Abstract
Little bustard seasonal movements are complex. Besides the long known fully long-distance migratory populations (i.e. those of western France, Russia, and Central Asia), there are also others fully migratory that perform shorter migrations, such as many from northern Spain, or partially migratory, such as those from central Spain. Moreover, there are other seasonal movements different from winter migration that entail a directional round trip after breeding (summer migration) or a combination of the latter with a winter directional migration from summer sites to wintering sites and return to breeding grounds. Summer movements are frequent in strongly seasonal Mediterranean areas of central and southern Iberia and usually involve trips to the north or to higher altitudes with less severe summer drought. Finally, there are resident populations in southern Iberia and other Mediterranean regions that perform short-distance movements, tracking the availability of food and other resources. Although such a variety of movements has been described only in the Iberian Peninsula, it may have been present in other climatically and geographically diverse regions of the species’ former range.
Because little bustards move frequently, they are exposed to threats that they may not find in their breeding grounds (which are often protected and relatively cared-after). Moreover, habitat selection outside the breeding season is not constrained by breeding requirements, and thus the diversity of habitats used by birds is greater, so they can be found in places where they rarely appear in spring, such as the proximity of linear infrastructures or irrigated farmland. However, non-breeding grounds are usually less protected than breeding sites. Threats faced by little bustards out of the breeding areas comprise land-use changes (including urbanization and intensively irrigated crops) and infrastructure development that lead to a strong decrease or full disappearance of large winter concentrations. In addition, powerline collision risk, poaching, or simple disturbance from human frequentation have been found to affect wintering little bustards negatively.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Introduction
The study of species’ post-breeding ecology has become a rapidly growing field in avian biology as researchers have realized its relevance, not only in completing our picture of species’ annual biological cycles, but also in our understanding of their breeding biology and population dynamics, and thereby for their conservation and management (Sherry and Holmes 1996; Newton 1998). Therefore, the identification of species’ requirements and threats outside of the reproductive season is crucial to the development of effective conservation strategies that take those requirements into account (Vickery et al. 2014). Birds inhabiting dynamic and seasonally changing landscapes such as cereal farmland in temperate and Mediterranean climates are faced with rapid oscillation in the availability of limiting resources, and knowing how they cope with these natural and human-induced changes beyond the breeding season is essential if their currently declining trends are to be reversed (Suárez 2004).
Birds and other animals usually respond to environmental changes through their propensity to move to a different area, offering individuals better survival or breeding opportunities (Newton 2010). This tendency to migrate is usually adaptive and thus genetically fixed in most populations. Consequently, migratory behaviour has evolved in many species whenever adequate selection pressures are present (Berthold 1993; Newton 2010). Moreover, experimental evidence has proved that migratory behaviour can evolve in a sedentary population or be lost in a migratory one in a relatively short time period (even decades in short-lived species) if selective advantages leading to enhanced breeding performance and/or survival are strong (Berthold et al. 1992; Pulido 2007; Pulido and Berthold 2010).
In the present chapter, we focus on two main and intrinsically linked aspects of little bustard post-breeding biology. First, we describe the little bustard seasonal movements and migration, highlighting the diversity of movement patterns found across the species’ distribution range. Then, we synthesize post-breeding habitat and resource use, which includes habitat selection and diet composition, as well as flocking behaviour and home range features. After reviewing little bustard post-breeding biology, we finish by discussing some of the main threats faced by the species, specifically outside the breeding season, as well as the conservation measures that can be applied to minimize them.
Little Bustard Seasonal Movements
Overview
Animals undertaking a regular annual return movement from reproductive to post-breeding areas are usually regarded as migratory (Bernis 1966a; Sinclair 1983; Terrill and Able 1988). In the case of birds, when this movement occurs after the breeding season in a restricted range of directions (usually north to south and vice versa in the higher latitudes of the Northern Hemisphere), associated with seasonal changes in the conditions for survival and involving variable distances (from tens to thousands of kilometres), it is known as typical or directional migration, and it usually produces massive displacements of birds and a corresponding geographical shift of populations (Newton 2010). However, bird species show a huge range of movement patterns (Berthold 1993; Newton 2010): at the opposite end of that range, populations whose individuals perform much shorter displacements, so that their distributions remain unchanged within and across years, are considered resident or sedentary (Newton 2010). Bird migratory behaviour is largely determined by genetic adaptation to local environmental conditions, which generates an enormous intraspecific variation, particularly in species with large distributions (Alerstam et al. 2003; Pulido 2007). Differences in migration behaviour may be found even within populations so that those where all individuals leave breeding localities are known as annual or fully migratory populations, while those where only some individuals migrate and others do not are called partially migratory (Chapman et al. 2011).
As introduced in chapter “The little bustard around the world: distribution, global conservation status, threats and population trends”, the little bustard’s world range, comprises both migratory and sedentary populations. In general terms, most populations currently representing the western sub-range are considered sedentary (Snow and Perrins 1998, see Table 1). Little bustard populations of central-western France are the exception since they are mainly fully migratory, using areas in central and southern Iberia as wintering quarters (Villers et al. 2010). Different authors have speculated about the possible wintering of European little bustards in North Africa in historical times, although no evidence based on radio/satellite tracking, ring recovery or direct observation of birds crossing the Mediterranean has ever been provided (see review in Palacín and Alonso 2009).
Remaining little bustard populations from the eastern sub-range, conversely, are considered mainly migratory (Snow and Perrins 1998). Birds from southern Russia and Central Asia have long been known to leave their breeding grounds during the harsh winters typical of those regions (e.g. Shlyakhtin et al. 2004), and large winter concentrations have been observed south of the Black and Caspian Seas (Gauger 2007; Sehhatisabet et al. 2012; Yousefi et al. 2017). In addition, there are historical records of massive autumn migration at bottlenecks along the main eastern flyways (Phillips-Wolley 1881; Heiss 2013). Nothing is known about the seasonal movements of the marginal populations that might still breed in Iran and other parts of the Middle East (see chapter “The little bustard around the world: distribution, global conservation status, threats and population trends”), although similar patterns to those found in western Mediterranean populations might be expected (Table 1).
Nevertheless, recent studies based on radio and satellite tracking have shown that little bustard seasonal movements can be quite diverse and that populations once considered sedentary in fact exhibit a variety of movement patterns associated with different local or regional-scale factors. Some of these populations are far from sedentary (García de la Morena et al. 2015). Such intraspecific diversity of movement has also been described in other bustards and steppe birds (e.g. Morales et al. 2000; Alonso et al. 2001; Palacín 2007; Limiñana et al. 2008; Combreau et al. 2011) and, as mentioned, is generally found in species whose extensive distributions encounter a large variety of seasonal environments (Newton 2010). On the other hand, in spite of the spatial segregation and different behaviour of the sexes during the breeding period (see chapter “Habitat selection and space use”), male and female little bustards form mixed post-breeding flocks and, although much of what we know from their movements are based on tagged males, there is no evidence of differential migration of the sexes, as described for other bustard species (e.g. Morales et al. 2000; Alonso et al. 2000). In this section, we review and discuss, in the framework of bird migration theory, the whole variety of seasonal movement patterns, from typical directional migration to strictly sedentary behaviour, shown by the little bustard across its entire range.
Long-Distance Directional Migration: Eastern Range
As stated above and reviewed in chapter “The little bustard around the world: distribution, global conservation status, threats and population trends”, basically all little bustard breeding populations found north of the Black and Caspian Seas, in the species’ eastern sub-range, are considered fully migratory (e.g. Martin et al. 2018). This conclusion is based on the following observations: (1) disappearance of birds from breeding grounds during winter to avoid snow cover or heavy frost, (2) the concentration of very large numbers of birds south of those seas, mainly in eastern Azerbaijan (150–200,000 individuals, Gauger 2007), and northern Iran (up to ca. 57,000 in recent years, Yousefi et al. 2017), and (3) the passage of very large numbers of migrating little bustards flying through the Besh Barmag bottleneck of the Caucasus migration flyway (Heiss 2013). Therefore, this seems to be a typical north–south autumn migration between breeding and wintering areas, likely comprising a few thousand kilometres.
