Abstract
Reproductive investment decision is an integral part of life-history theory. Differential allocation hypothesis predicts that females should increase investment when mated to high-quality males, conversely, reproductive compensation hypothesis predicts that females should increase investment when mated to low-quality males. Empirical research dominantly focuses on polygamous species and rarely on serially monogamous species. So, the question remains: which hypothesis does serially monogamous species fit? And if it fits reproductive compensation hypothesis, do females only compensate once or continuously for multiple times when mating to low-quality males? Here, we used a serially monogamous fish, the lined seahorse (Hippocampus erectus), to investigate the reproductive investment pattern of females in relation to male quality (measured by sexual attractiveness). We found that females allocated more resources into eggs when they mated to less-sexually-attractive males, indicating the investment pattern of lined seahorse falls in with the prediction of reproductive compensation hypothesis. This finding may imply that the sex role of seahorses is reversed, and female is the side imposed on a greater sexual selection pressure. On this basis, we compared the investment difference of females in two consecutive breeding events when mated to less-sexually-attractive males. We found that females allocated less resources into eggs in the second breeding than in the first one. Females reduced their reproductive compensation in the second breeding, which may be attributed to the improvement in the quality (e.g., paternal care ability) of their mates after the first breeding, thus eliminating the need for them to invest more in the second breeding.
Similar content being viewed by others
Introduction
Species showing serial monogamy refer to animals that have only one partner during multiple breeding seasons1. Although these animals do not make mate selection decisions frequently, their pressure on sexual selection is not weak, and has even been shown to be comparable to polygamous species in a simulation study on cichlid fish2. Serially monogamous species do not have the opportunity to correct a poor mate selection by mating to multiple partners as polygamous species do, so they have to be cautious and choosy, and put more effort into mate choice to maximize their lifetime reproductive success1,3.
Serially monogamous species are not always able to pair with high-quality partners as they wish, due to the shortage of high-quality resources and high cost of searching for them4,5. This means that some of them have to pair with ordinary (i.e., non-high-quality) or even low-quality partners1,6. Once establishing a mating relationship with low-quality partner, serially monogamous species would be faithful to the poor partner in the subsequent breeding events, which implies that they would basically lose the likelihood of mating to an expected high-quality partner in the future7. Considering that the offspring produced by low-quality partner often have potential viability deficits (e.g., small body size, nutritional and immune deficiency)8,9, how do serially monogamous species maximize their lifetime reproductive success when they are constrained to mate to low-quality partner has been a research hotspot. Life-history theory predicts that the quality of males could affect offspring fitness via direct and/or indirect effects, which would affect the investment of females in reproduction, and females should adjust their investment in a particular breeding attempt according to the expected pay-offs of that attempt10. Based on this theory, researchers have begun to pay attention to the reproductive investment of females showing serial monogamy in relation to male quality.
To date, studies on female reproductive investment in relation to male quality have dominantly focused on polygamous species. In the field, there are two hypotheses, one is the differential allocation hypothesis (DAH), and the other one is the reproductive compensation hypothesis (RCH). DAH assumes that females should weigh costs and benefits of investing into reproduction with a current partner against the expected quality of future partners, and predicts that they should invest more into reproduction when pairing with a high-quality partner11. On the contrary, RCH assumes that individual mating preferences predict offspring viability and that ecological and social constraints on the expression of mating preferences are common, and thus predicts that females should increase their reproductive investment to make up for lowered offspring viability when pairing with a partner of low quality12,13. Both hypotheses have been supported in a range of species. The house mouse14, the zebra finch15, the lance-tailed manakin16, the European waterfrog17, the banggai cardinal fish18 and the convict cichlid19 are on side of DAH. The pronghorn20, the house finch21, the mallard8 and the broad-nosed pipefish22 are in favor of RCH. In addition to these extensive empirical studies, there are also some studies that summarized the influencing factors, occurrence conditions and applicable populations of these two hypotheses. Harris & Uller23 pointed out that DAH is the mainstream investment pattern across the animal kingdom. Bolund et al.10 indicated that the likelihood of mating to a higher quality partner in the future and the degree of reproductive skew in the population are the two most decisive factors, and mating systems with high reproductive skew incline to DAH, while mating systems with low reproductive skew favor RCH.
To our knowledge, study on female reproductive investment in relation to male quality in serially monogamous species is rare and the zebra finch is the only case10. A major difference between polygamous species and monogamous species is that the former has the expectation of mating to a higher quality partner in the future, while the latter has almost none. Therefore, the question remains: will females showing serial monogamy adjust their reproductive investment based on the quality of their partners, just like that in polygamous species? If so, which hypothesis do they support? Moreover, the mating system of serially monogamous species is characterized by low reproductive skew24. According to Bolund et al.’s prediction that this type of mating system tends to support RCH10, if that’s really the case, do the females only compensate for one breeding event or compensate continually for multiple breeding events when they are constrained to mate to the males they do not prefer? These questions have attracted much attention but not been well studied yet.
Seahorses (family Syngnathidae, genus Hippocampus) are a group of fishes that show serial monogamy25,26,27. The bond of paired seahorses is stable and can last through multiple breeding seasons. The paired seahorses rarely change their partners unless one side of the pair has a health problem, disappears or dies28,29. Moreover, seahorses also show male-only parental care. Female seahorse deposits eggs into the male’s brood pouch where the eggs are fertilized. Then, the male provides care solely. He broods the embryos including respiratory gas exchange, osmoregulation, metabolic waste removal and nutrition provision, until independent young are released30. This form of care is termed as male pregnancy. In the present study, the lined seahorse (Hippocampus erectus) was employed as a representative of serially monogamous species to investigate the reproductive investment pattern of female in relation to male quality (measured by sexual attractiveness). The aim is to provide an empirical case to answer the above questions, thereby to broaden our understanding of reproductive investment pattern of the species with different mating systems.
Materials and methods
The study species
The seahorse used in the present study was the lined seahorse (H. erectus). Its serial monogamy had been verified by our previous study29. Similar to other seahorse species26,31, it takes at least 3–4 days of courtship for two receptive lined seahorses (one male and one female) that have never met before to mate successfully from their meeting. After mating, the male begins to brood embryos, which takes approximately 15 days from pregnancy to giving birth. During male pregnancy, the female also prepares eggs synchronously based on the male’s pregnancy stage, and the period required from egg deposition to the maturation of the next clutch of eggs is synchronized with the period required for male pregnancy. After giving birth, the male and the female can mate again in a short time.
