Abstract
Radio tracking of 142 males captured at 44 leks in Spain showed that partial migration of great bustard males in summer is a widespread behaviour in many Iberian populations, in contrast to their previously assumed sedentariness. A variable number of males migrated immediately after mating to summering areas with lower temperatures and human population densities and more trees and rainfall levels than the breeding sites. Birds selected there fields with trees and sunflower crops which provided shade during the hottest midday hours and protective cover against predators. Males breeding in areas with higher July temperatures had a higher tendency to migrate, and males from hotter, southern regions migrated longer distances than those from milder, northern regions and showed a preferred northward direction. These results confirmed various predictions from the weather sensitivity hypothesis, suggesting that summer migration of great bustard males represents primarily an adaptation to escape the summer heat of most breeding areas in central and southern Iberia. The hypothesis that males migrated to benefit from higher food availability at the summering areas could not be rejected by our results. Finally, migrating males also gained more tranquillity during the post-breeding moult due to the lower human population density at the summering areas. Summer migration of Iberian great bustard males may thus be interpreted as a form of behavioural thermoregulation which has not been described for other Palaearctic populations of this species or for other bird species breeding in temperate latitudes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In many migratory birds, the seasonal movements between breeding and non-breeding areas involve only a fraction of the population, whilst other individuals remain sedentary. This phenomenon has been called partial migration, to distinguish it from annual migration where all individuals of the population migrate from their breeding areas on an annual basis. The term differential migration refers to the situation in which some distinguishable classes of individuals (ages, sexes or others) differ in timing, distance travelled or both (Gauthreaux 1982; Ketterson and Nolan 1983; Lundberg 1988; Terrill and Able 1988). Within partial migrants, Terrill and Able (1988) proposed the term obligate partial migrants to refer to those individuals that migrate each year regardless of annual variation in environmental parameters or population density, and facultative partial migrants to define those individuals that may or may not migrate in a given year, depending largely on environmental conditions. Modern reviews have revealed that partial and differential migration are much more widespread than previously reported and indeed may be considered as the norm amongst migratory birds (Cristol et al. 1999; Berthold 2001; Newton 2008).
Furthermore, recent findings on genetic control of migration and a better understanding of the microevolutionary processes involved have suggested new interpretations for the evolution of migration (see Berthold 1996). For example, obligate partial migration can be viewed as an evolutionary stable strategy where two morphs, migrants and residents, occur in the population in a proportion that is adapted to local conditions (Lundberg 1987; Berthold 1984; Kaitala et al. 1993). The current view is that obligate migrants and non-migrants are probably present in all bird populations in different proportions, and given sufficient underlying genetic variation and strong enough selection pressure, this would enable any population to vary the migratory tendency and adapt to changing environmental conditions (Berthold 1999, 2001; Newton 2008). Amongst these environmental conditions determining migratory movements, seasonal variation in food availability is considered the most important. As a rule, birds migrate to exploit seasonal food abundances and to avoid seasonal food shortages. However, other variables may also be important in determining migratory movements during certain periods of the annual cycle. For example, some waterfowl perform moult migrations after breeding when they move to traditional sites that offer not only food but also safety during several weeks when birds remain flightless (e.g. Jehl 1990). Other seasonal movements not obviously related to food availability have often been described as partial or differential migration in some grouse species (Herzog and Keppie 1980; Schroeder 1985; Cade and Hoffman 1993; Schroeder and Braun 1993). Based on the absence of a clear directional trend in most of these cases, in a recent review, Newton (2008) classified them as dispersive migration, a term that he proposed to highlight that these movements have some features of dispersal (variable directions and usually short distances) and some of migration (return movement, consistency between years, sex differences), but no apparent relation to seasonal changes or latitudinal trends in food supplies. In this paper, we describe one of such particular types of migratory movements not clearly related to food availability but nevertheless classifiable as a true migratory movement, the summer migration in male great bustards Otis tarda. We propose that the post-mating migration of many Iberian populations of this species is primarily determined by the physiological stress caused by high summer temperatures at their breeding areas.
The great bustard has been traditionally considered as sedentary in the western and southern parts of its distribution range and migratory elsewhere (Glutz et al. 1973; Del Hoyo et al. 1996). The migratory status of the eastern European population of the species has been recently confirmed through satellite tracking of six females between their nesting areas east of Saratov, Russia to their wintering areas in southern Ukraine (Watzke et al. 2001; Watzke 2007). Some populations in central Europe are facultative migrants in response to extreme weather conditions during winter (Block 1996; Dornbusch 1981; Farago 1990; Streich et al. 2006). As for the western population, year-round surveys at various populations in Spain reported monthly changes in numbers, which suggested that some seasonal movements did occur (Alonso and Alonso 1990; Hidalgo and Carranza 1990; Hellmich 1991; Alonso et al. 1995). Later studies with radio-tagged birds showed that in this part of its distribution range, the species is a partial migrant, with sedentary and migratory birds of both sexes coexisting in the same populations, at least in two different regions of the Iberian Peninsula (Alonso et al. 1995, 2000, 2001; Morales et al. 2000). Although based on limited samples, preliminary results suggested the existence of a particular type of migratory movement, the summer migration, which was only performed by males, but showed marked differences between populations in the proportions of migrating birds and distances travelled. For example, in Villafáfila, northern Spain, only 69% males performed migratory movements to summering areas 7–20 km away from their leks (Morales et al. 2000), whereas in Madrid region, central Spain, all 22 radio-tagged males left their leks after mating and spent the summer at 9–167 km (average = 82 km; Alonso et al. 2001). Based on the absence of summer migration in females and on the different proportions of migrating males at the two study areas, we hypothesised that summer migrations of males might be related to particular environmental conditions affecting only males and more in certain populations than in others (Alonso et al. 2001; Palacín 2007). Specifically, due to their large size, males would have difficulties in dissipating body heat and balancing water budget (Calder and King 1974; Walsberg and King 1978; Searcy 1980; Dawson and O’Connor 1995; Blanckenhorn 2000), as well as in reducing their evaporative water loss (Tieleman and Williams 1999; Williams and Tielemann 2001). We proposed that male great bustards migrate in summer to cooler areas because they do not tolerate well the hot temperatures at the breeding sites (weather sensitivity hypothesis, see Jackes 1973; Young and Isbell 1991; Conradt et al. 2000).
