Abstract
We described the relationship between relatedness as full or maternal half siblings and expression of social play and other social behaviors in juvenile Belding’s ground squirrel (Urocitellus beldingi) litters and evaluated the possible role of play in establishing social bonds between juvenile females. We used microsatellite analysis to determine relatedness. Juvenile females did not interact preferentially with full over half siblings, suggesting that they may form bonds equally with full and half sisters. The probability that females will have a surviving full sister beyond the juvenile period may be low in U. beldingi, and establishing a cooperative relationship with a half sister may sometimes be the best available option in adulthood. As the proportion of females within litters increased, rates of play decreased, suggesting that low social play may be adequate for social bonding among females. Among juvenile male U. beldingi, play bouts lasted longer between full than half brothers; however, juvenile males did not interact preferentially with full brothers in play or other social interactions. Body mass differences were smaller between full than half brothers, and in both full and half brother pairings, play bouts lasted longest when body mass differences were small. Because male U. beldingi do not ordinarily interact with littermate siblings after emigrating from the natal area, it is unlikely that play behavior functions to establish long-term social bonds between full brothers. Rather, young males may favor play interactions with phenotypically similar partners who can provide optimal challenges in interactions that promote motor development.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Play is a characteristic behavior of mammals, occurring in young of nearly all mammalian species. Play behavior can be categorized as social, involving interaction with other individuals, or nonsocial, typically involving locomotor activity or interaction with objects (Fagen 1981; Burghardt 2005). Several hypotheses have been proposed suggesting adaptive functions of play behavior in young animals, including promotion of motor, cognitive, and social development, and development of versatility in coping with unfamiliar or unpredictable situations (Bekoff 1972, 1988; Poirier and Smith 1974; Caro 1988; Bekoff and Byers 1998; Lewis 2000; Špinka et al. 2001; Pellis et al. 2010). The expression of play behavior varies widely across mammalian species, and comparative studies indicate that specific functions of play also differ among species, including those that are closely related (Bekoff and Byers 1998; Burghardt 2005; Pellis and Pellis 2009). Functions of play behavior can include short- or long-term benefits to individuals (Held and Špinka 2011; Blumstein et al. 2013). Moreover, the forms of play behavior in which young animals engage can change as they progress through development (Gomendio 1988; Govindarajulu et al. 1993), and the mechanics and functions of play can be sexually dimorphic within species (Pellis et al. 1997; Paukner and Suomi 2008). The overall importance of play behavior for young animals during early development is suggested by its association with enhanced survival of young in some species (Fagen and Fagen 2004, 2009; Cameron et al. 2008).
A variety of adaptive explanations relating social play to social development have been proposed. For example, in common seals (Phoca vitulina), play behavior introduces young seals to unfamiliar individuals and helps integrate them into their social group (Wilson 1974). In gelada baboons (Theropithecus gelada), social play fosters relationships in which individuals support each other in agonistic interactions with other baboons (Mancini and Palagi 2009). In yellow-bellied marmots (Marmota flaviventris), social play helps young animals develop dominance relationships that persist into adulthood (Blumstein et al. 2013). In dogs (Canis lupus familiaris), play may contribute to social bonding, as preferences for specific play partners develop early and become more pronounced over time (Ward et al. 2008). In this work, we assessed social play and other social behaviors in juvenile Belding’s ground squirrels (Urocitellus beldingi; formerly Spermophilus beldingi, Helgen et al. 2009) and evaluated the possible role of social play behavior in promoting bonding between sisters.
Formation of social bonds with female relatives is important in many female mammals (Sterck et al. 1997; Wolff and Sherman 2007). Female philopatry is prevalent in mammals, with females typically remaining in their natal areas or natal groups throughout their lives and males emigrating to a new home area or social group prior to reproducing (Greenwood 1980; Lawson Hadley and Perrin 2007). Female philopatry typically results in clustering of related females. Cooperative interactions among females in kin clusters can be favored by kin selection, with contributions to indirect fitness increasing as the relatedness between cooperating individuals increases (Sherman 1977; Nunes 2007; Clutton-Brock and Lukas 2012). For example, sisters or mothers and daughters can share portions of their territories and cooperate in evicting intruders from the territories (Sherman 1977). Sisters and mothers and daughters can also help each other establish and maintain rank in social hierarchies (Holekamp and Smale 1991), and closely related females can cooperate in the care and rearing of young (Jennions and Macdonald 1994; König 1997; Solomon and French 1997).