The lined seahorse used in the present study was artificially cultivated and provided by the Seahorse Research Center of the East China Sea Fisheries Research Institute, Qionghai City, Hainan Province, China. The center housed various sizes of artificially cultivated lined seahorse. Seahorses with newly differentiated gender were collected and reared in the tanks, separated by gender. The gender of seahorses can be distinguished based on whether the male has a brood pouch. The rearing conditions were maintained with a water temperature of 25–26 ℃, a salinity of 30–31‰, a dissolved oxygen content of 6.0–6.5 mg/L, and a pH of 8.0–8.2, respectively. The rearing tanks were continuously flowed with fresh seawater for 12 h a day at a flow rate of 1.0–1.2 L/min. The collected seahorses were kept feeding frozen Mysis twice a day until they reached the required size of approximately 13 cm in body height.
Males with body height of 13–14 cm and well-developed brood pouch, and females with body height of 12–13 cm and well-developed gonad were picked out as the experimental seahorses. The experimental seahorses were stored in the holding tanks, still separated by gender, and still fed frozen Mysis twice a day.
Methods of sexual attractiveness improvement
In practice, feeding live Mysis can significantly improve the reproductive performance of seahorses. We assume that seahorses fed live Mysis should be more sexually attractive than seahorses fed frozen Mysis. Actually, we not only observed that compared to males fed frozen Mysis, males fed live Mysis exhibited brighter body color and more active courtship behavior, but also confirmed that feeding live Mysis did have an improvement on sexual attractiveness of males in our pilot experiment. In that experiment, 20 males were fed live Mysis, another 20 males were fed frozen Mysis, and 20 females were also fed frozen Mysis twice a day for 15 days. Afterwards, one live-Mysis-fed male, one frozen-Mysis-male and one female were placed together in a breeding tank (0.8 m in diameter and 0.8 m in depth) for observing the mate choice. These two males were distinguished by tagging their necks with thin cotton threads of different colors. Of these 20 females, 17 females mated to the live-Mysis-fed males, one female mated to the frozen-Mysis-fed males, and the remaining two females failed to mate (Binomial test, p < 0.001). Therefore, feeding live Mysis is indeed a workable method to improve male’s sexual attractiveness. Here, we designated the live-Mysis-fed males as the sexually attractive males and the frozen-Mysis-fed males as the less sexually attractive males.
Experimental design
In the present study, with the purpose to investigate the investment pattern of serially monogamous species, we first conducted a comparative experiment about the reproductive investment of a focal female seahorse in relation to two male seahorses with different sexual attractiveness. If the result shows that the investment pattern follows RCH, then we would conduct the second experiment about the difference in reproductive investment between two consecutive breeding events when females were constrained to mate to less sexually attractive males. The purpose of the second experiment is to explore whether the females only compensate once or continuously for multiple times, when they need to do so.
Indexes for measuring female reproductive investment
For females involved in maternal care, their reproductive investment includes two parts, gametic investment (e.g., egg size, egg number, yolk content) and care investment (e.g., care frequency, care duration). While for seahorse females, as they do not participate in maternal care, their reproductive investment is only reflected in gametic investment. Therefore, we only used gamete related indexes to measure the reproductive investment of female seahorse.
In theory, the number and size of eggs laid by a female are the most important gametic indexes for measuring her gametic investment10. However, for seahorse, part of the eggs transferred to the male’s pouch will rupture32, making it difficult to accurately count. Meanwhile, eggs come in various morphotypes with great differences in size and in proportion between each type33, also making it difficult to accurately measure the size of eggs.
As an alternative, we used some compositions in the eggs to measure and evaluate the gametic investment of female seahorses. Numerous studies have demonstrated that in addition to the size of eggs, the composition of eggs is also an important index for evaluating the quality of fish eggs. Egg proteins and lipids constitute the macro components in fish eggs and are the most important source of energy for developing embryos34. Eggs containing high contents of amino acids and lipids (n-3 highly unsaturated fatty acids (n-3 HUFAs), glycerolipids and glycerophospholipids, etc.) are widely considered to be of better quality and can improve embryonic development35,36,37. Besides, the micro components in fish eggs, such as immunoglobulins, hormones and pigments, also play a crucial role in embryonic development. They can promote yolk utilization and protect developing embryos against oxidative damage from free radicals and against pathogens34,38,39. Therefore, the indexes used in the present study included the compositions and contents of amino acids and n-3 HUFAs, the expression abundance of lipids and other metabolites, and the contents of testosterone, immunoglobulin M (Ig M) and carotenoid in the eggs.
Experiment 1: a focal female’s reproductive investment in relation to two males with different sexual attractiveness
Pairing and mating
According to the method described in 2.2, the male experimental seahorses in the holding tanks were divided into two groups. One group continued to feed frozen Mysis, and the males in this group were designated as less sexually attractive males; the other group switched to feed live Mysis, and the males in this group were designated as sexually attractive males. The female experimental seahorses in the holding tanks continued to feed frozen Mysis.
After 15 days, 40 females were selected and assigned to 40 breeding tanks, with one female per tank. For tanks 1−20, each female was first paired with a sexually attractive male. The mating status was observed daily, and the observation lasted for two weeks. If no mating was observed within two weeks, it was defined as mating failure. The criterion for mating success was to observe an obvious expansion of the male’s pouch (due to the reception of eggs) and an obvious contraction of the female’s abdomen (due to the expulsion of eggs) simultaneously. Once mating success was observed, the sexually attractive male was removed from the tank and anaesthetized with a solution of MS-222 (50 mg/L) in seawater for egg collection. Fifteen days after the removal of sexually attractive male, the female was then paired with a less sexually attractive male. When mating success occurred again, the less sexually attractive male was also removed and anaesthetized for egg collection. While for tanks 21−40, the operating procedure was the same as for tanks 1–20, but each female was first paired with a less sexually attractive male and then with a sexually attractive male. During the pairing and mating period, the seahorses in each breeding tank were fed frozen Mysis.
Due to mating failure or death of the seahorses during the experiment, a total of 22 focal females that finished two consecutive breeding events were obtained. The identities of these focal females were named after the tank numbers they were stayed, i.e., F1 (the female in tank 1), F2, F6, F8, F9, F12, F13, F16, F17, F20, F21, F23, F25, F26, F27, F29, F31, F33, F34, F36, F38 and F39, respectively.
Egg collection
The successfully mated male seahorse was removed from the tank and anaesthetized for egg collection. To collect eggs, a small incision was made using a sterilized scissors at the opening of the brood pouch. A Pasteur pipette was inserted into the incision to gently suck out the mixture of seminal fluid and eggs in the brood pouch. The mixture was passed through a 60-mesh screen and the eggs were collected. The collected eggs were rinsed with 0.01 M phosphate-buffered solution (PBS) and stored at −80 ℃ until determinations. After the eggs were removed, the male seahorse was immersed in a 10% povidone iodine seawater solution to disinfect the incision, and then was transferred to a new tank with fresh seawater to recover from anaesthesia. This male seahorse was no longer used for any experiment.