In spite of these reports, no attempt to radio or satellite-track little bustards during their migration movements in Central Asia has yet been made. Therefore, their precise migration routes, stopovers, and timing, as well as their degree of breeding and wintering site fidelity and possible pre-migratory movements, are unknown. These birds seem to stay on the Iranian wintering grounds from November to February (Sehhatisabet et al. 2012), and apparently through March at the Azerbaijani ones (Heiss 2013), although supplementation in this latter area by birds returning from further south or south-east (Iran) during the spring migration cannot be discarded. Information on dates of departure from, and arrival in, breeding areas is scarce. According to Shlyakhtin et al. (2004), little bustards breeding in the Transvolga region of southern Russia undertake their autumn migration from early October to early November, flying south to the northern Caspian region and then south-west to Ciscaucasia (northern Caucasus). These birds would likely join the contingents passing through the Besh Barmag bottleneck (Heiss 2013). Shlyakhtin et al. (2004) also report the arrival of little bustards on their nesting grounds in the second half of April, although this might occur as late as early May in higher latitudes such as northern Kazakhstan (Snow and Perrins 1998). Since these dates may imply some level of timing mismatch between departure from winter quarters and arrival at breeding sites, and the numbers passing through the bottleneck are far fewer in spring, different and less direct spring flyways (e.g. associated with prospecting trips) could exist and be revealed with the use of adequate tracking technology, as described in other Central Asian migratory birds (Terraube et al. 2012; Kessler et al. 2013).
Although the numbers mentioned above clearly suggest that Azerbaijan and Iran hold the bulk of the eastern winter population of the species, additional records indicate that other regions in Central Asia receive smaller but still significant numbers of wintering little bustards. In fact such information has existed since the early and mid-twentieth century (e.g. Zarudny 1915, Ivanov 1940, Maslov 1947), although its limited availability to non-Russian speakers and the real population changes that have occurred since then have not allowed greater clarity on the evolution of wintering numbers in the region. E.A. Kreutzberg (pers. comm.) reports a flock of 1500 little bustards near Termez, Uzbekistan, at the border with Afghanistan in February 2001. This observer reports smaller numbers (120–140 birds) at the same site the following winter and has compiled observations of small flocks (from a few individuals to a few tens) of migrating and wintering little bustards in Uzbekistan during the early twenty-first century. Observations of migrating birds mainly involve central Uzbekistan (Kyzylkum desert region), and wintering flocks tend to be located in the southern Surkhandarya province, close to the Afghan border. In this latter region, more recent observations of large flocks have been reported by A. Ten and V. Soldatov (pers. comm.), who found a winter roost with 1400 little bustards near the Amu Darya river and a flock of 1980 birds north-west of that area in January 2018. Gavrilov and Gavrilov (2005) report on the regular concentration of several hundreds of birds from the second half of August to early May in southern Kazakhstan, close to the Kirgiz border, although there are no more recent records from this area (E.A. Kreuzberg, pers. comm.). Finally, in south-western Tajikistan, the little bustard seemed to winter in relatively large numbers in the Javan valley where 1500–4000 individuals were regularly observed between 2004 and 2010 according to R.S. Muratov (pers. comm.), who reports however that the amount of birds wintering there decreased to 300–500 in later years. This observer also reports migration and irregular wintering in the Sir Darya valley in northern Tajikistan. Overall, these observations indicate the existence of several minor but still significant little bustard wintering areas in Central Asia, as well as migration flyways different from the main one that runs west of the Caspian Sea and through the Caucasus to Azerbaijan and northern Iran, which should be confirmed in the future through the use of satellite telemetry. Nevertheless, it is worth noting that the total latitudinal range of migration movements of little bustards in the eastern range is quite similar to that observed in the west (see below), ranging from around 50°N (e.g. northern Kazakhstan or former breeding areas in north-western Europe) to around 37°N (e.g. wintering grounds in Central Asia or southern Iberia).
Long-Distance Directional Migration: Western Range
In the western sub-range, little bustard populations of central-western France have always been known to desert their breeding areas in autumn (see, for example Snow and Perrins 1998), although some individuals may overwinter close to them (Villers et al. 2010). It is thus reasonable to assume that all populations historically breeding in central-western, northern, and eastern France performed similar movements. In the early twentieth century, little bustards also bred in south-western France, where they are now extinct; their winter movements were unknown. Although French populations were suspected of overwintering in Iberia (e.g. Bernis 1966b), their precise winter quarters were not revealed until radio, and satellite tracking was applied. Using data provided by 32 radio- and satellite-tracked birds tagged between 1997 and 2007, Villers et al. (2010) showed that the great majority of little bustards breeding in central-western France (fitted in Poitou-Charentes and Centre regions) perform a typical directional migration to overwinter in central and south-western areas of the Iberian Peninsula. Additional data from birds tracked after 2010 have confirmed these patterns.
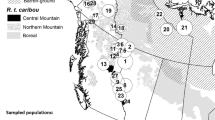
According to Villers et al. (2010) and other cited reports, five main wintering areas of French little bustards in Iberia can be distinguished (Fig. 1). One is the river Duero basin in the Spanish Northern Plateau. This area was also used by birds as stopovers on their further route south (Delgado et al. 2010; Villers et al. 2010). The second is the Spanish Southern Plateau, mainly the valleys of the rivers Tagus and Jarama, but also farther south in La Mancha region (Morales et al. 2002). The third is Extremadura, also in Spain, a large region with different wintering sites, located mainly in the Tagus basin, but also in its south-eastern confines, in La Serena (Delgado et al. 2010). A fourth wintering area is located around the Tagus estuary in Portugal. Finally, a fifth site was found in the Lleida plains in Catalonia (north-eastern Spain). Stopover sites used during both the autumn and spring migrations were also identified (Fig. 1). Stopovers were located mainly in northern Spain (both during autumn and spring migration, Villers et al. 2010), mostly in the Northern Plateau, but also in the Ebro valley (Morales et al. 2002; Delgado et al. 2010; Villers et al. 2010). However, some stopover sites were also identified in northern Extremadura during both autumn and spring migrations and the Southern Plateau during the spring migration (Delgado et al. 2010). A few birds also regularly stop at French sites before crossing the Pyrenees. During their stay in wintering areas, French little bustards join Iberian birds in wintering flocks (Delgado et al. 2010). At least some French birds perform smaller-scale movements, changing overwintering sites (Morales et al. 2002) in a rather nomadic way (sensu Newton 2010), as also often observed in Iberian birds.
Precise migration routes, stopovers, and wintering sites of French little bustards wintering in the Iberian Peninsula provided by GPS telemetry. Significant regions and geographical features mentioned in the text are shown. Based on Villers et al. (2010) and updated with unpublished data from V. Bretagnolle and A. Villers
Little bustards from central-western France leave their breeding grounds during October and early November. These departures involve most birds from a given area, which indicates that French little bustards migrate in flocks (Villers et al. 2010) as observed in the eastern populations. Using data from GPS-satellite transmitters, Villers et al. (2010) showed that migration to Iberian wintering quarters is generally accomplished in a single or two consecutive night flights (Villers et al. 2010). The flights were ca. 450 km long if they were direct and 330–380 km if consecutive, with total distances ranging from 600 to 1178 km (Villers et al. 2010). Two main flyways were identified and confirmed by more recent GPS-tagged birds (Fig. 1). One included birds flying over land and using passes in the western Pyrenees. The other included bustards that entered the Peninsula through the eastern Cantabric coast, crossing the Bay of Biscay in about 4 h. One particular individual flew at 64 km/h and at a maximum distance from the coast of 66 km (Villers et al. 2010). The existence of one or more sea flyways is consistent with the relatively high number of historical little bustard observations along the Spanish Cantabric coast compiled by García de la Morena (2015). Although, to date, only four migratory French little bustards have been tracked in at least 2 consecutive years (Villers et al. 2010, V. Bretagnolle and A. Villers unpublished data), data suggest high variability in migratory behaviour both between and within individuals, with some birds using different flyways in consecutive years, while others have crossed the Pyrenees via the exact same pass for 2 consecutive years, only to switch to a different route for another two spring migrations.
Directional migration, however, is not exclusive to French little bustards within the species’ western range. Using radio, satellite and GPS-satellite tracking, García de la Morena et al. (2015) have identified different populations in Iberia where little bustards perform a typical north–south autumn migration from their breeding or post-breeding sites to winter quarters several hundred kilometres distant. These populations breed in areas of northern and central Spain where spring and summer productivity is high, but winters are cold. Such localities spread over a large region ranging from Galicia, in north-western Spain, to the Ebro valley in the north-east, and comprise the Spanish Northern Plateau and the high-altitude plains (páramos) of the Central and Iberian ranges (Fig. 2). Birds tagged in these populations leave breeding or post-breeding summer grounds during late September, October, and November to spend the winter in thermo and meso-Mediterranean localities in the southern half of Iberia, from Alentejo (southern Portugal) to Extremadura and the Spanish Southern Plateau (Fig. 2a), where they join local populations in relatively large flocks (from tens to several hundreds, historically even a few thousands of individuals, Otero 1985; García de la Morena 2015). Birds return to their breeding sites during March and April (Fig. 3a), although some may reach lekking areas as late as June in Galicia. In this respect, it is worth noting that two adult males captured as breeders in Galicia were located as late as 15 May in Alentejo and Extremadura at lekking sites where local males were calling to attract mates. Their movements in this period, as revealed by GPS locations, suggested that they might be attempting breeding in those localities (own unpubl. data). These two birds later flew north to their capture sites, where other males were at their lekking territories throughout June. This movement behaviour resembles that of common quails (Coturnix coturnix), which in western Europe are known to track favourable reproductive environmental conditions (ecosystem productivity) from wintering quarters in northern Africa up to France and through Iberia, breeding successively along the way as those conditions are met (Sardà-Palomera et al. 2012). Nevertheless, these males may have still been too young to be definitely established as territorial breeders.