If the males’ partial migratory trend in summer is explained by their low tolerance to high summer temperatures, we predicted that over the species’ distribution range in Iberia, (a) summer temperatures should be one of the environmental variables showing highest differences between breeding and summering sites. Also, (b) males breeding in areas with higher summer temperatures should have a higher tendency to abandon them in summer. Finally, if there are any detrimental effects of summer heat, these would decrease with decreasing summer temperatures and would eventually disappear at a certain threshold where males would obtain no thermoregulation benefit from migrating to cooler areas. Thus, (c) males breeding at areas with lower summer temperatures should migrate shorter distances to reach summering areas with a tolerable temperature. In other words, given the latitudinal gradient in temperature in Spain where the mean July temperature increases with decreasing latitude at an average of slightly over 1°C every 200 km (De Castro et al. 2005), males breeding at southern latitudes should migrate preferably northwards and longer distances than those from northern latitudes.
Alternatively, males could migrate to look for better feeding grounds in summer when food availability decreases at the breeding areas due to the drying up of the vegetation, since great bustards are mainly vegetarian (Lane et al. 1999). This has been suggested as a possible cause of intermittent or summer migrations observed in juveniles of some species and irruptive or nomadic movements in others, both amongst birds (Berthold 2001; Newton 2008) and mammals (reviewed in Aublet et al. 2009). In the Mediterranean region, most of the herbaceous vegetation dries during summer (Blondel and Aronson 1999), and the peak of vegetation productivity is delayed at higher latitudes and altitudes (Margalef 1974). Some authors have suggested that this could determine the northward post-fledging dispersal of juveniles or adult movements in some birds in Iberia (García 2000; Olea 2001; Silva et al. 2007). To explore this possibility, we tested predictions equivalent to (a), (b) and (c) above using annual precipitation and altitude as proxy variables of vegetation greenness, i.e. food availability. In short, altitude and precipitation should differ more than temperature between lek and summering sites, and males breeding in areas with higher rainfall or located at higher altitudes should have a lower tendency to abandon them in summer and should migrate shorter distances. Here, we present the results on summer migration of a large sample of radio-tagged adult male great bustards from different leks over most of the species’ range in Spain. We radio-tracked males through several years and compared a number of environmental variables between breeding and summer sites. Our aim was to test the weather sensitivity hypothesis and its alternative food abundance sensitivity hypothesis.
Materials and methods
Capturing and tracking birds
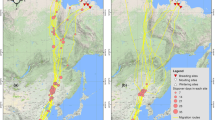
During winter 1991–2004, we captured 109 adult male great bustards using rocket nets. Another 33 male chicks were captured in July, when they were 3–10 weeks old and still dependent on their mothers, by chasing them down. In total, we recorded migration data from 142 males of 44 leks in 13 provinces, covering the whole distribution range of the species in Spain. Of these, 99 males were considered migratory because they left their lek areas (defined with ArcGIS 9 as the minimum convex polygons encompassing all locations of males and females during the breeding season, March–May) after mating and spent the summer and sometimes also the autumn and winter part of their yearly cycle outside their breeding ranges. For some males, we could not accurately determine their summering area due to insufficient number of radio locations, transmitter failure or death of the bird. This reduced our sample to 82 migratory males for the study of differences between spring and summer sites and to 128 males for the study of migration tendency. Each captured bird was fitted with a backpack radio transmitter (Biotrack, UK) using elastic band as harness material. In addition, birds were provided with polyvinyl chloride wing tags (juveniles) or dorsal tags glued to the transmitters (adults) for visual identification in the field. Wing or dorsal tags also enabled location of marked birds after transmitter batteries were exhausted (battery life was 4–5 years in the 2 × AA transmitters used for juveniles and up to 7–8 years in the 3 × AA model used in most adults). After marking, we located all radio-tagged individuals by triangulation using TR2 receivers-TS1 scanners from Telonics, USA and subsequent visual observation with 20–60× telescopes at least once, most frequently several times per month. The location of a bird was determined with a Garmin-12 GPS and a maximum error of 100 m. When a marked bird was not found from the ground, we used small aeroplanes (E-24 Bonanza, Beechcraft). During this study, we have spent >1,500 flight hours searching for birds over all Spain. Aerial tracking enabled us to obtain lek and summer locations of all marked birds, thus avoiding the bias derived from emigration outside the study area in dispersal and migration studies (see Koenig et al. 1996).