Determinations of gamete related indexes
The egg samples of eight focal females, four mated to the sexually attractive male first (IDs: F8, F9, F12 and F13) and four mated to the less sexually attractive male first (IDs: F26, F27, F29 and F31), were used for analysis of the composition and content of amino acids and n-3 HUFAs. The egg sample of each female was divided into two subsets, one for amino acid analysis and the other for n-3 HUFAs analysis. Amino acids were determined using an automatic amino acid analyzer (LA8080; Hitachi High-Tech, Tokyo, Japan). Briefly, the egg samples were vacuum freeze-dried and then were hydrolyzed in 6 M HCl. The hydrolysate was electric-blast dried at 110 ℃ for 22 h, and the resulting residue was dissolved in 1–2 ml of sodium citrate-hydrochloric acid buffer solution (pH 2.0) and filtered with a 0.22 μm filter membrane. Twenty microliters of the filtrate were taken to add onto the analyzer. Fatty acids were determined using capillary gas chromatography. Briefly, total lipids of the vacuum freeze-dried egg samples were extracted according to the method of Bligh and Dyer40. Then fatty acid methyl esters (FAMEs) were produced from the total lipids and methylated with boron trifluoride-methanol solution at 85 ℃ for 30 min. After cooling, 1 ml of n-hexane was added to the methylated FAMEs, shaken for two minutes and left to stand for one hour. Finally, 100 μl of the supernatant was taken to add onto a gas chromatograph (Agilent 7890A, Santa Clara, CA, USA) with a capillary column (TG-FAME, 50 m × 0.25 mm × 0.20 μm)41.
The egg samples of another eight focal females, three mated to the sexually attractive male first (IDs: F16, F17 and F20) and five mated to the less sexually attractive male first (IDs: F33, F34, F36, F38 and F39), were used for analysis of the content of Ig M, testosterone and carotenoid. They were determined using commercial ELISA kits (Abmart Shanghai Co., Ltd., Shanghai, China). Briefly, the egg samples were thawed and weighed first, then homogenized in nine volumes v/w (ml/g) of pre-cold 0.01 M PBS. The homogenate was centrifuged (3K16, Sigma, Osterode am Harz, Germany) at 3300 × g for 10 min at 4 ℃, and the supernatant was collected. Fifty microliters of the supernatant were added into a microplate, then horseradish peroxidase (HRP) labeled antibody, coloring solution and stop solution were successively added according to the manufacturer’s instructions. Finally, the absorbance values were measured at 450 nm with an automatic ELISA microplate reader (Molecular Devices Inc., Sunnyvale, CA, US). The concentration of egg soluble protein in the supernatant was determined by the method of Bradford42.
The egg samples of the remaining six focal females, three mated to the sexually attractive male first (IDs: F1, F2 and F6) and three mated to the less sexually attractive male first (IDs: F21, F23 and F25), were used for analysis of the expression abundance of lipids and other metabolites. They were determined using ultra-high performance liquid chromate- graphy tandem mass spectrometry (UHPLC-MS/MS). Briefly, 60 mg of the egg samples were homogenized in 200 μl of distilled water. Then the metabolites were extracted with 800 μl of methanol–acetonitrile solution (1:1, v/v) and vacuum freeze-dried. The extract was dissolved in 100 μl of acetonitrile–water (1:1, v/v), and 2 μl of this solution was taken to add onto an ACQUITY UPLC BEH Amide column (1.7 μm, 2.1 mm × 100 mm) (Waters Technologies Ireland Ltd., Wexford, Ireland) for hydrophilic interaction liquid chromatography with an Agilent 1290 Infinity UHPLC system. Detailed detection parameters and data processing were referenced to the study of Sun et al.43.
Experiment 2: investment difference of females in two consecutive breeding events when mated to less sexually attractive males
In experiment 1, we found that the investment pattern of female seahorse supports RCH. Therefore, we further conducted experiment 2.
In experiment 2, the ideal scenario would be that a female mated twice consecutively with the same less sexually attractive male, collecting eggs each time. However, the actual scenario is that it was impossible for a female to mate with the same male twice because the male was already injured in the first egg collection. Therefore, it is not workable to collect the eggs from the same less sexually attractive male twice in a row. As an alternative, we compared two parallel groups. The pairs (one female and one less sexually attractive male) were divided into two groups. Group I was for the collection of eggs at the first breeding event (i.e., only collection the first clutch of eggs), while group II was for the collection of eggs at the second breeding event (i.e., only collection the second clutch of eggs).
Specifically, seventy pairs (one female and one less sexually attractive male) of experimental seahorses were randomly picked out and assigned to 70 breeding tanks, one pair per tank. These tanks were divided evenly into two groups as mentioned above, tanks 1–35 as group I, and tanks 36–70 as group II. For group I, once mating success was observed, the successfully mated male was removed and anaesthetized for egg collection. While for group II, when mating success was observed, the successfully mated male was not removed, and he continued to stay with the female. He was only removed and anaesthetized for egg collection after giving birth and successfully mating with the female for the second time.
A total of 29 egg samples were collected in group I, and a total of 26 egg samples were collected in group II. Ten samples from each group were used for analysis of the compositions and contents of amino acids and n-3 HUFAs, another ten samples from each group were used for analysis of the contents of Ig M, testosterone and carotenoid, and the remaining six samples from each group were used for analysis of the expression abundance of lipids and other metabolites.
Statistical analysis
Statistical analysis was performed using SPSS statistical software (version 17.0, Chicago, Illinois). In experiment 1, the difference in gamete related indexes was analyzed using the paired sample t-test. While in experiment 2, the data were expressed as the mean ± standard deviation (SD), and the difference in gamete related indexes was analyzed using two independent sample t-test. Before running t-test, normality of data was tested using Shapiro–Wilk test. The p-value of 0.05 was taken as a significance level. In the data of amino acids and n-3 HUFAs, multiple tests were applied, and the value indicating significance level was corrected accordingly by the Bonferroni method as p/N, where p is 0.05 and N is the number of multiple tests44.
Ethics approval
Ethical approval for this study was obtained from the Committee on the Ethics of Animal Experiments of Chinese Academy of Fishery Sciences (Permit Number: 20200225). All procedures were conducted in compliance with the recommendations in the Guide for the Management and Use of the Experimental Animals of China Science and Technology Commission. This study was reported in accordance with ARRIVE guidelines.