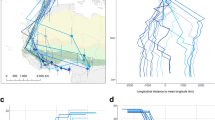
Simplified migration movements performed by some little bustards radio- and satellite-tracked in Iberia, and representing the two migration types described in this chapter. Stars show locations where breeding birds were captured, and red arrows indicate wintering or summering movements. (a) typical autumn migration; (b) summer migration by pure summer (dashed arrows) and winter-summer migrants (solid arrows). Ellipsoids roughly represent the areas where the different migration patterns described for Iberian little bustards are dominant (see text for details). Solid: the dominance of full autumn migration; pointed: the dominance of partial autumn migration; dashed: coexistence of partial autumn and summer migration, receiving winter migrants; dashed and pointed: the dominance of partial summer migration, receiving winter migrants. Based on García de la Morena et al. (2015) and García de la Morena (2015)
Migration patterns described for the little bustard in the Iberian Peninsula according to mean distance to breeding sites of 47 radio- and satellite-tracked birds (bars indicate 95% confidence intervals). (a) typical autumn migration (N = 6); (b) pure summer migration (N = 5); (c, d, e) summer-autumn migration (see text for differences, N = 15, 6, 7, respectively); (f) sedentary (N = 8). Based on García de la Morena et al. (2015)
Little bustards completely or almost completely leave their breeding or post-breeding sites in the northern half of Iberia, where they are basically spring and summer visitors, although, in some localities of the Spanish Northern Plateau, some wintering or stopover visitors may be observed (García de la Morena 2015 see also above). The only reported exceptions are populations in Catalonia, in the eastern Ebro valley, where breeding little bustards overwinter in irrigated alfalfa fields a few kilometres away, and only a few birds sporadically undertake long-distance migration to central and southern Iberia (S. Mañosa and co-workers’ unpublished data, Sampietro et al. 2013). In cases when individuals could be tracked for more than a year, they displayed between-year fidelity to both breeding and wintering grounds, although some visited different sites during the winter (García de la Morena et al. 2015, own unpubl. data). It can be therefore concluded that these northern or high-altitude central Iberian populations can be considered totally or partially migratory (Fig. 2a).
The average distance travelled by birds performing long-distance autumn migration in Iberia is 319 km (SD = 148), with a recorded maximum of 565 km (García de la Morena et al. 2015, own unpubl. data). These migration flights occur basically at night. As flapping flyers, little bustards can reach relatively high instant speeds. For example the mean speed of birds migrating from northern Spain to their wintering grounds in south-western Iberia was 85 km/h, but maximum speeds reached 98 km/h in a male flying with a tailwind during spring migration from his wintering site in western Andalusia to his breeding territory on the Iberian high plateau (García de la Morena 2015, authors’ own unpubl. data). French little bustards were regularly recorded flying over 90 km/h during migration, and one individual travelled as fast as 137 km/h during spring migration, probably thanks to a strong tailwind. Birds flew at a mean altitude (relative to ground level) of 326 ± 273 m, and flying altitude did not seem to be affected by relief, although variance in relative altitude tended to be lower when birds crossed higher areas (mean ± SD for ground altitudes <500 m.a.s.l. = 326 ± 320 m and mean ± SD for ground altitudes >1000 m.a.s.l. 319 ± 180 m).
Migration movements usually involved one stopover before crossing the main mountain ranges encountered by birds. Little bustards tagged in Galicia and the Northern Plateau crossed the Iberian Central Range using the natural passages of its western sector (altitudes 910–1275 m.a.s.l., see Fig. 2a). Stopovers on the autumn migration were located near the northern slopes of the range, while in spring, birds usually used stopovers near the southern slopes (own unpubl. data). Birds migrating from the Ebro valley used natural corridors of the Iberian System at ca. 1218 m.a.s.l., Fig. 2a). Although the wintering locations of tracked little bustards were concentrated in south-western Iberia (Alentejo, Extremadura and adjacent areas of the Southern Plateau and Andalusia), birds breeding in more eastern populations also wintered in more eastern quarters (Fig. 2a). These long-distance directional migrants represented 24% of the 71 birds monitored by García de la Morena et al. (2015).
Other Migration Patterns
García de La Morena et al. (2015) showed that Iberian little bustards might perform a second type of seasonal movement, not described in other parts of the species’ range and depicted in Fig. 2b. It is a post-breeding summer movement undertaken by birds breeding in areas of very low summer productivity typical of the semi-arid Mediterranean climate of the southern half of Iberia. Birds may also completely leave these breeding areas and fly to northern and/or higher-altitude localities, where summer productivity can still provide feeding resources (it should be recalled that the Spanish Northern Plateau is notably higher than the Southern Plateau: 850 vs. 550 m.a.s.l. on average, respectively; see also García de la Morena et al. 2015). In fact, many 10 × 10 km Atlas squares in the northern half of Spain possess exclusive summer records of the species (García de la Morena 2015). However, some summering sites were not located in more northerly or higher areas but simply coincided with places offering enhanced summer primary production (e.g. areas with deeper, more productive soils, irrigated farmland, Silva et al. 2007, own unpubl. data).
Individuals from the same breeding locality may visit quite different summering sites, and distances travelled are shorter than those recorded for autumn migration, reaching a reported maximum of 162 km (but most often only several tens of km). Unlike typical autumn migration, birds do not usually gather in large flocks but spend the summer in small dispersed groups and often solitarily (own unpubl. data). Therefore, although this summer migration shares some features with the typical directional migration (i.e. geographically biased spread of movement directions, complete disappearance from some breeding areas), it closely resembles the so-called dispersive migration described in different seabird species (Newton 2010). Since birds undergo a partial moult during this phase (Cramp and Simmons 1980), it also somehow parallels the moult migration performed by many waterfowl (Newton 2010), although the large concentrations observed in those species do not occur in the little bustard. Birds performing summer migration were a majority in the sample monitored by García de la Morena et al. (2015), representing 75% of all individuals. In fact, the vast majority (ca. 77%) of 10 × 10 km Atlas squares in Spain with the presence of the species at some time of the year do not hold little bustards during summer, which means that birds leave these squares to summer elsewhere (García de la Morena 2015; see Silva et al. 2007 for Portugal). This indicates that the summer movement may actually be a dominant pattern in Iberia. However, summer migrants tagged were mainly males, which moved from lekking sites while breeding females were still rearing their chicks. Therefore, female movement patterns in these populations are poorly known and might differ from those reported for males.
Summer migrants may follow three different patterns of movement after the summer period (García de la Morena et al. 2015, Fig. 3). Some return directly to their breeding areas, where they stay for the rest of the year (Fig. 3b), while others stay through the winter at summering localities (Fig. 3c) or fly south to overwinter before returning the following spring (Fig. 3d, e). The former ‘pure’ summer migrants may start leaving breeding sites in May and begin their return in September. These individuals usually breed in localities where conditions are adequate for foraging and survival also in the winter, common in the Spanish Southern Plateau and Extremadura. Those staying at summering sites through the winter return to breeding sites during March. Among birds moving in autumn to a different overwintering site, called summer–winter migrants by García de la Morena et al. (2015), these authors identified two groups, those travelling to summering sites further away from the breeding grounds than the wintering areas (Fig. 3d), and those moving to relatively nearby summering sites (Fig. 3e), but overwintering in much more distant areas. Birds in this latter group usually belong to breeding populations where winters are cold, as it is usually the case in central Spain, but are close to higher-altitude areas or even irrigated farmland that can provide food resources during the summer drought period.