Multiple paternity has been observed in U. beldingi, and juveniles typically have options for interacting with either full or half siblings (Hanken and Sherman 1981). Multiple paternity has also been observed in a wide range of litter- and twin-bearing mammals, including those characterized by social monogamy (e.g., Hanken and Sherman 1981; Goossens et al. 1998; Baker et al. 1999; Say et al. 1999; DeYoung et al. 2002; Kraaijeveld-Smit et al. 2002; Carling et al. 2003; Haynie et al. 2003; Glen et al. 2009; Sale et al. 2013). Various ideas have been proposed to explain the adaptive benefit multiple paternity. For example, greater phenotypic diversity among offspring might increase the likelihood that at least some offspring will be able to resist disease or successfully cope with environmental challenges or changes (Lacy 1997; Yasui 1998). Greater representation of males as fathers within litters might also decrease the probability of infanticide by males (Coulon et al. 1995). Multiple paternity may also serve as “bet hedging” to reduce inbreeding when females have unreliable access to outbred males as potential mates (Yasui 2001; Waser and De Woody 2006). Although multiple paternity increases genetic diversity within litters, it also reduces relatedness among littermates, thus potentially reducing opportunities for social interactions most strongly favored by kin selection.
In this work, we described the relationship between sibling relatedness within U. beldingi litters and the expression of social play and other social behaviors and evaluated the hypothesis that social play promotes social bonding between sisters. We defined social bonding in the context of our study as the establishment or reinforcement of preferences for partners in nonagonistic social interactions and focused on preferences for classes of partners (e.g., full sibling vs maternal half sibling) rather than preferences for individual partners. Play behavior in U. beldingi has been shown to promote motor development and improve motor coordination; however, whether play has a role in establishing social bonds between individuals is not known (Nunes et al. 2004a, b). Female U. beldingi are philopatric and act cooperatively with close female relatives as adults in mutual defense of territories and alarm calling to alert each other to potential danger (Sherman 1977). By contrast, all surviving male U. beldingi emigrate from the natal area before mating and do not ordinarily interact with close relatives in adulthood (Holekamp 1984, 1986). Thus, it may be especially important for young females to establish social bonds with sisters early in life in support of cooperative interactions later in life.
Cooperation among kin requires the ability to recognize related individuals, and robust kin recognition abilities have been observed in U. beldingi (Sherman 1980; Holmes and Sherman 1982; Holmes 1994; Mateo and Johnston 2000; Mateo 2003, 2010). In U. beldingi, littermate full sisters engage in fewer aggressive and more cooperative interactions as yearlings than do maternal half sisters raised in the same litter, suggesting that kinship importantly influences social relationships in female U. beldingi (Holmes and Sherman 1982). Unrelated U. beldingi raised in the same litter prior to weaning are less aggressive toward each other than are unrelated juveniles raised apart, suggesting that social rearing environment also influences social relationships between young squirrels (Holmes and Sherman 1982). Kin recognition in U. beldingi is mediated by self-referent phenotype matching involving odors, using one’s own cues to determine the relatedness of conspecifics (Mateo 2010). Phenotype matching has also been observed in other species such as spotted hyenas (Crocuta crocuta), and young hyenas express preferences in social interactions for full over half sibling littermates (Wahaj et al. 2004).
We predicted that if play promotes social bonding among juvenile female U. beldingi, then rates of play and other affinitive interactions should be highest in litters in which the proportion of females in the litter and the degree of relatedness among female littermates are highest. Moreover, females should play and engage in other affinitive interactions preferentially with full sisters over maternal half sisters (Smith et al. 2013). All other things being equal, kin selection should favor cooperative behavior later in life more between full than half sisters, and it should thus be important for juveniles to forge stronger relationships with full sisters. Finally, we predicted that if play behavior functions to establish social relationships important in adulthood, then there should be no preferences for full over maternal half siblings in play or other affinitive interactions between brothers or brothers and sisters because these sibling combinations do not ordinarily interact in adulthood (Holekamp 1984, 1986). Body mass can affect preferences for partners in play interactions between juvenile male U. beldingi, with young males expressing preferences for other males evenly matched in body mass (Nunes et al. 2004b); so, when data analyses suggested specific partner preferences among males, we further evaluated the possible role of body mass in the preferences.