Results
Experiment 1: a focal female’s reproductive investment in relation to two males with different sexual attractiveness
Amino acids
The compositions and contents of amino acids in the eggs provided by females to sexually attractive males and to less sexually attractive males are shown in Table 1. A total of 17 amino acids were detected, of which six amino acids showed significant differences in content between these two types of eggs. These six differential amino acids were glutamic acid (Glu, t = −5.954, df = 7, p = 0.001), cysteine (Cys, t = −4.333, df = 7, p = 0.003), isoleucine (Ile, t = −4.706, df = 7, p = 0.002), leucine (Leu, t = −18.278, df = 7, p < 0.001), lysine (Lys, t = −8.130, df = 7, p < 0.001) and arginine (Arg, t = −8.763, df = 7, p < 0.001), respectively. These six differential amino acids, along with the total amino acids (TAA, t = −6.060, df = 7, p < 0.001), all were significantly higher in the eggs provided to less sexually attractive males than those in the eggs provided to sexually attractive males, whether the females mated to less sexually attractive males first or later.
n-3 HUFAs
The compositions and contents of n-3 HUFAs in the eggs provided by females to sexually attractive males and to less sexually attractive males are shown in Table 2. A total of four n-3 HUFAs were detected, all of which showed significant differences in content between these two types of eggs. These four differential n-3 HUFAs were C18:3n3 (α-linolenic acid, t = −4.107, df = 7, p = 0.005), C20:3n3 (cis-11,14,17-eicosatrienoic acid methyl ester, t = −3.917, df = 7, p = 0.006), C20:5n3 (eicosapentaenoic acid, EPA, t = −4.006, df = 7, p = 0.005) and C22:6n3 (docosahexaenoic acid, DHA, t = −4.735, df = 7, p = 0.002), respectively. These four differential n-3 HUFAs, along with the total n-3 HUFAs (t = −6.060, df = 7, p < 0.001), all were significantly higher in the eggs provided to less sexually attractive males than those in the eggs provided to sexually attractive males, whether the females mated to less sexually attractive males first or later.
Ig M, testosterone and carotenoid
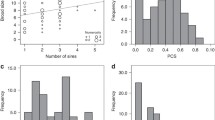
Whether the females mated to less sexually attractive males first or later, the testosterone content (t = −7.498, df = 7, p < 0.001) in the eggs they provided to less sexually attractive males was significantly higher than that in the eggs they provided to sexually attractive males (Fig. 1b). As for Ig M (t = 0.638, df = 7, p = 0.544) (Fig. 1a) and carotenoid (t = −0.472, df = 7, p = 0.652) (Fig. 1c), no significant difference in content was observed between these two types of eggs.
Lipids and other metabolites
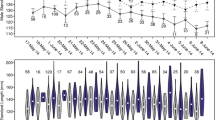
The metabolomic data show that there were a large number of significantly differential metabolites (SDMs) between the eggs provided to sexually attractive males and the eggs provided to less sexually attractive males. Specifically, the vast majority of these SDMs were expressed more abundantly in the eggs provided to less sexually attractive males than in the eggs provided to sexually attractive males, whether the females mated to less sexually attractive males first or later. Taking the top 30 SDMs as an example, the ratios of the number of abundance-upregulated SDMs to abundance-downregulated SDMs when females mated to less sexually attractive males were 24/6 (F1), 26/4 (F2), 17/13 (F6), 22/8 (F21), 21/9 (F23) and 24/6 (F25), respectively (Figs. 2, 3).
The metabolomic profiles of eggs provided by females F1, F2 and F6 to the sexually attractive male and the less sexually attractive male, respectively. The metabolites listed on the heatmap are the top 30 significantly differential metabolites (SDMs) with the highest expression abundance. The blue color on the heatmap represents a decrease in the abundance of the metabolite, while red represents an increase. Metabolite classes with pink, yellow and green shades are the increased SDMs present in all six females (F1, F2, F6, F 21, F23 and F25) when they mated to less sexually attractive males, while metabolite classes with orange and cyan shades are the decreased SDMs when they mated to less sexually attractive males. PGP glycerophosphoglycerophosphates, PI glycerophosphoinositols, PG glycerophospho- glycerols, PIM1 glycerophosphoinositolglycans, PS glycerophosphoserines, PC glycero- phosphocholines.
The metabolomic profiles of eggs provided by females F21, F23 and F25 to the sexually attractive male and the less sexually attractive male, respectively. The metabolites listed on the heatmap are the top 30 significantly differential metabolites (SDMs) with the highest expression abundance. The blue color on the heatmap represents a decrease in the abundance of the metabolite, while red represents an increase. Metabolite classes with pink, yellow and green shades are the increased SDMs present in all six females (F1, F2, F6, F 21, F23 and F25) when they mated to less sexually attractive males, while metabolite classes with orange and cyan shades are the decreased SDMs when they mated to less sexually attractive males. PIM1 glycerophosphoinositolglycans, PE glycerophosphoethanolamines, PS glycero- phosphoserines, PA glycerophosphates, PGP glycerophosphoglycerophosphates.
Many classes of lipids were involved in the top 30 SDMs, such as glycerophospholipids, sphingolipids, sterol lipids, prenol lipids and polyketides. The expression abundance of glycerophospholipids (marked with pink shade in the figures) and polyketides (yellow shade) remained consistently upregulated, while prenol lipids (orange shade) remained consistently downregulated in all analyzed females when they mated to less sexually attractive males. The subclasses of glycerophospholipids included glycerophosphoethanolamines (PE), glycerophosphoinositols (PI), glycerolphosphoglycerophosphates (PGP), glycerophosphoinositolglycans (PIM1), glycerophosphoglycerols (PG), glycerophosphoserines (PS), glycerophosphocholines (PC) and glycerophosphates (PA). The subclasses of polyketides included macrolides, lactone polyketides and flavonoids. In addition to lipids, other metabolites were also involved in the top 30 SDMs. The expression abundance of carboxylic acids and derivatives (mainly amino acids and peptides) (green shade) remained consistently upregulated, while benzene and substituted derivatives (cyan shade) remained consistently downregulated in the analyzed females when they mated to less sexually attractive males (Figs. 2, 3).
Experiment 2: investment difference of females in two consecutive breeding events when mated to less sexually attractive males
Amino acids
The compositions and contents of amino acids in the eggs provided by females to less sexually attractive males during the first and second breeding events are shown in Table 3. A total of 17 amino acids were detected, among which only the content of serine (Ser, t = 3.718, df = 18, p = 0.002) and total amino acids (TAA, t = 3.707, df = 18, p = 0.001) showed significant differences between these two events. Specifically, the levels of Ser and TAA in the second clutch of eggs both were significantly lower than those in the first clutch of eggs.
n-3 HUFAs
The compositions and contents of n-3 HUFAs in the eggs provided by females to less sexually attractive males during the first and second breeding events are shown in Table 4. A total of four n-3 HUFAs were detected, of which three showed significant differences in content between these two clutches of eggs. They were C20:3n3 (t = 3.654, df = 18, p = 0.002), C20:5n3 (EPA, t = 3.347, df = 18, p = 0.004) and C22:6n3 (DHA, t = 3.646, df = 18, p = 0.002), respectively. These three differential n-3 HUFAs, along with the total n-3 HUFAs in the second clutch of eggs were all significantly lower than those in the first clutch of eggs.