Sedentary Populations
Little bustard breeding localities where the species is present throughout the year (Fig. 3f), and thus can be considered sedentary, are spread across southern Iberia (Portuguese Alentejo, Extremadura, Spanish Southern Plateau, and Andalusia) and the Ebro valley (Silva and Pinto 2006; García de la Morena 2015), all under semi-arid meso- or thermo-Mediterranean climate conditions (García de la Morena et al. 2015). However, and at least in Spain, the number of 10 × 10 UTM squares monitored for national atlases where little bustards are strictly resident is a minority compared to those where they present a different movement status (García de la Morena 2015). These localities offer little bustards adequate habitat and resources throughout the year, and they either possess particularly benign climate conditions (e.g. thermo-Mediterranean areas with oceanic influence) or contain cultivated habitats exploitable by birds during both winter and summer, like irrigated alfalfa fields. In the study of García de la Morena et al. (2015), birds that never moved at a rate faster than 8 km/day were always considered to remain within the same area (Shimazaki et al. 2004) and thus classified as sedentary. These birds represented 11% of the total sample studied (N = 8). Nevertheless, it is important to highlight that, in all populations where sedentary individuals were tagged, at least some of them left the area in summer, thus undertaking dispersive summer migration (García de la Morena et al. 2015).
The little bustard is considered sedentary also in Sardinia and Mediterranean France (Snow and Perrins 1998). In Sardinia, published information about movements is completely lacking. In southern France, nearly 100 birds were tagged (VHF and GPS tags) over the course of two PhD studies (Wolff 2001; Devoucoux 2014), showing that patterns described for Spanish birds seem to hold also in this region. Post-breeding movements have been found for birds fitted with GPS in Costière de Nîmes (Devoucoux et al., unpubl.), as well as regular migration between Costières and La Crau (in both directions). However, the majority of birds are sedentary, staying in wintering locations within an 8 km radius. This non-migratory status has also been assumed for the virtually extinct populations of continental Italy and northern Africa, as well as for those relict ones in Turkey and the Middle East, including Iran (Snow and Perrins 1998). However, the latter are geographical and climatically heterogeneous regions, and it is possible that radio-tracking studies there might have eventually revealed a diversity of movement patterns similar to that found in Iberia.
Historical Hypotheses on the Evolution of Current Diversity of Little Bustard Migration Patterns
Little bustards seem well adapted to migration. Like all members of the Otididae family, they are suited to maintaining highly energy-demanding flapping flights thanks to their large heart relative to body size, well above the general allometric relationship described for birds (Bishop 1997). In addition, existing evidence from a translocation experiment, in which birds hatched from eggs collected in resident Spanish populations and released in post-breeding flocks of French migratory populations retained sedentary behaviour, indicating that migration is, at least partially, genetically controlled in the little bustard (Villers et al. 2010), and thus subject to natural selection. Therefore, the diversity of seasonal movement patterns described above for the little bustard, a species that occupies a vast range in the Palearctic and thus has faced different selection pressure regimes during its history, should not be a surprise.
However, the particular environmental and evolutionary changes through which such variation in movement patterns has developed remain almost entirely unknown, and explanatory hypotheses are largely speculative. In a phylogeographic study, García et al. (2011) used mitochondrial DNA markers to identify two historical periods of population expansion and genetic diversification in the little bustard: the cold periods just before and after the Last Glacial interstadial (127–111 Ky BP, see chapter “The little bustard and its family: an overview of relationships”). During such cold stadials, and throughout their Eurasian distribution range, little bustards must have left their northernmost breeding grounds to winter as far south as possible, where they probably joined local birds. When the climate warmed during the interglacial periods, forests expanded northwards, and steppe habitats must have been confined in certain continental climate areas of eastern Europe and Central Asia (e.g. Sümegi et al. 2013), as well as in some Mediterranean enclaves where saline or gypsum-rich soils, drought and continentality had preserved open vegetation habitats (e.g. saline basins in central Iberia; Suárez et al. 1992; Sainz-Ollero 2013). Such areas likely functioned as interglacial refugia for steppe-dwelling species, promoting the genetic isolation of populations (Hewitt 2001; García et al. 2011). During these phases, little bustard populations outside the Mediterranean Region (i.e. eastern Europe and Central Asia), which faced much harder winters, likely became totally migratory, using Mediterranean steppes as wintering grounds. Conversely, sedentary behaviour spread in Mediterranean populations. With the return of glacial conditions, all populations expanded and came into contact again. Such a scenario is consistent with the relatively higher haplotype diversity detected by García et al. (2011) in areas intermediate between central France, where little bustards are totally migratory, and southern Iberia, where sedentary birds are dominant. These areas correspond to populations breeding in the northern fringe of Iberia, from Galicia to Catalonia, where migration patterns are totally or partially migratory (see above). Indeed, García et al. (2011) infer that a genetic admixture occurred in those intermediate areas when birds from different interglacial refugia came into contact. This hypothetical past scenario, which is depicted in Fig. 4, could explain the diversity of movement strategies observed today in the western extreme of the species’ range, although the role of present-day declines and recent extinctions cannot be ruled out in explaining the genetic impoverishment of some populations (e.g. central France, García et al. 2011). Moreover, the scenario must be completed with genetic data from central Europe, Italy, and northern Africa.
Depiction of the hypothetical scenario explaining the evolution of migratory and sedentary little bustard populations in Europe during the Quaternary as suggested by García et al. (2011). Light blue area: regions covered by ice at the last Glacial Maximum (20,000–18,000 y BP). Pointed line: southern limit of permafrost. Solid line: limit of forested areas in Mediterranean Glacial refugia. The area between pointed and solid lines: open vegetation and steppes. Dashed circles: interstadial refugia (R1: Iberian refugium, R2: inferred north-eastern refugium). Dashed ellipsoid: genetic admixture area. Ice sheet, permafrost, and vegetation type covers are based on Tarbelet et al. (1998), and García-Antón et al. (2002) for the particular case of Iberia. See text for details
Post-Breeding Ecology
Although studies on flocking behaviour, space use and habitat needs of the little bustard out of the breeding season are still scarce, an important research effort has been made in recent years to improve knowledge of these topics and contribute to a more complete conservation strategy that integrates the problems faced by the species over the entire annual cycle. In what follows, we synthesize the main results and conclusions of that research, which concern aspects ranging from home range size variation and habitat selection at different scales to interspecific interactions and human-induced disturbance. It is important to keep in mind, however, that such information comes primarily from populations of the species’ western range. As in many other aspects of their biology, the post-breeding ecology of eastern little bustards remains largely unknown.
Flocking Behaviour and Space Use
Outside the breeding season, little bustards are gregarious birds, gathering in flocks of variable size that include males and females, as well as juvenile individuals. Flocking starts in early summer (later as latitude increases) when males abandon their lekking sites to associate with one another and with non-breeding individuals such as first-year males and unsuccessful females. These birds may also completely leave their breeding grounds and migrate to their post-breeding summer areas or directly to their winter quarters (see Sect. “Other migration patterns”). At this time, most breeding females are still rearing their chicks in isolation (Tarjuelo et al. 2013), but as chicks grow and their size approaches that of adult females, families also gather in larger groups that may eventually be joined by adult males and non-breeding individuals (Cramp and Simmons 1980, own unpubl. data). These post-breeding summer flocks are usually small, ranging from a few birds to several tens, although they can eventually be much larger. In migratory populations, basically, all local individuals congregate in summer flocks before departing for the wintering grounds. For example groups of up to 200 individuals are regularly observed in Poitou-Charente, central-western France (V. Bretagnolle and A. Villers, unpublished data). In the 1950s and 1960s, such flocks frequently peaked at thousands of birds (Boutin and Métais 1995).
In wintering quarters that receive migrants from different breeding populations, the size of flocks and their level of aggregation increase as autumn progresses. For example in a Spanish wintering area of the mid-Tagus valley (see below), flocks attained their maximum size (200–300 individuals) in early December, when the number of existing flocks oscillated between one and two (own unpubl. data). Between January and February, these compact groups tended to split into smaller ones, while the total number of wintering birds decreased in March when little bustards started to return to breeding areas (own unpubl. data). Flock size also varied daily (own unpubl. data). The smallest flocks were observed early in the morning (ca. 100 individuals) when birds were still on the roosting sites, but their size increased steadily over the day to reach a maximum during the evening foraging and roosting periods (over 150 individuals). This circadian increase in flock size may be related to anti-predator strategies like enhanced vigilance or dilution of predation risk at roosts. In fact, the smaller morning flock size suggests group fragmentation overnight, perhaps caused by the attacks of nocturnal predators like red foxes (Vulpes vulpes). More detailed studies are needed to understand the origin of circadian variation in flock size.