Methods
From May through July 2012–2013, we studied a population of U. beldingi in a 50-ha meadow near Tioga Pass in Mono County, California, USA (37° 55′ N, 119° 15′ W). This species is diurnal, inhabits alpine and subalpine meadows in the western USA, and hibernates 8–9 months each year from late summer through spring (Jenkens and Eshelman 1984). Females typically enter estrus within a week after emerging from hibernation and mate on only 1 day per year, thus bearing at most 1 l per year (Morton and Gallup 1975). Gestation lasts 24–25 days. Young remain underground in natal burrows during lactation and first emerge from the natal burrow when they are about 25–28 days old, near the time of weaning (Holekamp et al. 1984; Nunes et al. 1999). Juveniles engage in play behavior during their first 2 weeks above ground, after which rates of play decline substantially as juvenile roam farther from the natal burrow. Most play interactions (>97 %) occur between littermates (Nunes et al. 1999).
Squirrels were captured in live traps (Tomahawk Live-Trap Company, Hazelhurst, Wisconsin, USA) baited with peanut butter. Traps were checked every 30 min or less during trapping sessions. At their first capture, squirrels were fitted with monel metal ear tags (National Band and Tag Co., Newport, Kentucky, USA) for permanent identification. Ear tags of juveniles were painted different colors with nail polish prior to application to aid in identification of individuals during behavioral observations. We used six different colors of ear tags (orange, blue, green, yellow, pink, and purple). When a litter consisted of more than six juveniles, more than one juvenile in the litter was fitted with pink or orange ear tags. Thus, 17 different color combinations of ear tags were possible overall in dyadic pairings of juveniles. Also, at squirrels’ first capture, a 1–2-mm sliver of skin tissue was collected from the outer rim of the ear with surgical scissors for genetic analysis. Tissue samples were kept temporarily on ice until they could be transferred to −20° for storage. Collection of tissue samples caused only momentary discomfort and did not result in bleeding. The fur of squirrels was marked with unique symbols using Nice ‘n Easy blue black #124 hair dye (Clairol, Stamford, Connecticut, USA) to aid in visual identification of individuals during behavioral observations. Body mass of squirrels was measured with spring balance scales (Avinet, Dryden, New York, USA). The maternal burrows of lactating females were observed daily from elevated posts such as rocks or hilltops to determine the date on which their young first emerged from the natal burrow. Young were trapped within 2 days of their first appearance above ground during which time they remain close to the natal burrow and can be unambiguously assigned to mothers (Holekamp 1984). Distances between burrows from which juveniles emerged were calculated using scaled maps obtained from the US Geological Survey. All work with U. beldingi in this study followed humane guidelines published for mammals (Sikes et al. 2011).
Observation of behavior
We observed the behavior of 198 free-living juvenile U. beldingi from a total of 34 litters in which there were at least three juveniles and squirrels thus had a choice of partners in social interactions. We used dyadic pairings of littermates as the basic unit of interest in behavioral observations. Specifically, we were interested in whether some dyadic pairings in social interactions were more likely or engaged in longer interactions than others based on the sex and relatedness of juveniles in the pairing. Moreover, we did not evaluate the direction of interaction in dyads. That is, we were not interested in which juvenile in a dyad initiated an interaction, but rather in whether behavioral interactions were more frequent or lasted longer among specific classes of dyads. We observed the behavior of a total of 493 dyadic pairings of littermates. Observations included a total of 1868 play, 1823 affiliative, and 637 investigative interactions (see below) between littermates, and also 45 play, 25 affiliative, and 27 investigative interactions among 40 nonlittermate dyads. Analyses of behavior focused on interactions between littermates.
