, Lundberg A, Glynn C (1986) Female pied flycatchers choose territory quality and not male characteristics. Nature 323:152–153
Amundsen T (2003) Fishes as models in studies of sexual selection and parental care. J Fish Biol 63(Suppl A):17–52
Andersson M (1994) Sexual selection. Princeton University Press, Princeton, NJ
Arnqvist G, Lowe R (2005) Sexual conflict. Princeton University Press, Princeton, NJ
Barber I, Arnott SA, Braithwaite VA, Andrew J, Huntingford FA (2001) Indirect fitness consequences of mate choice in sticklebacks: offspring of brighter males grow slowly but resist parasite infections. Proc R Soc Lond B 268:71–76
Candolin U (1999) Male–male competition facilitates female choice in sticklebacks. Proc R Soc Lond B 266:785–789
Candolin U (2001) Male–male competition ensures honest signaling of male parental ability in the three-spined stickleback (Gasterosteus aculeatus). Behav Ecol Sociobiol 49:57–61
Candolin U (2004) Opposing selection on a sexually dimorphic trait through female choice and male competition in a water boatman. Evolution 58:1861–1864
Casalini M, Agbali M, Reichard M, Konečná M, Bryjová A, Smith C (2009) Male dominance, female choice and intersexual conflict in the rose bitterling (Rhodeus ocellatus). Evolution 63:366–376
Clutton-Brock TH, Parker GA (1995) Sexual coercion in animal societies. Anim Behav 49:1345–1365
Côte IM, Hunte W (1989) Male and female mate choice in the redlip blenny: why bigger is better. Anim Behav 38:78–88
De Fraipont M, FitzGerald GJ, Guderlay H (1993) Age-related differences in reproductive tactics in the three-spined stickleback, Gasterosteus aculeatus. Anim Behav 46:961–968
Dziminski MA, Roberts JD, Simmons LW (2008) Fitness consequences of parental compatibility in the frog Crinia georgiana. Evolution 62:879–886
Eaton RC, Farley RD (1974) Spawning cycle and egg production of zebrafish, Brachydanio rerio, in the laboratory. Copeia 1974:195–204
Eberhard WG (1996) Female control: sexual selection by cryptic female choice. Princeton University Press, Princeton, NJ
Engqvist L (2007) Sex, food and conflicts: nutrition dependent nuptial feeding and pre-mating struggles in scorpionflies. Behav Ecol Sociobiol 61:703–710
Genade T, Benedetti M, Terzibasi E, Roncaglia P, Valenzano DR, Cattaneo R, Cellerino A (2005) Annual fishes of the genus Nothobranchius as a model system for aging research. Aging Cell 4:223–233
Haas R (1976a) Behavioral biology of the annual killifish, Nothobranchius guentheri. Copeia 1976:81–91
Haas R (1976b) Sexual selection in Nothobranchius guentheri (Pisces: Cyprinidontidae). Evolution 30:614–622
Head ML, Hunt J, Jennions MD, Brooks R (2005) The indirect benefits of mating with attractive males outweigh the direct costs. PLoS Biol 3:e33
Houde AE (1997) Sex, color, and mate choice in guppies. Princeton University Press, Princeton, NJ
Huber JH (2000) Killi-Data 2000. Updated checklist of taxonomic names, collecting localities and bibliographic references of oviparous Cyprinodont fishes (Cyprinodontiformes). Cybium, Paris
Jacob A, Nusslé S, Britschgi A, Evanno G, Müller R, Wedekind C (2007) Male dominance linked to size and age, but not to ‘good genes’ in brown trout (Salmo trutta). BMC Evol Biol 7:207
Kangas N, Lindström K (2001) Male interaction and female mate choice in the sand goby, Pomatoschistus minutes. Anim Behav 61:425–430
Kokko H (2005) Treat 'em mean, keep 'em (sometimes) keen: evolution of female preferences for dominant and coercive males. Evol Ecol 19:123–135
Kokko H, Jennions MD, Brooks R (2006) Unifying and testing models of sexual selection. Annu Rev Ecol Evol Syst 37:43–66
Kolm N (2001) Females produce larger eggs for large males in a paternal mouthbrooding fish. Proc R Soc Lond B 268:2229–2234
Kolm N, Olsson J (2003) Rapid matching of egg size to mate attractiveness in the Banggai cardinalfish. J Fish Biol 63:144–151
Kortet R, Vainikka A, Rantala MJ, Myntti J, Taskinen J (2004) In vitro embryo survival and early viability of larvae in relation to male sexual ornaments and parasite resistance in roach, Rutilus rutilus L. J Evol Biol 17:1337–1344
Mank JE, Avise JC (2006) The evolution of reproductive and genomic diversity in ray-finned fishes: Insights from phylogeny and comparative analysis. J Fish Biol 69:1–27
Milinski M, Bakker TCM (1990) Female sticklebacks use male colouration in mate choice and hence avoid parasitized males. Nature 344:330–333