Ig M, testosterone and carotenoid
Compared with the first clutch of eggs, although the contents of testosterone (t = 1.824, df = 18, p = 0.085, Fig. 4b) and carotenoid (t = 0.594, df = 18, p = 0.560, Fig. 4c) decreased slightly in the second clutch of eggs, there was no statistically difference between these two clutches of eggs. As for Ig M, no significant difference in content was observed as well between these two clutches of eggs (t = −1.206, df = 18, p = 0.243, Fig. 4a).
The contents (mg or μg/g egg soluble protein) of Ig M (mean ± SD: 0.488 ± 0.048 vs 0.514 ± 0.048), testosterone (3.985 ± 0.185 vs 3.847 ± 0.152) and carotenoid (3.581 ± 0.194 vs 3.532 ± 0.175) in the eggs provided by females (N = 10) to the less sexually attractive males for the first and second breeding, respectively.
Lipids and other metabolites
The metabolomic data show that there were a large number of SDMs between the first and the second clutch of eggs. Taking the top 20 SDMs as example, compared with the first clutch of eggs, the expression abundance of glycerophospholipids (marked with pink shade in the figures) and polyketides (yellow shade) was downregulated, and the expression abundance of prenol lipids (orange shade) and benzene and substituted derivatives (cyan shade) was upregulated in the second clutch of eggs (Fig. 5). In general, the lipids and other metabolites upregulated in the eggs provided to less sexually attractive males in Experiment 1 were downregulated in the second clutch of eggs in Experiment 2, and vice versa.
The metabolomic profile of eggs provided by females to the less sexually attractive males for the first and the second breeding, respectively. The metabolites listed in the heatmap are the top 20 significantly differential metabolites (SDMs) with the highest relative expression abundance. The blue color on the heatmap represents a decrease in the abundance of the metabolite, while red represents an increase. Metabolite classes with pink and yellow shades are the metabolites that were upregulated expressed in the eggs when females mated to the low-quality males in the Experiment 1, but were downregulated expressed in the eggs of the second breeding in the Experiment 2. Metabolite classes with orange and cyan shades are the metabolites that were downregulated expressed in the eggs when females mated to the low-quality males in the Experiment 1, but were upregulated expressed in the eggs of the second breeding in the Experiment 2. PA glycerophosphates, PC glycerophosphocholines, PE glycerophosphoethanolamines.
Discussion
The results of the present study clearly indicate that the reproductive investment pattern of the lined seahorse (H. erectus) follows the assumptions of RCH as the females allocated more resources into eggs when they mated to the males of low sexual attractiveness or low quality. Moreover, the compensation investment by the females mainly focuses on the first breeding event, and will decrease in the second event.
Egg amino acids, acting the precursors of many biological compounds (notably protein) and the substrates for energy production, play a crucial role in embryonic development of marine teleost. Lack or low content of one or more amino acids have been proven to lead to growth retardation, dysplasia and even death of developing embryos36,37. Egg lipids not only provide the primary energy for developing embryos, but also are involved in many biological functions, such as n-3 HUFAs (especially EPA and DHA) involvement in embryonic pigmentation, organogenesis (e.g., eyes and brain) and neurological development; and glycerophospholipids and polyketides involvement in embryonic immune regulation and lipid metabolism regulation36,38. Eggs containing high contents of amino acids and lipids are widely considered to be of better quality and can improve embryonic development34. In addition, sex steroids, such as testosterone, also play a crucial role in embryonic development. They can increase protein synthesis during embryonic development through the promotion of polyamine synthesis38. In experiment 1, when a female mated to a less sexually attractive male, a large number of substances related to embryonic development, such as amino acids, carboxylic acids and derivatives (peptides), n-3 HUFAs, other lipids (glycerophospholipids and polyketides) and testosterone, in the eggs she provided to the male significantly increased. This finding is in line with the prediction that offspring produced by low-quality males may have potential viability deficits and females should increase their egg investment or care investment to compensate for the deficits. Therefore, the investment pattern of the lined seahorse supports RCH.
The finding that the lined seahorse supports RCH may help elucidate its sex role from the perspective of reproductive investment. To date, there is still no consensus on which category of sex role seahorses belong to, which may be largely caused by the rare mating system of seahorses themselves. Seahorses show serial monogamy, which determines that males and females have almost identical potential reproductive rate (PRR)45. PRR is an important basis for classifying sex role, and the sex with higher PRR commonly faces more intense intraspecific competition and greater sexual selection pressure46. The lack of sex difference in PRR in the seahorses makes it difficult to classify their sex role from the perspective of PRR. Nowadays, the classification of the sex role of seahorses is mostly from the perspectives of operational sex ratio (OSR), intrasexual competition intensity and mate size preference45,47,48. In existing studies, some classified it as conventional based on female’s preference for larger males, and males being more active in courtship and having a shorter reproductive interval than females45,49,50. While others classified it as reversed based on male’s preference for larger females and female-biased OSR47,51,52. In the lined seahorse, male’s preference for larger females and female-biased OSR were also observed in the captive population (personal observation). In addition, we also observed that in pairs with more females and fewer males, females often experience abnormal ovulation (i.e., deposition their eggs directly into the water instead of the males’ pouch, and this behavior should be a consequence of female-female competition), while in pairs with fewer females and more males, females do not ovulate abnormally (unpublished data). This observation may suggest that the intensity of intrasexual competition by females is stronger than that by males. Therefore, combined with male’s preference for larger females, female-biased OSR and intense female-female competition, we speculate that the sex role of the lined seahorse is reversed. In the present study, the results of experiment 1 may further support our speculation. That females allocated more resources into eggs when mated to low-quality males may indicate that they have a high tolerance for male quality. In the presence of high-quality males, females are more inclined to choose high-quality males (see the result of pilot experiment), while in the presence of only low-quality males, females are also willing to mate with them because can compensate for potential viability deficits in their offspring by increasing egg investment. The high tolerance for mate quality may further suggest that compared to mate quality, females are more concerned about mating opportunity. How to mate with the available males as soon as possible to gain the reproductive success may be a top priority for females. Therefore, the results of experiment 1 may support that the lined seahorse undergoes a sex-role reversal, and the female faces a greater pressure on sexual selection53,54.