Behavioral observations were conducted throughout the day between 0700 and 1800 h from elevated posts such as boulders or hilltops. We observed juveniles during the 2-week period following their first emergence from the natal burrow. During this 2-week span, juveniles within a litter tended to emerge synchronously from the natal burrow at the beginning of periods of activity. To ensure that all littermates were available as social partners during observations, we conducted behavioral observations for a litter only when all juveniles in the litter were above ground and within view. Litters were observed for an average of 10.3 ± 0.8 (SEM) total hours on an average of 8.1 ± 0.3 (SEM) different days. During observations, we recorded all occurrences of specific behaviors (Altmann 1974; Martin and Bateson 2007), focusing on social play behaviors and affinitive behaviors potentially associated with promoting cohesiveness between individuals, such as affiliation and social investigation. Start and stop times of behavioral interactions were noted so that their durations could be calculated. Specific behaviors recorded during observations are described below.
Social play behavior:
-
Wrestling—The juvenile faces a partner, in a ventrum to ventrum clench or embrace, and pecks at the partner’s neck, throat, cheeks, or abdomen without inflicting bite wounds.
-
Tackling—The juvenile jumps or pounces on a partner, either from a stationary or running start.
-
Boxing—The juvenile bats with the forepaws at a partner, who may reciprocate.
-
Chasing—The juvenile follows and pursues a partner while both juveniles are running.
-
Mounting and play copulation—The juvenile climbs on the back of a partner and places the forepaws around the partner’s chest or abdomen, grasping in the mouth the skin of the partner’s neck, cheek, or back, and aligning the pelvis with the partner’s pelvis; the juvenile and partner may both lie on their sides on the ground; no intromission occurs.
Affinitive behavior:
-
Affiliation—The juvenile remains within 0.5 m of another juvenile for >10 s without engaging in other social interactions.
-
Investigation—The juvenile engages in olfactory examination of the partner with the head extended and nose within 2 cm of the partner, or walks around the partner with the head oriented toward the partner.
For littermate dyads, we determined the number of play, affiliative, and investigative interactions that would be expected if juveniles were randomly partnering with littermates in these interactions. Expected frequencies of interactions for dyads were calculated by dividing the total number of interactions observed in a litter by the number of dyadic pairings possible in the litter. Observed values of interactions for each possible dyadic pairing in a litter were divided by expected values to create a measure of preferences for specific partners. We calculated rates of play, affiliative, and investigative behavior for each dyad in a litter as the number of interactions between juveniles in the dyad divided by the total number of hours the juveniles were observed. Observations were recorded only when all juveniles in a litter were active; so, each juvenile was observed the same amount of time as its littermates.
Paternity analysis
DNA was isolated from tissue samples using DNeasy kits (Qiagen, Valencia, California, USA). Microsatellites were identified and developed by Genetic Identification Services (GIS, Chatsworth, California, USA) specifically for U. Beldingi. Primers were designed by GIS for 67 loci identified using Designer PCR 1.03. To create the library, DNA fragments from U. beldingi were ligated into the HindIII site of the pUC19 plasmid, which was then transformed into Escherichia coli. Of the 67 loci, 17 were clearly polymorphic, and seven of the polymorphic loci amplified reliably and could be used for genotyping (Table 1). Both multiplex and singleplex polymerase chain reaction (PCR) parameters were optimized for these seven microsatellites.
The 5′ end of forward primers used in PCR was tagged with the fluorescent markers HEX or 6FAM to facilitate differentiation of fragments in later analyses. Type-it PCR kits (Qiagen, Valencia, California, USA) were used to perform multiplex PCR. Markers B108 and D106 were paired for multiplexing, and markers C4 and D4 were paired. Markers B12 and D108 had the same annealing temperature but could not be paired for multiplexing due to overlap in specific fragment lengths. The mix for each sample included 1 μl of isolated DNA, forward and reverse primers both in 0.2 μM concentrations, 12.5 μl of the supplied master mix, and water to bring total volume to 25 μl. The protocol for multiplex PCR involved (1) a 5-min 95 °C hot start, (2) 35 cycles of [30 s at 95 °C for melting, 60 s at the appropriate annealing temperature (Table 1), and a 30-s extension at 72 °C], and (3) a 30-min final extension at 60 °C. Taq Polymerase PCR kits (TaKaRa, Otsu, Japan) were used for singleplex PCR. The singleplex PCR protocol also involved a 25-μl reaction; however, 0.5 μM concentrations of the primers were used for B6, B108, C4, D4, and D108, and 0.2 μM concentrations were used for B12 and D106. The mix for each sample included 1 μl of isolated DNA, 2.5 μl of the buffer provided in kits, 2 μl of dNTPs, 0.125 μl of Ex Taq polymerase, and water to bring the total volume to 25 μl. The protocol for singleplex PCR involved (1) a 5-min 94 °C hot start, (2) 35 cycles of [40 s at 94 °C, 40 s at the appropriate annealing temperature (Table 1), and a 30-s 72 °C extension], and (3) a 3-min 72 °C final extension. PCR products were analyzed via capillary electrophoresis on the ABI 3730xl DNA Analyzer, with the 400HD ROX size standard at the University of California at San Francisco. Output was given in the form of fragment lengths of each microsatellite allele. A total of 356 individual squirrels were genotyped using the seven loci, including all juveniles, yearlings, and adults captured in the study area. Numbers of alleles present, heterozygosity, and Fis statistics were calculated for the 356 squirrels using Genepop 4.2 software (Rousset 2008).