These flocks tended to routinely commute between roosts and foraging sites (Wolff 2001, own unpubl. data). The former were usually ploughs or stubbles (irrigated cereals, maize) where birds probably seek high visibility to detect potential terrestrial predators, while foraging areas were basically located in large pivot-irrigated alfalfa fields, a preferred habitat where birds find both food and cover (see section “Overview”). In La Crau (southern France), roosts are often located in highly grazed grasslands, while foraging areas are often in rapeseed or mixed cereal and alfalfa crops. The size of these routine core areas (defined using 75 and 50% kernel area estimators) was much smaller than the total range of flock movements, measured as maximum convex polygons (MCP). For example over the course of the 2003–2004 wintering season, the mean size of core areas used by flocks in the mid-Tagus valley was estimated as 91 and 366 ha for 75 and 50% kernels, respectively, while the mean MCP size was 540 ha (own unpubl. data). The larger size of MCPs than estimated core areas usually reflects displacements caused by direct disturbance (generally human-induced) or changes in habitat availability (e.g. ploughing of preferred fields). Overall, the range of flock movements decreased from autumn (October–December) to winter (January–March) as the availability of alfalfa also decreased in the area (due to ploughing), although core areas notably increased in March (own unpubl. data), presumably associated with the start of pre-breeding migration. The fact that little bustard winter movements are mainly confined to a relatively small core range, even when the preferred habitats are largely available, suggests an influence of the species’ social dynamics (conspecific attraction, site fidelity) on its habitat and space use. In this line of evidence, Cuscó et al. (2018) found that individual space use of GPS satellite-tracked female little bustards in the irrigated wintering areas of the Ebro valley was markedly constrained by spatial variables related to social and historical factors, which explained 47% of the variance in the probability of individual female occurrence.
Post-Breeding Habitat Requirements
Landscape Scale Preferences
Little bustards breeding in agricultural landscapes in western Europe continue to be linked mainly to such habitat after the breeding season. For example Suárez-Seoane et al. (2008) used landscape scale variables to spatially model and compare the potential breeding and winter distributions of little bustard males in central Spain and found high levels of niche overlap and habitat connectedness between seasons. Moreover, breeding habitat was a good predictor of winter distribution, although the converse relationship was weaker. This means that the preference of breeding birds for diversified agricultural landscapes (see chapter “Habitat selection and space use”) generally holds also for the rest of the year. This has clear conservation implications, suggesting that preserving breeding sites closer to wintering grounds would allow the protection of a larger share of the total range. However, the species’ habitat niche seems to present fewer constraints during the post-breeding period, thus increasing its width (Suárez-Seoane et al. 2008), which may reflect the absence of the more compelling habitat requirements related to reproduction and/or the need for birds to use a wider range of habitats to satisfy their food demands. Indeed, according to the study in question, breeding distribution was predicted only by habitat variables, while winter distribution was determined more by climate variables. Therefore, little bustard winter distribution in central Spain was determined not only by landscape composition variables such as the cover of dry arable crops and long-term fallows but also by mean rainfall and net radiation (Suárez-Seoane et al. 2008).
Non-breeding habitat must basically fulfil two key requirements with impact on survival: the need for food to overcome the adverse conditions associated with summer drought (in Mediterranean regions), winter cold, or both, and security from predators and other sources of disturbance (caused by humans, including poaching). For example little bustards may be more tolerant of the proximity of human infrastructures outside the breeding season than in spring (García de la Morena et al. 2007; Silva et al. 2007), if it implies better feeding or security conditions. They may also be less dependent on diverse landscapes with complementary habitats if a single habitat providing both food and security against predators is largely available. One such habitat may be stubbles (cereals, rapeseed, legumes) from late summer up to ploughing time (Silva et al. 2004, 2007; Faria and Silva 2010), in which birds can find sprouted weeds and crop plants (Bravo et al. 2017), along with shelter provided by standing straws (Martín et al. 2010; García de la Morena 2015). Another is fallows of various ages: Bravo et al. (2017) found that in wintering areas with a significant fallow cover, little bustards consumed a remarkable diversity of wild plants, particularly Cruciferae, Compositae, Leguminosae, and Papaveraceae, although cultivated legumes were also consumed if present. A third relevant habitat for wintering little bustards is alfalfa, particularly in irrigated landscapes (Cuscó et al. 2018, own unpubl. data), which provides anti-predator cover (García de la Morena 2015) as well as abundant protein-rich food in the form of leaves and shoots. It is worth noting here that, although alfalfa forms the bulk of the species’ diet in irrigated wintering sites, wild plants like Cruciferae still have a significant role, accounting for around 25% of diet composition (Bravo et al. 2017).
Security from predators and human disturbance can also be provided by the topographic features of some wintering areas, where birds can benefit from the use of gentle hilltops for surveillance if they also offer an appropriate vegetation structure, as shown by Silva et al. (2004) in Alentejo. In synthesis, post-breeding areas must guarantee food resources and protection from disturbance and predation to support the little bustard, although whether they are fully suitable or not often depends on smaller-scale factors as already pointed out and described below.
Microhabitat Requirements
Little bustards must balance food availability with anti-predator safety when selecting suitable habitats for overwintering. The study by Silva et al. (2004) on winter habitat selection showed that wintering little bustards select food-rich habitats offering intermediate vegetation height that allows both visual surveillance and concealment. In the extensive dry farmland of Alentejo, such microhabitat structure is provided by grazed fallows and stubbles. However, in other post-breeding and wintering areas, other agricultural substrates can play that role. Such is the case of irrigated alfalfa fields in the mid-Tagus valley (central Spain) and Catalonia (García de la Morena 2015; Cuscó et al. 2018). Therefore, wintering little bustards select certain features of vegetation structure rather than habitat typologies, as has also been shown for the breeding season (see chapter “Habitat selection and space use”). For example García de la Morena (2015) shows that in the food-rich irrigated alfalfa fields of the Tagus valley, little bustards prioritize anti-predator cover, selecting higher and denser vegetation than the mean, as well as seeking good visibility by maximizing their distance to topographic horizons. Similar microhabitat features were selected in fallow fields of the dry farmland of the Spanish Southern Plateau, where sprouted weeds also provided abundant food for wintering birds (García de la Morena 2015). Therefore, very similar microhabitat selection patterns were found in two wintering sites dominated by quite different habitats and agricultural systems.
The little bustard in Iberia often shares wintering habitat with the smaller-sized pin-tailed sandgrouse (Pterocles alchata), frequently forming mixed flocks (Cramp and Simmons 1980). The studies by Martín et al. (2010) and García de la Morena (2015) have shown that little bustard microhabitat selection patterns do not differ between single-species and mixed-species flocks, while sandgrouse associated with bustards can access food resources in habitats they would otherwise avoid. For example, single-species sandgrouse flocks were very rarely seen in stubbles or alfalfa fields because vegetation there is too high to allow them anti-predator surveillance. However, mixed-species flocks were always found in those habitats, so that pin-tailed sandgrouse could exploit their abundant food resources while benefiting from little bustard surveillance effort and greater detection ability due to its larger size. This pattern was found in both dry cereal farmland and irrigated alfalfa fields (García de la Morena 2015). In this context, the little bustard would behave as the leader species (Sridhar et al. 2009), whereas the sandgrouse would be a follower species (García de la Morena 2015). Therefore, while this interspecific interaction is beneficial for the pin-tailed sandgrouse, it is rather neutral for the little bustard and could be considered a form of commensalism (see chapter “Interspecific relationships”). Nevertheless, certain advantages could also be expected for bustards, such as predator confusion or predation risk dilution due to enlarged group size (Terborgh 1990; Quinn and Cresswell 2005). Further research would be required to assess these possibilities.
Little Bustard Conservation in Winter
In this final section of the chapter, we review the main threats and conservation problems faced by the little bustard outside the breeding season, which can be grouped in two categories: direct human disturbance and landscape modification. The threats posed by these processes on little bustards are direct consequences of the species’ winter behaviour and ecology described in the preceding sections and their interaction with humans and human land management. Therefore, we believe the reader will appreciate this overview of winter conservation issues after considering the species’ post-breeding ecology, given the specific threats faced by the species during winter (more general threats and those specific of breeding populations are addressed in chapter “Threats affecting little bustards: human impacts”).
Direct Human Disturbance and Shooting
While post-breeding little bustards may be able to minimize predation risk by raptors and terrestrial enemies through the landscape scale and microhabitat selection behaviour described above, they might still have to deal with other sources of disturbance, such as those associated with human activity in an increasingly humanized countryside. Human activities in farmland areas in autumn and winter are diverse, ranging from different agricultural labours (ploughing, agrochemical application, sowing, mowing) to leisure activities (walking with or without dogs, running, biking), including, of course, various forms of hunting. Humans engaged in these and other activities can be perceived as predators by animals, which often modify their behaviour accordingly.