Møller AP, Alatalo RV (1999) Good-genes effects in sexual selection. Proc R Soc Lond B 266:85–91
Nakagawa S, Cuthill IC (2007) Effect size, confidence interval and statistical significance: a practical guide for biologists. Biol Rev 82:591–605
Nakatsuru K, Kramer DL (1982) Is sperm cheap? Limited male fertility and female choice in the lemon tetra (Pisces, Characidae). Science 216:753–755
Neff BD, Pitcher TE (2005) Genetic quality and sexual selection: an integrated framework for good genes and compatible genes. Mol Ecol 14:19–38
Ophir AG, Galef BG Jr (2003) Female Japanese quail that ‘eavesdrop’ on fighting males prefer losers to winners. Anim Behav 66:399–407
Pitcher TE, Neff BD (2006) MHC class IIB alleles contribute to both additive and non-additive genetic effects on survival in Chinook salmon. Mol Ecol 15:2357–2365
Pitcher TE, Neff BD, Rodd FH, Rowe L (2003) Multiple mating and sequential mate choice in guppies: females trade up. Proc R Soc Lond B 260:1623–1629
Preston BT, Stevenson IR, Pemberton JM, Wilson K (2001) Dominant rams lose out by sperm depletion. Nature 409:681–682
Qvarnström A (1997) Experimentally increased badge size increases male competition and reduces male parental care in the collared flycatcher. Proc R Soc Lond B 264:1225–1231
Qvarnström A, Forsgren E (1998) Should female prefer dominant males? Trends Ecol Evol 13:498–503
Reichard M, Bryja J, Ondračková M, Dávidová M, Kaniewska P, Smith C (2005) Sexual selection for male dominance reduces opportunities for female mate choice in the European bitterling (Rhodeus sericeus). Mol Ecol 14:1533–1542
Reichard M, Le Comber SC, Smith C (2007) Sneaking from a female perspective. Anim Behav 74:679–688
Reichard M, Polačik M, Sedláček O (2009) Distribution, colour polymorphism and habitat use of the African killifish, Nothobranchius furzeri, the vertebrate with the shortest lifespan. J Fish Biol 74:198–212
Rudolfsen G, Figenshou L, Folstad I, Nordeide JT, Søreng E (2005) Potential fitness benefits from mate selection in the Atlantic cod (Gadus morhua). J Evol Biol 18:172–179
Smith C, Douglas A, Jurajda P (2002) Sexual conflict, sexual selection and sperm competition in the spawning decisions of bitterling, Rhodeus sericeus. Behav Ecol Sociobiol 51:433–439
Spence R, Smith C (2006) Mating preference of female zebrafish, Danio rerio, in relation to male dominance. Behav Ecol 17:779–783
Valdesalici S, Cellerino A (2003) Extremely short lifespan in the annual fish Nothobranchius furzeri. Proc R Soc Lond B 279:S189–S191
Warner RR (1987) Female choice of sites versus mates in a coral reef fish, Thalassoma bifasciatum. Anim Behav 35:1470–1478
Warner RR, Shapiro DY, Marcanato A, Petersen CW (1995) Sexual conflict—males with the highest mating success convey the lowest fertilization benefits to females. Proc R Soc Lond B 262:135–139
Watters JV (2005) Can alternative male tactics ’fighter’ and ‘sneaker’ be considered ‘coercer’ and ‘cooperator’? Anim Behav 70:1055–1062
Wedekind C, Muller R, Spicher H (2001) Potential genetic benefits of mate selection in whitefish. J Evol Biol 14:980–986
Welch AM, Semlitch RD, Gerhardt HC (1998) Call duration as an indicator of genetic duality in male gray tree frogs. Science 280:1928–1930
Wildekamp RH (2004) A world of killies: atlas of the oviparous cyprinidontiform fishes of the world, volume IV. American Killifish Association, Elyria
Acknowledgments
The study was supported by Czech Science Foundation (206/06/P152). Both authors hold a license for conducting experimental work on vertebrates and all experiments complied with current legal regulations in the Czech Republic. The research was approved by the ethical committee of the Czech Academy of Sciences and the Institute of Vertebrate Biology. The authors thank Carl Smith for comments on the manuscript. M. P. and M. R. conceived and designed the research and wrote the paper. M. P. performed the experiments and collected the data, which were analyzed by M. R.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by J. Krause
Rights and permissions
About this article
Cite this article
Polačik, M., Reichard, M. Indirect fitness benefits are not related to male dominance in a killifish. Behav Ecol Sociobiol 63, 1427–1435 (2009). https://doi.org/10.1007/s00265-009-0798-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00265-009-0798-2