We extensively trapped adult males in our study site and in areas adjacent to the study site throughout the summer to consider as potential fathers. Adult males trapped in the same summer that juveniles were born were included as potential fathers in determination of paternity. Assignments of paternity for juveniles were made manually by comparing the alleles of juveniles at each of the seven microsatellite markers with the alleles of their mothers and alleles of potential fathers. A paternal allele in a juvenile was identified as an allele not present in the mother, an allele homozygous in the juvenile, or either of the two heterozygous alleles in a juvenile identical to the mother’s alleles at that locus (Baker et al. 1999). Candidate fathers for a juvenile were eliminated if they lacked any paternal alleles. Determinations of paternity made manually were verified with determinations using Cervus software (Field Genetics, London, UK), which uses full likelihood and exclusion to assign parentage. The overall exclusionary power of Cervus in determining paternity with the mother known was 0.989 for all seven microsatellite loci combined. Confidence intervals for Cervus were set at 0.95 for identifying the most likely father. All manual determinations of paternity corresponded to those made by Cervus. Paternity was established for all juveniles included in the study. Full siblings within a litter were defined as having the same father and maternal half siblings as having different fathers. Unless otherwise specified, we use “full sibling” and “half sibling” to denote littermate full sibling and maternal half sibling U. beldingi. We also used Cervus to analyze Hardy-Weinberg equilibrium with a Χ2 goodness of fit method.
Statistical analysis
We used a mixed linear model to evaluate whether the proportion of females or degree of relatedness among juveniles in a litter were associated with rates of play, affiliative, or investigative interactions among dyadic pairings of littermates. Multiple paternity within litters was used as a measure of relatedness. To account for differences in the number of juveniles within litters, the mean number of young per father represented within a litter was used to gauge multiple paternity rather than the mean number of males who sired young in a litter. The proportion of females in a litter was not correlated with the mean number of juveniles per sire represented in the litter (Pearson’s r = −0.051, P = 0.260). Litters and dyads nested within litters were included in the model as random effects.
As an index of partner preferences in social interactions, we evaluated ratios of observed numbers of play, affiliative, and investigative interactions among dyadic pairings of juveniles to numbers that would be expected if littermates randomly paired with each other. We used a mixed linear model to evaluate these data. Sibling status (full, half) and sexes of juveniles in dyadic pairings (female-female, female-male, and male-male) were included as fixed effects in the model. The combination of ear tag colors in dyads was also included as a fixed effect to assess the possible influence of ear tag color on partner preferences. Dyadic pairing and litter were included as random effects in the model, with dyads nested within litters.
To further assess partner preferences in social interactions, we evaluated the duration of play and affiliative interactions among dyadic pairings of littermates using a mixed linear model. Individual interactions were used as the unit of observation in the model. Investigative interactions typically lasted only 1–2 s and so were not included in this analysis. Sibling status and sexes in dyads were included as fixed effects in the models, and litter and dyad nested within litter were included as random effects.
To assess the possible influence of body mass on partner preferences among brothers, we evaluated body mass differences within dyadic pairings of littermates using a mixed linear model. Sibling status and sexes in dyads were included as fixed effects in the models, and litter and dyad nested within litter were included as random effects.