Variables analysed
Each male was radio-tracked during 1–9 years (average 3.7 years). For each bird, we calculated activity centres for spring (breeding season, between mid March and mid May) and summer (area where the bird settled during late June–August), by averaging yearly mean locations, using only data after each bird had established as a breeding adult at a lek site, which, in great bustard males, occurs at the age of 3–4 years (Alonso et al. 1998). After settling as breeding adults, all males used year after year the same lek and 94% migrated every year to exactly the same summer site (Palacín 2007). Around these spring and summer locations for each bird, we established circles of 2-km radius within which we calculated mean values of all environmental variables used to characterise these sites. Our radio tracking data show that home ranges of most males are usually smaller than these circles both in spring and summer, and thus, the latter represent adequately the environmental requirements of birds. The environmental variables used as predictors of the habitat requirements of male great bustards were selected based on our knowledge of the ecology and behaviour of the species, processed using GIS, and rasterised in IDRISI (Eastman 2000) to either 1-km2 resolution (topographic and climatic variables) or 1 ha (land cover variables). We analysed several sets of variables that could help identify environmental differences between sites used by the males in spring and summer. Three variables described the topographic structure of the habitat and could determine microclimatic differences between areas used in both seasons: altitude (in m a.s.l.), slope (degrees), roughness (% variation in slope estimated as the mean of all standard deviations of altitudes in each pixel, calculated from digital terrain model). To describe the climatic conditions, we used the mean monthly temperatures in January, April and July, the mean annual temperature and the mean annual rainfall (in mm). These variables were obtained from the models with 1-km2 resolution produced for the whole Spanish territory using interpolation techniques: trend surfaces, multiple regression with predictors derived from a digital elevation model and kriging (Bustamante 2003). A third set of variables describing the habitat used by the birds were obtained from the Geographic Database on Land Use of the European Union Corine Land Cover 2000, from satellite imagery scale 1:100,000 with 1-ha resolution (European Topic Centre on Terrestrial Environment, http://www.dataservice.eea.eu.int). We considered the following substrate types: urban areas, dry cereal farmland, irrigated farmland, vineyards, fruit tree plantations, olive groves, pastureland, dry farmland mosaic (mosaic formed by small vineyards and olive groves with interspersed small cereal fields), non-arboreal natural vegetation, arboreal vegetation (including mostly open-wooded oak tree ‘dehesas’ and uncultivated areas with natural arboreal or scrub vegetation) and areas with sclerophyll vegetation. A fourth group of variables was used to describe the degree of landscape humanisation: human population density (number of inhabitants of the village closest to the central coordinate of the circle divided by the surface of the municipality in square kilometres (data for 2003 from the Instituto Nacional de Estadística, http://www.ine.es/nomen/nomena.jsp, and the Dirección General del Catastro, http://www.catastro.minhac.es/).
Migration tendency and direction
To explore the migration tendency of great bustard males, we used a logistic regression model with a binary response (migratory vs non-migratory) for the behaviour of each of the 128 individuals for which we had adequate data. To examine differences in migration direction, we grouped the birds into two latitudinal subdivisions (19 northern birds, 63 southern birds, respectively, north and south of the Sistema Central, the main mountain chain crossing central Iberia from west to east) and used the Rayleigh and Watson–Williams tests.
Habitat selection
To determine habitat use by migratory males during summer, we recorded the substrate type where each marked male was found between 1999 and 2003 and assigned it to one of the following categories: fallow; ploughed field; cereal stubble, usually winter sown wheat Triticum aestivum or barley Hordeum vulgare; arboreal vegetation, mostly open-wooded ‘dehesas’ of evergreen oak Quercus ilex or fields with almond trees Prunus dulcis; vineyards Vitis vinifera; olive groves Olea europaea; sunflower Helianthus annuus; legumes, usually vetch Vicia sativa or alfalfa Medicago sativa grown as a fodder crop mixed with barley; or uncultivated land, stony ground with broom Retama sphaerocarpa a major feature. We established habitat availability by means of a transect starting from the track closest to each location of a marked bird and recording the substrate type encountered immediately to the left and right of our vehicle. For each marked male, the proportion in each substrate was calculated by dividing the substrate total by the number of patches counted. The total length covered by these transects was 411.2 km and the number of fields 4,805. From the substrate availability and usage data, we calculated habitat selection indices (IS; Ivlev 1961) as IS = (usage − availability)/(usage + availability). Values of IS vary between −1 (substrate avoided) and +1 (maximum positive selection). Values equal or close to zero indicate no preference for the corresponding substrate type.
Statistical analyses
Exploratory univariate analyses of the differences between spring and summer values of each variable in the circles of 2-km radius were carried out using Wilcoxon’s matched pairs test. Next, a Hotelling’s T 2 test [multivariate analysis of variance (MANOVA) one-sample test] with Wilk’s lambda estimation was used to test whether, considering all variables together, the difference between spring and summer in the environmental characteristics of locations used by males in both seasons could be considered significantly distinct from zero (Winer et al. 1962; see, e.g. Rubio and Carrascal 1994; Waldorp et al. 2006; Wallace et al. 2006). The H 0 predicts no differences between spring and summer in the set of variables describing the characteristics of the 2-km circles used by the 82 males. Prior to the multivariate analysis, all variables were transformed to attain equal variance and normality, and variables that were highly correlated (r > 0.65) with other predictors were excluded. Since there was a high correlation amongst all temperature variables and a high negative correlation between temperature and altitude, in the multivariate analysis, we excluded altitude and all temperatures except the mean temperature in July. After testing the significance of the multivariate model, univariate post hoc analyses were performed to obtain the F values for each variable. These post hoc univariate analyses of variance are protected against the probability of type I error (Winer et al. 1962). Using as predictors the four variables (after appropriate log transformation) that showed a significant difference in the MANOVA comparing spring and summer sites used by the migrating males (see Table 2), we performed a logistic regression to explain the behaviour of each individual. We used the binomial distribution (migratory vs non-migratory) and logit link function, the type 3 test, and corrected for overdispersion of the data. To further explore the relative importance of each explanatory variable, we used the corrected Akaike’s information criterion (ΔAICc < 2) to select the best models from a set of candidate models with different combinations of predictor variables. The preferred migration direction was established after performing a Rayleigh test of uniformity, and latitudinal differences were tested with the Watson–Williams F test. STATISTICA 6.0 (StatSoft 2001) software was used for all analyses, except those related to migration directions which were analysed with the program Oriana (Kovach Computing Services 2004).