Hunting is a major source of disturbance for wintering birds, both game and non-target species (Madsen and Fox 1995), although very few studies have paid attention to the effect of hunting on protected species. In one such study, Casas et al. (2009) showed that small game hunting altered the spatial distribution and behaviour of little bustards gathered in pre-migratory flocks in cereal farmland of central-western France. These authors compared flock activity on hunting days with that of days before and after hunting and found that flights occurred only on hunting days. Little bustards also increased the time devoted to vigilance on hunting days relative to days before and after and decreased the time spent resting. However, foraging time was not directly affected by hunting activity but varied with flock size, increasing with the number of individuals but decreasing in the largest flocks, presumably due to interference among birds (Sansom et al. 2008). Overall, these changes represent important alterations of the process of energy storage to prepare for their migration to Iberia, and even the lack of a negative response in foraging time could be partly due to a compensation effect to restore the energy lost in flights (Blanc et al. 2006). Also, as a consequence of hunting, little bustards concentrate in hunting-free areas set up as reserves for the game. Although these areas can adequately work as refuges for a threatened non-game species like the little bustard, they might not be large enough to fulfil all the requirements of birds throughout this preparatory migration period (Casas et al. 2009).
Besides these behavioural alterations, Tarjuelo et al. (2015) proved the existence of a stress response to increased human presence during weekends in a little bustard wintering area in the Spanish Southern Plateau. Not only does hunting take place mainly on weekends in this study area, but also other leisure activities then occur, such as walking with dogs, biking, and different agricultural activities. Tarjuelo et al. (2015) quantified corticosterone metabolites in little bustard faeces collected before, during and after weekends as a measure of physiological stress response to disturbance. The short-term release of glucocorticoids allows the reallocation of energy to improve survival possibilities (Sapolsky et al. 2000), although their continued secretion due to prolonged exposure to stressors reduces reproductive capacity and immunological competence (Romero et al. 2009). Apart from the behavioural changes observed in other studies (i.e. Casas et al. 2009), Tarjuelo et al. (2015) found a significantly higher physiological stress response during weekends, with increased levels of faecal corticosterone metabolites associated with an increase in human activities related to hunting. Therefore, the effect of prolonged hunting disturbance (from October to February plus the weeks of ‘media veda’ or half-season in countries like Spain, from mid-August to mid-September) on little bustards at wintering sites might adversely affect these populations (Tarjuelo et al. 2015), which still must face their return to breeding areas and the subsequent effort of reproduction.
Beyond behavioural and physiological stress responses, direct mortality caused by illegal hunting or poaching has been proved to be a major cause of human-induced mortality in the little bustard. Based on 139 radio, GPS or satellite-tracked little bustards, Marcelino et al. (2017) found that 32% of all known mortality records were due to poaching. Although those authors could not analyse mortality rates seasonally, it is reasonable to assume that most of that mortality occurs in the non-breeding season (summer, autumn, and winter), during hunting periods.
The impact of hunting during wintering and migration (legal and illegal), although not quantified, also seems to be very important in the eastern sub-range of the species. Iñigo and Barov (2010) list poaching on wintering grounds among the main global threats for the little bustard, with an estimated 30,000 migrant little bustards illegally hunted during migration, mainly in Azerbaijan. The killing of migrant birds has also been recorded in Lebanon in recent years (Ramadan-Jaradi et al. 2017), indicating high winter hunting pressure also in the Middle East (Collar et al. 2017). Such high hunting pressure is also reported for the above-mentioned wintering sites in Central Asia, where little bustards have traditionally been hunted by local people using various means of capture (E.A. Kreuzberg, A. Ten, V. Soldatov and R.S. Muratov, pers. comm.). In Iran, hunting pressure seems to have shaped the species’ winter distribution since flocks are mainly found within borderland areas surveyed by the military and thus free of hunter presence (Yousefi et al. 2017). More recently, increasing use of falconry for hunting little bustards in Azerbaijan and other wintering areas has been detected (J. Burnside pers. comm.). The impact of this pressure on population trends has not been assessed, but it may help explain the mentioned decrease in wintering numbers in some wintering sites from Central Asia.
Landscape Transformations
Little bustard wintering areas in the species’ western sub-range have experienced significant land-use changes in the last two decades. In dry farmland areas, practices like early stubble ploughing or old fallow elimination clearly reduce their suitability as non-breeding habitat for little bustards (Silva et al. 2004; García de la Morena 2015). Nevertheless, the strongest transformations at the landscape level generally occur on irrigated farmland, where existing suitable crops may be totally replaced by alternative cultures depending on market-regulated demands. For example the pivot-irrigated alfalfa fields of the central Tagus valley in Spain, a traditional little bustard non-breeding site (from summer to late winter; García de la Morena 2015), have been largely replaced by other crops, such as irrigated olive groves, that are unsuitable as habitat for the species (Morales et al. 2015). In addition, sprinklers, which are clearly avoided by birds (pers. obs.), have replaced pivots in most remaining alfalfa fields. As a result, the number of birds wintering in the area has decreased from around 1000 in the late 1990s (García de la Morena et al. 2007) to ca. 50 in 2014 (own unpubl. data). Similar changes could potentially take place in other irrigated wintering sites, like those of the Ebro valley in Catalonia, where nearly the entire Catalonian population concentrates after breeding (Mañosa et al. 2015). It is, therefore, important that such non-breeding sites are shielded from the expansion of crops that are completely unsuitable for the little bustard such as fruit-tree orchards, nowadays increasing their cover in the Ebro valley.
It is also important to recall that irrigated landscapes involve high human influence and are prone to anthropogenic disturbances like those described above. In addition, irrigation facilities require high densities of electric powerlines, which increase the risk of bird collision (Silva et al. 2010), another significant cause of mortality in the little bustard (Marcelino et al. 2017), which is also a threat during migration (Voronova et al. 2012 in Collar et al. 2017). In any case, a significant proportion of the little bustard population in Western Europe summers and winters in irrigated farmland, where birds can spend up to 8 months of their year, so appropriate management of these areas is required, even if they are excluded from SPAs and other protection measures. In this context, the maintenance of preferred feeding habitats (i.e. alfalfa fields) with compatible irrigation systems (pivots, gravity irrigation), anti-poaching vigilance to keep disturbance to a minimum, creation of hunting reserves in areas used by bustards, and burying dangerous power lines represent desirable measures for these areas. Most of these recommendations also hold for non-breeding sites in dry farmland, where some of them are already included in current EU agri-environmental schemes (e.g. delayed stubble ploughing). Given the importance of leguminous plants in the diet of little bustards wintering in Iberia (Bravo et al. 2017), increasing the cover of dry leguminous crops (vetch, chickpea, or rain-fed alfalfa) would notably improve the quality of these landscapes as non-breeding habitat for the species.
Beyond Western Europe, very little is known about the fate of little bustard wintering areas in Azerbaijan, Iran, and other Asian regions (Table 1). However, given the large number of birds known to overwinter in some of them (see above and chapter “The little bustard around the world: distribution, global conservation status, threats and population trends”), as well as the recent agricultural changes (i.e. the return of intensive agriculture) reported in breeding grounds (Kamp et al. 2011), particular attention should be paid to these areas. Little bustard winter habitat in Azerbaijan is mainly grazed steppe, and relevant overgrazing problems favouring desertification have already been detected (Gauger 2007). The habitat occupied by the wintering flocks reported from Central Asia by E.A. Kreuzberg, A. Ten, V. Soldatov and R.S. Muratov (pers. comm.) is basically irrigated farmland (winter crops or cotton and cereal stubbles) on the banks and floodplains of main rivers, such as the Amu Darya in Uzbekistan, or the Javan and Sir Darya in Tajikistan. This habitat use clearly resembles that of western birds, which largely occupy irrigated crops in winter. As regards northern Iran, little bustards wintering there use mainly marsh steppe and rice stubbles (Sehhatisabet et al. 2012), although very little information about the potential threats associated with agricultural management in that region is available.