Differential investment of females when mated to males with different qualities has also been observed in other fish species. Employing body size as the measure of quality (large size representing high quality and small size representing low quality), researchers found that females of the banggai cardinal fish (Pterapogon kauderni) produced larger eggs for large males18, females of the broad-nosed pipefish (Syngnathus typhle) allocated more protein into the eggs for small males22, and females of the convict cichlid (Amatitlania siquia) increased maternal care during the larval stage when mated to large males19. In the present study, two males provided to females had similar body sizes but different sexual attractiveness, and the presence of differentiated investment indicates that females of the lined seahorse may be able to detect males’ qualities without relying on males’ body sizes. Additionally, from experiment 1, we also found that mating order did not affect the females’ investment decision. Similar result also appeared in the broad-nosed pipefish (S. typhle), whether mating to large males first or later, it did not affect females’ willingness to invest, nor did it affect any egg parameters related to reproductive investment22. These findings in H. erectus and S. typhle indicate that female investment decision mainly depends on the quality of males, rather than the mating order of males.
As for how females mechanically adjust their egg investment, some speculations can be made from their reproductive characteristics. When two receptive seahorses (one male and one female) that have never met before meet, it takes at least 3–4 days of courtship to mate successfully from their meeting26,31. This period is not only a process of courtship and mutual familiarity, but also may be a process for females to evaluate male quality and adjust their egg investment. In addition, although the eggs of females involved in courtship have already matured, they have not yet been hydrated before mating, and egg hydration usually occurs 1–2 days before mating26. Therefore, the allocation of resources by females to eggs is likely to occur during the hydration process.
The extrinsic (e.g., health status, territorial defense ability, parental ability) and intrinsic (genetic) qualities of males are the two most decisive factors for females to choose them as mates. Females obtain high direct or indirect benefits for their offspring by selecting males with good extrinsic or intrinsic qualities10,55. In the present study, we found that females preferred males fed with live Mysis in our pilot experiment. This finding, on one hand, indicates that live Mysis improved the extrinsic quality of males. As evidence, their body color becomes brighter and courtship behavior becomes more active. On the other hand, it may also suggest that there was not much difference in intrinsic quality of these males, resulting in females having to prefer males with good extrinsic quality. Of course, we cannot rule out the possibility of differences in intrinsic quality of these males. In the presence of differences, females insisted on choosing males with good extrinsic quality rather than males with good intrinsic quality, which may be related to the genetic quality (e.g., the compatibility with female’s own genes) only being determined after mating1,56.
In experiment 2, a large number of substances related to embryonic development, such as amino acids, n-3 HUFAs, other lipids (glycerophospholipids and polyketides), decreased in the eggs provided for the second breeding event, compared with those provided for the first breeding event. This finding indicates that the level of the second investment by females reduced or they no longer invested, in other words, the higher resource investment only limits to the first breeding event. There are at least two reasons that could explain why females invest less in the second breeding. Firstly, reproductive investment is a high-cost process, which may result in females not having enough resources to support the continuous investment. According to the reports, females that spawn more eggs or larger eggs in response to male quality may reduce their somatic reserves, which would limit their growth between the current and subsequent breeding events, thereby reducing their future reproductive potential57,58,59. Secondly, the sexual attractiveness, paternal-care ability, collaboration ability with the mate, or other abilities of the low-quality males may be improved after the first breeding, which makes females do not need to invest more in the second breeding. As evidence, in our previous study, we found that the sexual attractiveness of the initially non-preferred male seahorses (H. erectus) would be greatly improved with an increase in the number of mating60. In addition, in practice, the quality of newborn juveniles in the second clutch of the same male (H. erectus) is often better than that in the first clutch61. In the case where the investment of females to the second clutch did not increase but decreased (i.e., the results of experiment 2), the improvement in quality of the second clutch of newborns is likely attributed to promotion of their father’s care ability. In addition to the two reasons discussed above, whether other reasons, such as reduced intrasexual competition leading to a reduced reproductive investment (i.e., once a female establishes a mating relationship with a male, the intrasexual competition she faced in subsequent breeding events would sharply decrease due to the characteristics of monogamy), needs to be investigated in the future.
Data availability
All the supporting data generated during the current study are either included in the text or included in the supplementary materials.
References
Kvarnemo, C. Why do some animals mate with one partner rather than many? A review of causes and consequences of monogamy. Biol. Rev. 93, 1795–1812. https://doi.org/10.1111/brv.12421 (2018).
Sefc, K. M. Variance in reproductive success and the opportunity for selection in a serially monogamous species: Simulations of the mating system of Tropheus (Teleostei: Cichlidae). Hydrobiologia 615, 21–35. https://doi.org/10.1007/s10750-008-9563-1 (2008).
Ihle, M., Kempenaers, B. & Forstmeier, W. Fitness benefits of mate choice for compatibility in a socially monogamous species. PLoS Biol. 13(9), e1002248. https://doi.org/10.1371/journal.pbio.1002248 (2015).
Byers, J. A., Byers, A. A. & Dunn, S. J. A dry summer diminishes mate search effort by pronghorn females: Evidence for a significant cost of mate search. Ethology 112, 74–80. https://doi.org/10.1111/j.1439-0310.2006.01127.x (2006).
Schacht, R. & Bell, A. V. The evolution of monogamy in response to partner scarcity. Sci. Rep. 6, 32472. https://doi.org/10.1038/srep32472 (2016).
Silva, K., Almada, V. C., Vieira, M. N. & Monteiro, N. M. Female reproductive tactics in a sex-role reversed pipefish: Scanning for male quality and number. Behav. Ecol. 20, 768–772. https://doi.org/10.1093/beheco/arp058 (2009).
Dillard, J. R. & Westneat, D. F. Disentangling the correlated evolution of monogamy and cooperation. Trends Ecol. Evol. 31, 503–513. https://doi.org/10.1016/j.tree.2016.03.009 (2016).
Bluhm, C. K. & Gowaty, P. A. Reproductive compensation for offspring viability deficits by female mallards, Anas platyrhynchos. Animal Behaviour 68, 985–992. https://doi.org/10.1016/j.anbehav.2004.01.012 (2004).
Sandvik, M., Rosenqvist, G. & Berglund, A. Male and female mate choice affects offspring quality in a sex-rolereversed pipefish. Proc. Royal Soc. B 267, 2151–2155. https://doi.org/10.1098/rspb.2000.1262 (2000).
Bolund, E., Schielzeth, H. & Forstmeier, W. Compensatory investment in zebra finches: Females lay larger eggs when paired to sexually unattractive males. Proc. Royal Soc. B 276, 707–715. https://doi.org/10.1098/rspb.2008.1251 (2009).
Sheldon, B. C. Differential allocation: Tests, mechanisms and implications. Trends Ecol. Evol. 15, 397–402. https://doi.org/10.1016/S0169-5347(00)01953-4 (2000).