Results
Differences between spring and summer sites
Univariate exploratory analysis showed that spring sites were located at lower altitudes than summer sites and had higher mean temperatures, lower mean annual rainfall, larger surfaces of dry cereal farmland, smaller surfaces of arboreal vegetation and higher human population densities (Table 1). Considering all environmental variables together, there were significant differences between spring and summer sites (Hotelling’s T 2 test, F 19,63 = 3.80, P < 0.001). The model explained a 53.4% of the variance in environmental structure (Wilk’s lambda = 0.466, F 19,63 = 3.80, P < 0.001). The variables showing significant effects are shown in Table 2. Summer sites had lower temperatures and human population densities, higher rainfall and more trees than sites used in spring. Repeating the MANOVA with latitude (northern vs southern Spain) as a factor, the model was also significant (Hotelling’s T 2 test, F 19,62 = 1.82, P = 0.041), with a significant effect of latitude (F 19,62 = 2.29, P = 0.007). The three most significant variables were still July temperature, human population density and rainfall; arboreal vegetation did not reach the 0.05 significance level. Birds from southern Spain (n = 63 males) chose summering sites with lower July temperatures (23.2°C, SD = 1.6), lower human population densities (39.8 inhabitants/km2, SD = 73.7) and more rainfall (4,850.4 mm, SD = 777.2) than those at their breeding sites (respectively, 24.3°C, SD = 1.1; 106.2 inhabitants/km2, SD = 126.9; 4,429.2 mm, SD = 503.8), whereas breeding and summering sites of birds from northern Spain (n = 19 males) did not differ in the values of these variables (summer: 21.7°C; SD = 1.1; 10.9 inhabitants/km2, SD = 5.2; 4,124.2 mm, SD = 472.5; spring: 21.7°C, SD = 0.9; 12.9 inhabitants/km2, SD = 7.8; 4,089.2 mm, SD = 349.5). The critical temperature threshold seemed to lay around 22–23°C (see mean July temperature in Table 1).
Migration tendency, direction and distance
The tendency of a male to migrate in summer was positively related to the mean July temperature at the breeding areas, with no significant effects of human population density, mean annual rainfall and surface of arboreal vegetation (Table 3). Mean July temperature was included in all eight models selected as best subsets using ΔAICc < 2 (in models 1 and 2 as the first variable, in model 5 as the only variable; the eight models were highly significant, P < 0.001), confirming its higher relevance as compared to human population density (included in four models: 1–4), rainfall (four models: 3, 4, 6 and 7) and arboreal vegetation surface (three models: 2, 4 and 7). The distance migrated by each male (minimum 6.3 km, maximum 246.6 km) was positively correlated with mean July temperature (r = 0.399, P < 0.001, n = 82) and precipitation at the breeding sites (r = 0.238, P = 0.031, n = 82) and inversely correlated with latitude (r = −0.441, P < 0.001, n = 82) and altitude (r = −0.346, P = 0.001, n = 82). To completely discard any pseudoreplication effect caused by the similarity in the values of these variables for all males of the same lek, we repeated the analyses using mean values of all variables for each of the 36 leks. The results were the same: r = 0.745, P < 0.001, n = 36 leks for July temperature; r = 0.373, P = 0.025, n = 36 leks for rainfall; r = −0.688, P < 0.001, n = 36 leks for latitude; and r = −0.404, P = 0.015, n = 36 leks for altitude. There was an interesting case of a male marked at a lek in central Spain that instead of flying a long distance northwards like other males of the same lek (131–196 km, latitudinal migration) climbed 646 m in altitude to nearby mountains (altitudinal migration, only 47 km away from the lek) where it spent the summer, at 1,052 m altitude, in a high mountain scrubland habitat quite atypical for this species. The preferred migratory direction of males from southern Spain was northeast (mean = 46°, Z = 32.71, n = 68, P < 0.001, Rayleigh test), whereas the mean direction for the males from northern Spain was east–southeast (mean = 105°, Z = 2.58, n = 14, P = 0.073), the difference being significant (F = 8.52, P = 0.004, Watson–Williams test). Finally, all adult males were either sedentary or migratory during the years they were studied, and most (94%) used the same post-breeding areas year after year.
Habitat selection during summer
At the summer gathering places, males selected positively sites with arboreal vegetation and sunflower fields. The two other substrates showing high percent use values were fallows or ploughed fields and stubbles, each with a 19.3% use (Table 4). All other substrate types were used much less than expected from their availability figures.
Discussion
Our study showed that in many Iberian great bustard populations, males are partial migrants in summer and not sedentary as previously assumed. A variable number of males of each breeding region migrated once the mating period was over to areas with lower mean temperature, more arboreal vegetation and precipitation and less human population where they spent the hottest summer months (June–September). The results confirmed various predictions derived from the weather sensitivity hypothesis. First, July temperature was the environmental variable showing highest differences between breeding and summering sites (Table 2). Second, males breeding in regions with higher summer temperatures had a higher tendency to migrate. As a consequence, summer displacements were more frequent in southern Iberian populations than in northern ones. And third, the distance migrated by males from southern latitudes was longer and their migration direction significantly oriented to north, in contrast to the shorter and not directional movements of males from northern latitudes. These latitudinal differences support the hypothesis that the post-mating migration of Iberian great bustard males could primarily be an adaptation to evade the hot summer temperatures of breeding sites at southern latitudes and so reduce their endogenous heat burden during summer.