References
Alerstam T, Hedenström A, Åkesson S (2003) Long-distance migration: evolution and determinants. Oikos 103:247–260
Alonso JA, Martín CA, Morales MB, Alonso JC, Lane SJ (2001) Long distance seasonal movements of male Great Bustards Otis tarda in Central Spain. J Field Ornithol 72:504–508
Alonso JC, Morales MB, Alonso JA (2000) Partial migration, lek fidelity and nesting area fidelity in female great bustards Otis tarda. Condor 102:127–136
Bernis F (1966a) La migración en aves. Tratado teórico y práctico. Sociedad Española de Ornitología, Madrid
Bernis F (1966b) Aves migradoras ibéricas. Sociedad Española de Ornitología, Madrid
Berthold P (1993) Bird migration: a general survey. Oxford University Press, Oxford
Berthold P, Helbig AJ, Mohr G, Querner U (1992) Rapid microevolution of migratory behaviour in a wild bird species. Nature 360:668–670
Bishop CM (1997) Heart mass and the maximum cardiac output of birds and mammals: implications for estimating the maximum aerobic power input of flying animals. Philos Trans R Soc Lond B Biol Sci 352:447–456
Blanc R, Guillemain M, Mouronval J-B, Desmonts D, Fritz H (2006) Effects of non-consumptive leisure disturbances to wildlife. Rev Ecol Terre Vie 61:117–133
Boutin JM, Métais M (1995) L’outarde canepetière. Eveil Editeur, Saint-Yrieix
Bravo C, Cuscó F, Morales MB, Mañosa S (2017) Diet composition of a declining steppe bird, the Little bustard (Tetrax tetrax), in relation to farming practices. Avian Ecol Conserv 12(1):3. https://doi.org/10.5751/ACE-00938-120103
Casas F, Mougeot F, Viñuela J, Bretagnolle V (2009) Effects of hunting on the behaviour and spatial distribution of farmland birds: importance of hunting-free refuges in agricultural areas. Anim Conserv 12:346–354
Chapman BB, Brönmark C, Nilsson JA, Hansson LA (2011) The ecology and evolution of partial migration. Oikos 120:1764–1775
Collar NJ, Baral HS, Batbayar N, Bhardwaj GS, Brahma N, Burnside RJ, Choudhury AU, Combreau O, Dolman PM, Donald PF, Dutta S, Gadhavi D, Gore K, Goroshko OA, Hong C, Jathar GA, Jha RRS, Jhala YV, Koshkin MA, Lahkar BP, Liu G, Mahood SP, Morales MB, Narwade SS, Natsagdorj T, Nefedov AA, Silva JP, Thakuri JJ, Wang M, Zhang Y, Kessler AE (2017) Averting the extinction of bustards in Asia. Forktail 33:1–26
Combreau O, Riou S, Judas J, Lawrence M, Launay F (2011) Migratory pathways and connectivity in Asian houbara bustards: evidence from 15 years of satellite tracking. PLoS One 6:e20570
Cramp S, Simmons KEL (1980) Handbook of the birds of Europe the Middle East and North Africa: the birds of the Western Paleartic. In: Cramp S, Simmons KEL (eds) Hawks to Bustards. Oxford University Press, London
Cuscó F, Cardador L, Bota G, Morales MB, Mañosa S (2018) Interindividual consistency in habitat selection patterns and spatial range constraints of female little bustards during the non-breeding season. BMC Ecol 18:56
Delgado MP, Morales MB, García de la Morena EL (2010) Seguimiento de la invernada de sisones comunes franceses en España (2005–2009). Informe Final. Unpublished report. Sociedad Española de Ornitología, Madrid
Devoucoux P (2014) Conséquence et impacts prévisibles d’une perte d’habitat majeure sur une espèce menacée aux exigences écologiques complexes: dynamique de la population d’outardes canepetières des Costières de Nîmes et construction de la Ligne à Grand Vitesse CNM. Octubre 2014. PhD thesis, University of Poitiers, Poitiers
Faria N, Silva JP (2010) Habitat selection of the little bustard during the beginning of the agricultural year. Ardeola 57:363–373
García de la Morena EL (2015) Ecología y movimientos migratorios del sisón común (Tetrax tetrax) fuera del periodo reproductor. PhD Thesis, Autónoma University of Madrid, Madrid
García de la Morena EL, Morales MB, De Juana E, Suárez F (2007) Surveys of wintering little bustards (Tetrax tetrax) in central Spain: distribution and population estimates at regional scale. Bird Conserv Int 17:23–34
García de la Morena EL, Morales MB, Bota G, Silva JP, Ponjoan A, Suárez F, Mañosa S, De Juana E (2015) Migration patterns of Iberian Little Bustards Tetrax tetrax. Ardeola 62:95–112
García JT, Mañosa S, Morales MB, Ponjoan A, García de la Morena EL, Bota G, Bretagnolle V, Dávila JA (2011) Genetic consequences of interglacial isolation in a steppe bird. Mol Phylogenet Evol 61:671–676
García-Antón M, Maldonado-Ruiz J, Morla-Juaristi C, Sainz Ollero H (2002) Fitogeografía histórica de la península Ibérica. In: Pineda F, Casado MA, Montalvo J (eds) La Diversidad Bilógica de España. Pearson Educación, Madrid, pp 45–63
Gauger K (2007) Occurrence, ecology and conservation of wintering Little BustardsTetrax tetrax in Azerbaijan. Arch Nat Landschaftsforschun 46:5–22
Gavrilov EI, Gavrilov AE (2005) The birds of Kazakhstan. Tethys Special Bulletin, Almaty
Heiss M (2013) The importance of Besh Barmag bottleneck (Azerbaijan) for Eurasian migrant birds. Acta Ornithol 48:151–164
Hewitt GM (2001) Speciation, hybrid zones and phylogeography—or seeing genes in space and time. Mol Ecol 10:537–549
Iñigo A, Barov B (2010) Action plan for the Little Bustard Tetrax tetrax in the European Union. SEO-BirdLife and BirdLife International for the European Commission, Madrid
Kamp J, Urazaliev R, Donald PF, Hölzel N (2011) Post-Soviet agricultural change predicts future declines after recent recovery in Eurasian steppe bird populations. Biol Conserv 144:2607–2614
Kessler AE, Batbayar N, Natsagdorj T, Batsuur’ D, Smith AT (2013) Satellite telemetry reveals long-distance migration in the Asian great bustard Otis tarda dybowskii. J Avian Biol 44:311–320
Limiñana R, Soutullo A, López-López P, Urios V (2008) Pre-migratory movements of adult Montagu’s harriers Circus pygargus. Ardea 96:81–90
Madsen J, Fox AD (1995) Impacts of hunting disturbance on waterbirds: a review. Wildl Biol 1:193–207
Mañosa S, Bota G, Estrada J, Cuscó F (2015) Una oportunidad para el sisón en Cataluña. Quercus 356:24–35
Marcelino J, Moreira F, Mañosa S, Cuscó F, Morales MB, García de la Morena EL, Bota G, Palmeirim JM, Silva JP (2017) Tracking data of the Little Bustard Tetrax tetrax in Iberia shows high anthropogenic mortality. Bird Conserv Int. https://doi.org/10.1017/S095927091700051X
Martín CA, Casas F, Mougeot F, García JT, Viñuela J (2010) Positive interactions between vulnerable species in agrarian pseudo- steppes: hábitat use by pin-tailed sandgrouse depends on its association with the little bustard. Anim Conserv 13:383–389
Martin TE, Guerin R, Fages F, Martineau A, Hingrat Y (2018) Breeding populations of Great Bustard and Little Bustard in South Kazakhstan province, Republic of Kazakhstan. Sandgrouse 40:138–143
Morales MB, Alonso JC, Alonso JA, Martín E (2000) Migration patterns in male great bustards (Otis tarda). Auk 117:493–498
Morales MB, Suárez F, García de la Morena EL, De Juana E (2002) Movimientos estacionales y conservación de aves esteparias: el ejemplo del sisón. Quercus 193:493–498
Morales MB, Arroyo B, Traba J (2015) El declive del sisón en el centro de España. Quercus 356:36–43
Newton I (1998) Population limitations in birds. Academic Press, London
Newton I (2010) Bird migration. Collins New Naturalist Library, London
Otero C (1985) Techniques for the capture of Little Bustards during the Autumn/Winter season. Bustard Studies 2:171–172
Palacín C (2007) Comportamiento Migratorio de la Avutarda Común en la Península Ibérica. PhD Thesis Complutense University of Madrid, Madrid
Palacín C, Alonso JC (2009) Probable population decline of the Little Bustard Tetrax tetrax in North-West Africa. Ostrich 80:165–170
Phillips-Wolley C (1881) Sport in the Crimea and Caucasus. Bentley and son, London
Pulido F (2007) The genetics and evolution of avian migration. Bioscience 57:165–174
Pulido F, Berthold P (2010) Current selection for lower migratory activity will drive the evolution of residency in a migratory bird population. Proc Natl Acad Sci U S A 107:7341–7346