Gowaty, P. A. et al. The hypothesis of reproductive compensation and its assumptions about mate preferences and offspring viability. Proc. Natl. Acad. Sci. U. S. A. 104, 15023–15027. https://doi.org/10.1073/pnas.0706622104 (2007).
Gowaty, P. A. Reproductive compensation. J. Evol. Biol. 21, 1189–1200. https://doi.org/10.1111/j.1420-9101.2008.01559.x (2008).
Gowaty, P. A., Drickamer, L. C. & Schmid-Holmes, S. Male house mice produce fewer offspring with lower viability and poorer performance when mated with females they do not prefer. Animal Behaviour 65, 95–103. https://doi.org/10.1006/anbe.2002.2026 (2003).
Wilson, K. M. & Burley, N. T. Female differential allocation in response to extrapair offspring and social mate attractiveness. Ecol. Evol. 11(12), 7278–7291. https://doi.org/10.1002/ece3.7560 (2021).
Sardell, R. J. & DuVal, E. H. Differential allocation in a lekking bird: Females lay larger eggs and are more likely to have male chicks when they mate with less related males. Proc. Royal Soc. B 281, 20132386. https://doi.org/10.1098/rspb.2013.2386 (2014).
Reyer, H., Frei, G. & Som, C. Cryptic female choice: Frogs reduce clutch size when amplexed by undesired males. Proc. Royal Soc. B 266, 2101–2107. https://doi.org/10.1098/rspb.1999.0894 (1999).
Kolm, N. Females produce larger eggs for large males in a paternal mouthbrooding fish. Proc. Royal Soc. B 268, 2229–2234. https://doi.org/10.1098/rspb.2001.1792 (2001).
Robart, A. R. & Sinervo, B. Females increase parental care, but not fecundity, when mated to high-quality males in a biparental fish. Animal Behaviour 148, 9–18. https://doi.org/10.1016/j.anbehav.2018.11.012 (2019).
Byers, J. A. & Waits, L. Good genes sexual selection in nature. Proc. Natl. Acad. Sci. U. S. A. 103, 16343–16345. https://doi.org/10.1073/pnas.0608184103 (2006).
Navara, K. J., Hill, G. E. & Mendonca, M. T. Yolk androgen deposition as a compensatory strategy. Behav. Ecol. Sociobiol. 60, 392–398. https://doi.org/10.1007/s00265-006-0177-1 (2006).
Goncalves, I. B. et al. Reproductive compensation in broad-nosed pipefish females. Proc. Royal Soc. B 277, 1581–1587. https://doi.org/10.1098/rspb.2009.2290 (2010).
Harris, W. E. & Uller, T. Reproductive investment when mate quality varies: Differential allocation versus reproductive compensation. Philos. Trans. Royal Soc. B 364, 1039–1048. https://doi.org/10.1098/rstb.2008.0299 (2009).
Johnstone, R. A. & Cant, M. A. Models of reproductive skew: Outside options and the resolution of reproductive conflict. In Reproductive Skew in Vertebrates: Proximate and Ultimate Causes (eds Hager, R. & Jones, C. B.) 3–23 (Cambridge University Press, 2009).
Rose, E., Small, C. M., Saucedo, H. A., Harper, C. & Jones, A. G. Genetic evidence for monogamy in the dwarf seahorse, Hippocampus zosterae. J. Hered. 105, 922–927. https://doi.org/10.1093/jhered/esu050 (2014).
Vincent, A. C. J. & Sadler, L. M. Faithful pair bonds in wild seahorses, Hippocampus whitei. Animal Behaviour 50, 1557–1569. https://doi.org/10.1016/0003-3472(95)80011-5 (1995).
Woodall, L. C., Koldewey, H. J. & Shaw, P. W. Serial monogamy in the European long-snouted seahorse Hippocampus guttulatus. Conserv. Genet. 12, 1645–1649. https://doi.org/10.1007/s10592-011-0253-6 (2011).
Kvarnemo, C., Moore, G. I., Jones, A. G., Nelson, W. S. & Avise, J. C. Monogamous pair bonds and mate switching in the Western Australian seahorse Hippocampus subelongatus. J. Evol. Biol. 13, 882–888. https://doi.org/10.1046/j.1420-9101.2000.00228.x (2000).
Lin, T., Liu, X. & Zhang, D. Does the female seahorse still prefer her mating partner after a period of separation?. J. Fish Biol. 99, 1613–1621. https://doi.org/10.1111/jfb.14867 (2021).
Stölting, K. N. & Wilson, A. B. Male pregnancy in seahorses and pipefish: Beyond the mammalian model. BioEssays 29, 884–896. https://doi.org/10.1002/bies.20626 (2007).
Vincent, A. C. J. A role for daily greetings in maintaining seahorse pair bonds. Animal Behaviour 49, 258–260. https://doi.org/10.1016/0003-3472(95)80178-2 (1995).
Ripley, J. L. & Foran, C. M. Differential parental nutrient allocation in two congeneric pipefish species (Syngnathidae: Syngnathus spp.). J. Exp. Biol. 209, 1112–1121. https://doi.org/10.1242/jeb.02119 (2006).
Planas, M., Quintas, P., Chamorro, A. & Silva, C. Female maturation, egg characteristics and fatty acids profile in the seahorse Hippocampus guttulatus. Animal Reprod. Sci. 122, 66–73. https://doi.org/10.1016/j.anireprosci.2010.07.008 (2010).
Brooks, S., Tyler, C. R. & Sumpter, J. P. Egg quality in fish: What makes a good egg?. Rev. Fish Biol. Fish. 7, 387–416. https://doi.org/10.1023/A:1018400130692 (1997).
Bruce, M. P., Shields, R. J., Bell, M. V. & Bromage, N. R. Lipid class and fatty acid composition of eggs of Atlantic halibut, Hippoglossus hippoglossus (L.), in relation to egg quality in captive broodstock. Aquac. Res. 24, 417–422. https://doi.org/10.1111/j.1365-2109.1993.tb00565.x (1993).
Fyhn, H. J. & Serigstad, B. Free amino-acids as energy substrate in developing eggs and larvae of the cod Gadus morhua. Mar. Biol. 96, 335–341. https://doi.org/10.1007/BF00412514 (1987).
Ohkubo, N., Sawaguchi, S., Nomura, K., Tanaka, H. & Matsubara, T. Utilization of free amino acids, yolk protein and lipids in developing eggs and yolk-sac larvae of Japanese eel Anguilla japonica. Aquaculture 282, 130–137. https://doi.org/10.1016/j.aquaculture.2008.06.017 (2008).
Srivastava, R. K. & Brown, J. A. Assessment of egg quality in Atlantic salmon, Salmo salar, treated with testosterone: Biochemical composition. Can. J. Zool. 71, 109–115. https://doi.org/10.1139/z93-016 (1993).