The alternative hypothesis suggesting that males migrated to search for favourable feeding grounds could not be completely rejected by our results, though it seemed less plausible as the main explanation. First, rainfall was not significant in the model explaining the migration tendency of males, contrary to the expectation that males breeding in areas with lower rainfall levels should migrate in higher proportion to look for more humid post-breeding areas. Second, the expected higher migration tendency of males breeding at lower altitudes, i.e. in areas more likely to dry up in summer, did not reach significance. Third, the longer migrations of males from leks with higher precipitation was opposite to the expectation that they would need to migrate shorter distances to find nearby favourable feeding grounds. Finally, habitat structure was quite similar between breeding and summering sites. These results suggest that the possible effect of food abundance on migration trends of great bustard males is probably weaker than the effect of temperature. However, because precipitation and altitude are only indicators of the greenness of herbaceous vegetation, and thus only crude estimators of food availability, we cannot completely discard that males also obtain some food-related benefits at the summering areas.
Earlier studies had shown a clear differential migration pattern by sex in this species, with summer migration present only in males and involving different proportions of males in different populations (Alonso et al. 2000, 2001; Morales et al. 2000; Palacín 2007). Based on these studies, we suggested that this differential migration pattern was probably due to the higher sensitivity of males to summer heat and proposed sexual size dimorphism as the major ultimate cause, in a similar way as suggested for many behavioural and ecological patterns in other dimorphic vertebrates (Ruckstuhl and Neuhaus 2000; Ruckstuhl and Clutton-Brock 2005). Sexual selection would have determined an increase of males’ weight beyond the optimum for the very hot summer temperatures of southern Iberia (see Searcy 1980), and males from this region would have developed a mechanism to compensate for the heat stress by migrating in summer to cooler sites. The partial migration pattern analysed in the present study is consistent with this suggested sensitivity of males to high summer temperatures. The idea that summer represents a critical season particularly for males is supported by our results on year-round values of mortality which in males reached highest values in July and were significantly higher than in females (based on a sample of 123 males and 130 females radio-tracked through several years, own unpublished data).
The constancy exhibited by all adult males in their migratory or sedentary pattern and their remarkable site fidelity to post-breeding areas in consecutive years suggests an obligate partial migration system with two strategies, migratory and resident, that could be either inherited or learned from conspecifics (Palacín 2007). In a subsample of males studied in detail, we found no differences in age, weight, secondary sexual characters indicative of social status or mating success (Morales et al. 2000; Magaña 2007), which suggests that these two strategies are not condition-dependent and have probably balanced payoffs. In most Iberian populations, both phenotypes, migratory and resident, are present, and their proportions seem to be in line with local environmental conditions (evolutionarily stable strategy, see Lundberg 1987; Berthold 1999; Newton 2008). Such an obligate partial migration system with variable proportions of migrants and sedentary birds has been recently proposed to be possibly universal in birds, allowing selection to act at any time towards complete migratory or complete sedentary behaviour and enabling species to adapt to changing conditions (see Berthold 1999; reviewed in Newton 2008).
Directional post-breeding movements to specific summering areas have not been described for northern European or Asian populations of great bustards (Dornbusch 1981; Farago 1990; Block 1996; Morales and Martín 2002; Streich et al. 2006), probably because at these latitudes, the summer temperature is not high enough, resembling the situation in northern Spain. This supports our interpretation that summer migration is a relatively recent adaptation of male great bustards to the very hot climate of southern latitudes in Iberia and probably Morocco, which represent the southern limit of the species’ distribution range and the front edge of its expansion from central Europe (Pitra et al. 2000; Alonso et al. 2009). This adaptation could delay the predicted extinction of great bustard populations in southern Iberia as a consequence of climate change by the end of the current century (Huntley et al. 2007). In another Palaearctic bustard, the little bustard Tetrax tetrax, preliminary data suggest that some males from southern Iberia migrate in summer northwards, whereas similar movements have not been reported for little bustards at higher latitudes (García et al. 2004; Silva 2009).
Post-breeding displacements resembling those described in our study have been reported, although not appropriately demonstrated, for other large bustards and might be common in regions subjected to high summer temperatures (Kori bustards Ardeotis kori, T and L Osborne, personal communication; Del Hoyo et al. 1996; great Indian bustards Ardeotis nigriceps, Australian bustards Ardeotis australis and Arabian bustards Ardeotis arabs, D White, in Rahmani and Manakadan 1986; Urban et al. 1986; Del Hoyo et al. 1996). Migration to other areas during the hottest part of the summer have been observed in other bird species that inhabit deserts only during the favourable season, but few studies have examined the advantages of such movements (Williams and Tieleman 2001). Finally, temperature-related summer migrations have not been described for bird species living in temperate regions (Berthold 2001; Berthold et al. 2003). Amongst mammals, the space use and foraging patterns are usually thought to be determined by distribution and seasonal changes in food availability, but recently, altitudinal migrations of male ibex (Capra ibex) have been interpreted, as in our case, as driven by thermoregulation rather than by spatial differences in forage quality (Aublet et al. 2009).
The difference in temperature between breeding and summering areas of great bustards was small (maximum 3.6°C), but considering that the interval between normal and injuriously high body temperatures is less than 6°C in most birds (Calder and King 1974) and that it is narrowed even further with the hyperthermia that can develop with vigorous activity and/or exposure to high temperatures and intense solar radiation (Taylor et al. 1971; Dawson 1984), any reduction in ambient temperature may be crucial. Two additional variables contributed to such reduction. First, the higher precipitation at the summering sites probably helps reduce water loss through evaporation (Dawson and O’Connor 1995). Second, a higher availability of trees enables great bustard males to look for shade during hot midday hours (Palacín 2007; personal observation), facilitating thermoregulation. At the summering sites, great bustards indeed selected fields with trees and sunflower crops, both of which provide protective cover against predators and shade. This contrasts with the species’ habitat selection during other seasons when they prefer open farmland with unobstructed visibility (Alonso and Alonso 1990; Martínez 1991; Lane et al. 2001).