Quinn JL, Cresswell W (2005) Escape response delays in wintering redshank, Tringa totanus, flocks: perceptual limits and economic decisions. Anim Behav 69:1285–1292
Ramadan-Jaradi G, Itani F, Serhal A (2017) Interesting bird records from Lebanon. Sandgrouse 39:187–192
Romero LM, Dickens MJ, Cyr NE (2009) The Reactive Scope Model: a new model integrating homeostasis, allostasis, and stress. Horm Behav 55:375–389
Sainz-Ollero H (2013) Steppes across the world. An overview with emphasis on the Iberian Peninsula. In: Morales MB, Traba J (eds) Steppe ecosystems. Biological diversity, management and restoration. NOVA Publishers, New York, pp 1–25
Sampietro J, Rivas JL, Sanz J, Albero JC, Pelayo E, Gajón A, García de la Morena E (2013) Desplazamientos a larga distancia de machos de sisón común (Tetrax tetrax) reproductores en Aragón. Rocín Anuario Ornitol Aragón 8:35–52
Sansom A, Cresswell W, Minderman J, Lind J (2008) Vigilance benefits and competition costs in groups: do individual redshanks gain an overall foraging benefit? Anim Behav 75:1869–1875
Sapolsky RM, Romero LM, Munck AU (2000) How do glucocorticoids influence stress responses? Integrating permissive, suppressive, stimulatory, and preparative actions. Endocr Rev 21:55–89
Sardà-Palomera F, Puigcerver M, Brotons L, Rodríguez-Teijeiro JD (2012) Modelling seasonal changes in the distribution of common quail Coturnix coturnix in farmland landscapes using remote sensing. Ibis 154:703–713
Sehhatisabet ME, Abdi F, Ashoori A, Khaleghizadeh A, Khani A, Rabiei K, Shakiba M (2012) Preliminary assessment of distribution and population size of wintering Little Bustards Tetrax tetrax in Iran. Bird Conserv Int 22(279):28
Sherry TW, Holmes RT (1996) Winter habitat quality, population limitation and conservation of Neotropical-Neartic migrant birds. Ecology 77:36–48
Shimazaki H, Tamura M, Darman Y, Andronov V, Parilov MP, Nagendran M, Higuchi H (2004) Network analysis of potential migration routes for oriental white storks (Ciconia boyciana). Ecol Res 19:683–698
Shlyakhtin GV, Tabachishin VG, Khrustov AV, Zav’yalov EV (2004) Ecological segregation of bustards (Otididae) in the north of the lower Volga region: evolutionary and adaptive aspects. Russ J Ecol 35:247–253
Silva JP, Pinto P (2006) Final report of action 2 of the project LIFE02NAT/P/8476: conservation of the little bustard in Alentejo. Institute for Nature Conservation. Available via http://portal.icnb.pt/NR/rdonlyres/783CA43B-7C0E-4FA4-8286-F4EB120C4A49/0/Life_SISAO.pdf. Accessed 17 Jan 2011
Silva JP, Pinto M, Palmeirim JM (2004) Managing landscapes for the little bustard Tetrax tetrax. Lessons from the study of winter habitat selection. Biol Conserv 117:521–528
Silva JP, Faria N, Catry T (2007) Summer habitat selection and abundance of the threatened little bustard in Iberian agricultural landscapes. Biol Conserv 139:186–194
Silva JP, Santos M, Queirós L, Leitão D, Moreira F, Pinto M, Leqoc M, Cabral JA (2010) Estimating the influence of overhead transmission power lines and landscape context on the density of little bustard Tetrax tetrax breeding populations. Ecol Model 221:1954–1963
Sinclair ARE (1983) The function of distance movements in vertebrates. In: Swingland IR, Greenwood PR (eds) The ecology of animal movement. Clarendon Press, Oxford, pp 240–258
Snow DW, Perrins CM (1998) The birds of the Western Palearctic (concise edition) vol 1. Non-Passerines. Oxford University Press, Oxford
Sridhar H, Beauchamp G, Shanker K (2009) Why do birds participate in mixed-species foraging flocks? A large-scale synthesis. Anim Behav 78:337–347
Suárez F (2004) Aves y agricultura en España peninsular. Una revisión sobre el estado actual de conocimiento y una previsión de futuro. In: Tellería JL (ed) La ornitología hoy. Homenaje al profesor Francisco Bernis Madrazo. Editorial Complutense, Madrid, pp 223–265
Suárez F, Sainz H, Santos T, González Bernáldez F (1992) Las estepas ibéricas. MOPT, Madrid
Suárez-Seoane S, García de la Morena EL, Morales MB, Osborne PE, De Juana E (2008) Maximum entropy niche-based modelling of seasonal changes in little bustard (Tetrax tetrax) distribution. Ecol Model 219:17–29
Sümegi P, Szilágyi G, Gulyás S, Jakab G, Molnár A (2013) The late quaternary paleoecology and environmental history of the Hortobágy, an unique mosaic alkaline steppe from the heart of the Carpathian basin, Central Europe. In: Morales MB, Traba J (eds) Steppe ecosystems. Biological diversity, management and restoration. NOVA Publishers, New York, pp 165–193
Tarbelet P, Fumagalli L, Wust-Saucy AG, Cosson JF (1998) Comparative phylogeography and postglacial colonization routes in Europe. Mol Ecol 7:453–464
Tarjuelo R, Delgado MP, Bota G, Morales MB, Traba J, Ponjoan A, Hervás I, Mañosa S (2013) Not only habitat but also sex: factors affecting spatial distribution of Little Bustard Tetrax tetrax families. Acta Ornithol 48:119–128
Tarjuelo R, Barja I, Morales MB, Traba J, Benítez-López A, Casas F, Arroyo B, Delgado MP, Mougeot F (2015) Effects of human activity on physiological and behavioral responses of an endangered steppe bird. Behav Ecol 26:828–838
Terborgh J (1990) Mixed flocks and polyspecific associations: costs and benefits of mixed groups to birds and monkeys. Am J Primatol 21:87–100
Terraube J, Mougeot F, Cornulier T, Verma A, Gavrilov A, Arroyo B (2012) Broad wintering range and intercontinental migratory divide within a core population of the near-threatened pallid harrier. Divers Distrib 18:401–409
Terrill SB, Able KP (1988) Bird migration terminology. Auk 105:205–206
Vickery JA, Ewing SR, Smith KW, Pain DJ, Bairlein F, Skorpilov J, Gregory RD (2014) The decline of Afro-Palaearctic migrants and an assessment of potential causes. Ibis 156:1–22
Villers A, Millon A, Jiguet F, Lett JM, Attie C, Morales MB, Bretagnolle V (2010) Migration of wild and captive-bred Little Bustards Tetrax tetrax: releasing birds from Spain threatens attempts to conserve declining French populations. Ibis 152:254–261
Wolff A (2001) Changements agricoles et conservation de la grand avifaune de plaine: Etude des rélations espèce-habitats à differentes échelles chez l’Outarde canepetière. Dissertation. Université Montpellier II. Montpellier
Yousefi M, Kafash A, Malakoutikhah S, Ashoori A, Khani A, Mehdizade Y, Ataei F, Ilanloo SS, Rezaie HR, Silva JP (2017) Distance to international border shapes the distribution pattern of the growing Little Bustard Tetrax tetrax winter population in northern Iran. Bird Conserv Int. https://doi.org/10.1017/S0959270917000181
Acknowledgements
Elena A. Kreuzberg, Anna Ten and Valentin Soldatov kindly provided recent and historical records of migrating and wintering little bustards in Uzbekistan and Kazakhstan. Dr. R. Sh. Muratov, from the Institute of Zoology and Parasitology of the Academy of Sciences of the Republic of Tajikistan, also kindly provided records from that country. We are thankful to all of them for this valuable information that otherwise would have remained out of our reach. Recent records provided by A. Ten and V. Soldatov were obtained with the help of the following additional observers: P Lampila and T. Eskelin. José Luis Tellería and Yves Hingrat kindly revised and commented on a previous version of this chapter. Finally, we are in debt with Nigel J. Collar, who thoroughly revised the English.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2022 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Morales, M.B., Mañosa, S., Villers, A., García de la Morena, E.L., Bretagnolle, V. (2022). Migration, Movements, and Non-breeding Ecology. In: Bretagnolle, V., Traba, J., Morales, M.B. (eds) Little Bustard: Ecology and Conservation. Wildlife Research Monographs, vol 5. Springer, Cham. https://doi.org/10.1007/978-3-030-84902-3_7
Download citation
DOI: https://doi.org/10.1007/978-3-030-84902-3_7
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-84901-6
Online ISBN: 978-3-030-84902-3
eBook Packages: Earth and Environmental ScienceEarth and Environmental Science (R0)