Tyndale, S. T., Letcher, R. J., Heath, J. W. & Heath, D. D. Why are salmon eggs red? Egg carotenoids and early life survival of Chinook salmon (Oncorhynchus tshawytscha). Evol. Ecol. Res. 10, 1187–1199 (2008).
Bligh, E. G. & Dyer, W. J. A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 37, 911–917. https://doi.org/10.1139/o59-099 (1959).
Zhou, H. et al. Identification of key nutrients for gonadal development by comparative analysis of proximate composition and fatty/amino acid profile in tissues and eggs of Chinese sturgeon (Acipenser sinensis Gray, 1835). J. Appl. Ichthyol. 33(5), 885–891. https://doi.org/10.1111/jai.13401 (2017).
Bradford, M. M. A rapid and sensitive method for quantification of microgram quantities of protein utilizing the principle of protein-dye-binding. Anal. Biochem. 72, 248–254. https://doi.org/10.1016/0003-2697(76)90527-3 (1976).
Sun, B., Sun, B., Zhang, B. & Sun, L. Temperature induces metabolic reprogramming in fish during bacterial infection. Front. Immunol. 13, 1010948. https://doi.org/10.3389/fimmu.2022.1010948 (2022).
McLaughlin, M. J. & Sainani, K. L. Bonferroni, Holm, and Hochberg corrections: Fun names, serious changes to p values. PM R 6(6), 544–546. https://doi.org/10.1016/j.pmrj.2014.04.006 (2014).
Vincent, A. C. J., Ahnesjö, I., Berglund, A. & Rosenqvist, G. Pipefishes and seahorses: Are they all sex role reversed?. Trends Ecol. Evol. 7, 237–241. https://doi.org/10.1016/0169-5347(92)90052-D (1992).
Clutton-Brock, T. H. & Vincent, A. C. J. Sexual selection and the potential reproductive rates of males and females. Nature 351, 58–60 (1991).
Mattle, B. & Wilson, A. B. Body size preferences in the pot-bellied seahorse Hippocampus abdominalis: Choosy males and indiscriminate females. Behav. Ecol. Sociobiol. 63(10), 1403–1410. https://doi.org/10.1007/s00265-009-0804-8 (2009).
Wilson, A. B. & Martin-Smith, K. M. Genetic monogamy despite social promiscuity in the pot-bellied seahorse (Hippocampus abdominalis). Mol. Ecol. 16, 2345–2352. https://doi.org/10.1111/j.1365-294X.2007.03243.x (2007).
Masonjones, H. D. & Lewis, S. M. Differences in potential reproductive rates of male and female seahorses related to courtship roles. Animal Behaviour 59(1), 11–20. https://doi.org/10.1006/anbe.1999.1269 (2000).
Vincent, A. C. J. Seahorses exhibit conventional sex roles in mating competition, despite male pregnancy. Behaviour 128, 135–151. https://doi.org/10.1163/156853994X00082 (1994).
Naud, M. J., Curtis, J. M. R., Woodall, L. C. & Gaspar, M. B. Mate choice, operational sex ratio and social promiscuity in a wild populations of the long snouted seahorse Hippocampus guttulatus. Behav. Ecol. 20, 160–164. https://doi.org/10.1093/beheco/arn128 (2009).
Kvarnemo, C., Moore, G. I. & Jones, A. G. Sexually selected females in the monogamous Western Australian seahorse. Proc. Royal Soc. B 274, 521–525. https://doi.org/10.1098/rspb.2006.3753 (2007).
Bateman, A. J. Intra-sexual selection in Drosophila. Heredity 2, 349–368 (1948).
Roth, O., Scharsack, J. P., Keller, I. & Reusch, T. B. H. Bateman’s principle and immunity in a sex-role reversed pipefish. J. Evol. Biol. 24, 1410–1420. https://doi.org/10.1111/j.1420-9101.2011.02273.x (2011).
Neff, B. D. & Pitcher, T. E. Genetic quality and sexual selection: An integrated framework for good genes and compatible genes. Mol. Ecol. 14(1), 19–38. https://doi.org/10.1111/j.1365-294X.2004.02395.x (2005).
Kosman, E. T. & Levitan, D. R. Sperm competition and the evolution of gametic compatibility in externally fertilizing taxa. Mol. Hum. Reprod. 20, 1190–1197. https://doi.org/10.1093/molehr/gau069 (2014).
Lavery, R. J. & Keenleyside, M. H. Parental investment of a biparental cichlid fish, Cichlasoma nigrofasciatum, in relation to brood size and past investment. Animal Behaviour 40, 1128–1137. https://doi.org/10.1016/S0003-3472(05)80179-4 (1990).
Skinner, A. M. J. & Watt, P. J. Strategic egg allocation in the zebra fish, Danio rerio. Behav. Ecol. 18(5), 905–909. https://doi.org/10.1093/beheco/arm059 (2007).
Evans, J. P., Box, T. M., Brooshooft, P., Tatter, J. R. & Fitzpatrick, J. L. Females increase egg deposition in favor of large males in the rainbowfish, Melanotaenia australis. Behav. Ecol. 21(3), 465–469. https://doi.org/10.1093/beheco/arq006 (2010).
Lin, T., Liu, X., Zhang, D. & Li, S. Extensive parental care experience of male seahorses increases their future mating attractiveness. Curr. Zool. 69, 106–108. https://doi.org/10.1093/cz/zoac017 (2023).
Lin, Q., Li, G., Qin, G., Lin, J. & Feng, P. The dynamics of reproductive rate, offspring survivorship and growth in the lined seahorse, Hippocampus erectus Perry, 1810. Biol. Open 1, 391–396. https://doi.org/10.1242/bio.2012398 (2012).
Acknowledgements
We are very grateful to all the staff of Seahorse Research Center for the assistance in seahorse rearing and management.
Funding
The present study was supported by the National Natural Science Foundation of China (Grant No. 32172950), the Natural Science Foundation of Shanghai (Grant No. 21ZR1479 -800), the Key Research and Development Projects of Hainan (Grant No. ZDYF2022XDNY -350) and the Central Public-interest Scientific Institution Basal Research Fund of CAFS (Grant No. 2023TD56).
Author information
Authors and Affiliations
Contributions
D.Z. and T.L. designed the experiments, analyzed and interpreted the data; T.L. and X.L. performed the experiments including experimental animal management, sample collection and determination, and data generation; S.L., F.S. and K.J. assisted in sample collection and determination; T.L. wrote the manuscript and D.Z. revised the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Lin, T., Liu, X., Li, S. et al. Females increase reproductive investment when mated to less sexually attractive males in a serially monogamous fish. Sci Rep 14, 19020 (2024). https://doi.org/10.1038/s41598-024-70007-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-70007-3
- Springer Nature Limited