In southern Spain, midday temperatures in July may be up to >15°C cooler in the shade than in the sun, and resting in the shade may have profound effects in the birds’ thermoregulation (see Wolf et al. 1996; Wolf and Walsberg 1996). The tendency to minimise activity and seek shade during the middle of hot days is a common behaviour in desert birds (Austin 1976, 1978; Dawson 1984; Combreau and Smith 1997; Dean et al. 1999; Tieleman 2005), and behavioural thermoregulation through resting has also been described for some mammals (Dussault et al. 2004; Maloney et al. 2005; Aublet et al. 2009). Finally, our frequent observations of great bustards panting at midday in summer even in the shade suggest that they are subjected to considerable heat stress. At high temperatures, evaporation via skin and normal breathing may become insufficient to dissipate the metabolic heat, and evaporative cooling is augmented by panting and gular flutter (Calder and King 1974; Dawson and O’Connor 1995).
Apart from the temperature-related differences, summering sites had lower human population densities than breeding areas. In a species with few natural predators that lives in cereal farmland close to human habitations, this probably reflects a selection for areas with smallest number of disturbances. Great bustards go through a post-breeding moult between June and September, including a serially descendant replacement of the flight feathers. Males are then particularly vulnerable to predators and spend extra energy moulting and thus should seek for quiet areas. From this point of view, summer migrations of male great bustards resemble moult migrations of many ducks, geese, flamingos, auks and waders (Jehl 1990; Berthold 2001; Newton 2008).
In conclusion, our results suggest that the summer migration of great bustard males represents primarily an adaptation to escape the very hot summer temperatures in most breeding areas of central and southern Iberia, with the possible additional benefit of a higher tranquillity during the moulting period. Summer migration of Iberian great bustard males may thus be interpreted as a form of behavioural thermoregulation which has not been described for other populations of this species or for other bird species breeding in temperate latitudes.
References
Alonso JC, Alonso JA (1990) Parámetros demográficos, selección de hábitat y distribución de la Avutarda en tres regiones españolas. ICONA, Madrid
Alonso JC, Alonso JA, Martín E, Morales MB (1995) Range and patterns of great bustard movements at Villafafila, NW Spain. Ardeola 42:69–76
Alonso JC, Alonso JA, Martín E, Morales MB (1998) Proximate and ultimate causes of natal dispersal in the great bustard. Behav Ecol 9:243–252
Alonso JC, Morales MB, Alonso JA (2000) Partial migration, and lek and nesting area fidelity in female great bustards. Condor 102:127–136
Alonso JA, Martín CA, Alonso JC, Morales MB, Lane SJ (2001) Seasonal movements of male great bustards in central Spain. J Field Ornithol 72:504–508
Alonso JC, Martín CA, Alonso JA, Lieckfeldt D, Magaña M, Palacín C, Pitra C (2009) Genetic diversity of the great bustard in Iberia and Morocco: risks from current population fragmentation. Conservation Genetics 10:379–390
Aublet J-F, Festa-Bianchet M, Bergero D, Bassano B (2009) Temperature constraints on foraging behaviour of male Alpine ibex (Capra ibex) in summer. Oecologia 159:237–247
Austin GT (1976) Behavioral adaptations of the verdin to the desert. Auk 93:245–262
Austin GT (1978) Daily time budget of the postnesting verdin. Auk 95:247–251
Berthold P (1984) The control of partial bird migration in birds: a review. Ring 10:253–265
Berthold P (1996) Control of bird migration. Chapman and Hall, London
Berthold P (1999) A comprehensive theory for the evolution, control and adaptability of avian migration. Ostrich 70:1–12
Berthold P (2001) Bird migration. A general survey, 2nd edn. Oxford University Press, Oxford
Berthold P, Gwinner E, Sonnenschein E (2003) Avian migration. Springer, Berlin
Blanckenhorn WU (2000) The evolution of body size: what keeps organisms small? Quart Rev Biol 75:385–407
Block B (1996) Wiederfunde von in Buckow ausgewilderten Großtrappen (Otis t. tarda L., 1758). Nat.schutz Landsch.pfl Brandenburg 1(2):76–79
Blondel J, Aronson J (1999) Biology and wildlife of the Mediterranean region. Oxford University Press, Oxford
Bustamante J (2003) Cartografía predictiva de las variables climáticas: comparación de distintos modelos de interpolación de la temperatura en España peninsular. Graellsia 59:359–376
Cade BS, Hoffman RW (1993) Differential migration of blue grouse in Colorado. Auk 110:70–77
Calder WA, King JR (1974) Thermal and caloric relations of birds. In: Farner DS, King JR (eds) Avian biology. Academic, New York, pp 260–413
Combreau O, Smith TR (1997) Summer habitat selection by houbara bustards introduced in central Saudi Arabia. J Arid Environ 36:149–160
Conradt L, Clutton-Borck TH, Guinness FE (2000) Sex differences in weather sensitivity can cause habitat segregation: red deer as an example. Anim Behav 59:1049–1060
Cristol DA, Baker MB, Carbone C (1999) Differential migration revisited: latitudinal segregation by age and sex class. In: Nolan V Jr, Ketterson ED, Thompson CF (eds) Current ornithology, vol 15. Plenum, New York, pp 33–88
Dawson WR (1984) Physiological studies of desert birds: present and future considerations. J Arid Environ 7:133–155
Dawson WR, O’Connor TP (1995) Energetic features of avian thermoregulatory responses. In: Carey C (ed) Avian energetics and nutritional ecology. Chapman and Hall, New York, pp 85–124
De Castro M, Martín-Vide J, Alonso S (2005) El clima de España: pasado, presente y escenarios de clima para el siglo XXI. In: Oficina Española de Cambio Climático (ed) Impactos del Cambio Climático para España. Ministerio de Medio Ambiente, Madrid, pp 1–64
Dean WRJ, Miltonw SJ, Jeltsch F (1999) Large trees, fertile islands, and birds in arid savannah. J Arid Environ 41:61–78
Del Hoyo J, Elliot A, Sargatal J (1996) Handbook of the birds of the world, vol 3. Lynx, Barcelona
Dornbusch M (1981) Bestand, Bestandsförderung und Wanderungen der Großtrappe (Otis tarda). Naturschutzarb. Berlin–Brandenburg 17:22–24
Dussault C, Ouellet JP, Courtois R, Huot J, Breton L, Larochelle J (2004) Behavioural responses of moose to thermal conditions in the boreal forest. Ecoscience 11:321–328
Eastman JR (2000) Idrisi for Windows. User’s guide, version 32. Clark Laboratories, Clark University, Worcester, MA
Farago S (1990) The effect of heavy winters on bustard (Otis tarda) populations in Hungary. Alatt Közl 76:51–62
García J (2000) Dispersión premigratoria del cernícalo primilla Falco naumanni en España. Ardeola 47:197–202
García EL, Morales MB, De Juana E, Suárez F (2004) Does Spanish little bustards migrate? New data on long distance movements. In: Centre Tecnologic Forestal de Catalunya (ed) International Symposium on Ecology and Conservation of Steppe-land Birds, Lleida, p 79
Gauthreaux SA (1982) The ecology and evolution of avian migration systems. In: Farner DS, King JR, Parkes KC (eds) Avian biology, vol 6. Academic, New York, pp 93–168
Glutz UN, Bauer KM, Bezzel E (1973) Handbuch der Vögel Mitteleuropas vol 5. Akademische Verlagsgesellschaft, Frankfurt a.M
Hellmich J (1991) La avutarda en Extremadura. Alytes 2:1–167
Herzog P, Keppie DM (1980) Migration in a local population of spruce grouse. Condor 82:366–372
Hidalgo SJ, Carranza J (1990) Ecología y comportamiento de la avutarda (Otis tarda). Universidad de Extremadura, Cáceres
Huntley B, Green RE, Collingham YC, Willis SG (2007) A climatic atlas of European breeding birds. Lynx Edicions, Barcelona
Ivlev VS (1961) Experimental ecology of the feeding of finches. Yale University Press, New Haven
Jackes AD (1973) The use of wintering ground by red deer in Ross-shire, Scotland. M.Phil. thesis, University of Edinbourgh, Edinbourgh
Jehl JR (1990) Aspects of the molt migration. In: Gwinner E (ed) Bird migration: physiology and ecophysiology. Springer, Berlin, pp 102–116
Kaitala A, Kaitala V, Lundberg P (1993) A theory of partial bird migration. Am Nat 142:59–81
Ketterson ED, Nolan V (1983) The evolution of differential bird migration. In: Johnston RF (ed) Current ornithology, vol 1. Plenum, New York, pp 357–402
Koenig WD, Vuren DV, Hooge PN (1996) Detectability, philopatry and the distribution of dispersal distances in vertebrates. Trends Ecol Evol 11:514–517
Kovach Computing Services (2004) Oriana software, version 2.0. Anglesey, Wales. http://www.kovcomp.co.ik/oriana/oribroc.html
Lane SJ, Alonso JC, Alonso JA, Naveso MA (1999) Seasonal changes in diet and diet selection of great bustards (Otis t. tarda) in north-west Spain. J Zool 247:201–214
Lane SJ, Alonso JC, Martín CA (2001) Habitat preferences of great bustard Otis tarda flocks in the arable steppes of central Spain: are potentially suitable areas unoccupied? J Appl Ecol 38:193–203
Lundberg P (1987) Partial bird migration and evolutionary stable strategies. J Theor Biol 125:351–360
Lundberg P (1988) The evolution of partial migration in birds. TREE 3:172–175
Magaña M (2007) Comportamiento reproductivo de la Avutarda Común. PhD thesis, Universidad Complutense, Madrid
Maloney SK, Moss G, Cartmell T, Mitchell D (2005) Alteration in diel activity patterns as a thermoregulatory strategy in black wildebeest (Connochaetes gnou). J Comp Physiol A 191:1055–1064
Margalef R (1974) Ecología. Omega, Barcelona
Martínez C (1991) Patterns of distribution and habitat selection of a great bustard (Otis tarda) population in northwestern Spain. Ardeola 38:137–147
Morales MB, Martín CA (2002) Great bustard. In: BWP Update 4. Oxford University Press, Oxford, pp 217–232
Morales MB, Alonso JC, Alonso JA, Martin E (2000) Migration patterns of great bustard males. Auk 117:493–498
Newton I (2008) The migration ecology of birds. Academic, London
Olea PP (2001) Postfledging dispersal in the endangered lesser kestrel Falco naumanni. Bird Study 48:110–115
Palacín C (2007) Comportamiento migratorio de la Avutarda Común en la Península Ibérica. PhD thesis, Universidad Complutense, Madrid
Pitra C, Lieckfeldt D, Alonso JC (2000) Population subdivision in European great bustards inferred from mitochondrial and nuclear DNA sequence variation. Mol Ecol 9:1165–1170
Rahmani AR, Manakadan R (1986) Movement and flock composition of the great Indian bustard Ardeotis nigriceps (Vigors) at Nanaj, Solapur District, Maharashtra, India. J Bombay Nat Hist Soc 83:17–31
Rubio JL, Carrascal LM (1994) Habitat selection and conservation o fan endemic Spanish lizard Algyroides marchi (Reptilia, Lacertidae). Biol Conserv 70:245–250
Ruckstuhl KE, Clutton-Brock TH (2005) Sexual segregation and the ecology of the two sexes. In: Ruckstuhl KE, Neuhaus P (eds) Sexual segregation in vertebrates. Cambridge University Press, Cambridge, pp 3–7
Ruckstuhl KE, Neuhaus P (2000) Sexual segregation in ungulates: a new approach. Behaviour 137:361–377
Schroeder MA (1985) Behavioural differences of female spruce grouse undertaking short and long migrations. Condor 87:281–286
Schroeder MA, Braun CE (1993) Partial migration in a population of greater prairie chickens in northeastern Colorado. Auk 110:21–28
Searcy WA (1980) Optimum body sizes at different temperatures: an energetic explanation of Bergmann’s rule. J Theor Biol 83:579–593
Silva JP (2009) Sisao. Seguimento de aves via satélite. In: http://seguimentodeaves.domdigital.pt/sisao/ Accessed 4 Feb 2009
Silva JP, Faria N, Catry T (2007) Summer habitat selection and abundance of the threatened little bustard in Iberian agricultural landscapes. Biol Conserv 139:186–194
StatSoft (2001) STATISTICA version 6. Tulsa, USA
Streich WJ, Litzbarski H, Ludwig B, Ludwig S (2006) What triggers facultative winter migration of great bustard (Otis tarda) in Central Europe? Eur J Wildlife Res 52:48–53
Taylor CR, Dmi’el R, Fedak M, Schmidt-Nielsen K (1971) Energetic cost of running and heat balance in a large bird, the rhea. Am J Physiol 221:597–601
Terrill SB, Able KP (1988) Bird migration terminology. Auk 105:205–206
Tieleman BI (2005) Physiological, behavioral, and life history adaptations of larks along an aridity gradient: a review. In: Bota G, Morales MB, Mañosa S, Camprodon J (eds) Ecology and conservation of steppe-land birds. Lynx Edicions, Barcelona, pp 49–67
Tieleman BI, Williams JB (1999) The role of hyperthermia in the water economy of desert birds. Physiol Biochem Zool 72:87–100
Urban EK, Fry CH, Keith S (1986) The birds of Africa, vol 2. Academic, London, UK
Waldorp LJ, Grasman RP, Huizenga HM (2006) Goodness-of-fit and confidence intervals of approximate models. J Math Psychol 50:203–213
Wallace C, Chapman JM, Clayton DG (2006) Improved power offered by a score test for linkage disequilibrium mapping of quantitative-trait loci by selective genotyping. Am J Hum Genet 78:498–504
Walsberg GE, King JR (1978) The relationship of the external surface area of birds to skin surface area and body mass. J Exp Biol 76:185–189
Watzke H (2007) Results from satellite telemetry of great bustards in the Saratov region of Russia. Bustard Stud 6:83–98
Watzke H, Litzbarski H, Oparina HS, Oparin ML (2001) Der Zug von Grosstrappen Otis tarda aus der Region Saratov (Russland)-erste Ergebnisse der Satellitentelemetrie im Rahmen eines Schutzprojektes. Vogelwelt 122:89–94
Williams JB, Tieleman BI (2001) Physiological ecology and behavior of desert birds. In: Nolan V Jr (ed) Current ornithology, vol 16. Kluwer, New York, pp 299–353
Winer BJ, Brown DR, Michels KM (1962) Statistical principles in experimental design. McGraw-Hill, New York
Wolf BO, Walsberg GE (1996) Thermal effects of radiation and wind on a small bird and implications for microsite selection. Ecology 77:2228–2236
Wolf BO, Wooden DM, Walsberg GE (1996) The use of thermal refugia by two small desert birds. Condor 98:424–428
Young TP, Isbell SA (1991) Sex-differences in giraffe feeding ecology—energetic and social constraints. Ethology 87:79–89
Acknowledgements
We thank M. Magaña and B. Martín for their collaboration during fieldwork. Additional help during bird captures and controls was provided by E. Martín, M. B. Morales, S. J. Lane and D. González. We also thank P. Sastre for his assistance to obtain GIS variables, LM Carrascal for his support during statistical analyses and two anonymous referees for their comments on an earlier version of the manuscript. We are especially grateful to the 42 Group of the Spanish Air Forces for their generous collaboration in locating radio-tagged birds. The field work was financed by the Dirección General de Investigación (projects PB91-0081, PB94-0068, PB97-1252 and BOS2002-01543), Instituto Nacional para la Conservación de la Naturaleza, Dirección General de Conservación de la Biodiversidad and Consejería de Medio Ambiente, Junta de Andalucía.
Ethical standards
The procedures followed in this study comply with the current Spanish laws.
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by: E. Korpimäki
Rights and permissions
About this article
Cite this article
Alonso, J.C., Palacín, C., Alonso, J.A. et al. Post-breeding migration in male great bustards: low tolerance of the heaviest Palaearctic bird to summer heat. Behav Ecol Sociobiol 63, 1705–1715 (2009). https://doi.org/10.1007/s00265-009-0783-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00265-009-0783-9