We also used a mixed linear model to further evaluate the duration of play interactions between brothers. In this analysis, status as full or half brothers and categories of magnitude in dyadic mass differences, expressed as a percentage of the smaller juvenile’s body mass, were included as fixed effects. Litter and dyad nested within litter were included as random effects. Individual interactions were used as the unit of observation.
Post hoc comparisons between full and half siblings within dyadic groupings based on sex were performed using a mixed linear model in which sibling status was included as a fixed effect, and litter and dyad nested within litter were included as random effects. Significance thresholds for multiple pairwise comparisons were adjusted using the sequential Bonferroni method (Rice 1989). Post hoc comparisons within other categorical variables were performed with Tukey’s HSD tests. When analyses indicated a significant association between continuous variables, linear regression was used to evaluate the magnitude of the association.
Statistical tests were conducted with Systat 13 (Systat Software, Inc., Chicago, Illinois, USA). For ease of interpretation, values of variables prior to transformation are presented in figures. Mean values are presented ±1 SEM. Probabilities were considered significant when P ≤ 0.05.
Results
The seven microsatellite loci characterized in our study ranged from 2 to 4 base pairs in size of repeating subunits and 129 to 316 base pairs in overall fragment length. None of the loci deviated significantly from Hardy-Weinberg equilibrium at the P < 0.05 level, both before and after Bonferroni correction (Marshall et al. 1998; Kalinowski et al. 2007). The number of different alleles present at each locus are summarized in Table 1. A total of 59 alleles across all ground squirrels in the population were detected in the seven markers, with a range of 4 (B6, D106) to 18 (C4) alleles per locus. Tests for heterozygote excess and deficiency within the population were not significant (Table 1). The estimated level of inbreeding (FIS) within the population over all loci produced a value of −0.0111, indicating that the population is panmictic with a low level of inbreeding.
Most behavioral interactions observed among juvenile U. beldingi occurred between littermates. Only 2.3 % (45/1916) of play interactions, 1.4 % (25/1849) of affiliative interactions, and 4.1 % (27/664) of investigative interactions occurred between juveniles from different litters. Most nonlittermate interactions occurred between juveniles with different fathers. Among nonlittermates, only 13.3 % (6/45) of play interactions, 16.0 % (4/25) of affiliative interactions, and 18.5 % (5/27) of investigative interactions occurred between juveniles with the same father. Due to the very small sample of social interactions involving paternal half siblings, we focused behavioral analyses on full sibling and maternal half sibling littermates.
We assessed whether rates of social interaction varied with the degree of relatedness among juveniles in a litter or with the relative proportion of sexes in the litter. The number of juveniles per sire represented in the litter was used as a gauge of relatedness among juveniles. Rates of neither play, affiliative, nor investigative behavior varied significantly with the number of young per father in a juvenile’s litter (Table 2). However, rates of play, affiliative, and investigative interactions each varied as a function of the proportion of females in a litter (Table 2). We used linear regression to evaluate the magnitude of association between the ratio of females in a litter and measures of social interaction. The slope of the regression line between the ratio of females and rates of interaction was −0.15 for play behavior (F 1, 491 = 4.0, P = 0.045), was 0.54 for affiliative behavior (F 1, 491 = 35.8, P < 0.001), and was 0.22 for investigative behavior (F 1, 491 = 15.0, P < 0.001). We further evaluated the relationship between rates of behavior and the proportion of females in a litter, classifying juveniles as being from a female-biased litter (proportion of females 0.67–1.00), unbiased litter (proportion of females 0.34–0.66), or male-biased litter (proportion of females 0–0.33). As litters became more female biased, rates of play decreased significantly (Fig. 1a, F 2, 425 = 3.56, P = 0.029), but rates of affiliative (Fig. 1b, F 2, 425 = 5.28, P = 0.005) and investigative (Fig. 1c, F 2, 425 = 5.01, P = 0.007) increased.
Relationship between the proportion of females in U. beldingi litters and rates of a play, b affiliative, and c investigative interactions between littermates. Rates of behavioral interactions were calculated for each possible dyadic pairing of juveniles within litters. Different lower case letters indicate significant differences between groupings of dyads. Sample sizes indicate the number of dyadic pairings within groups
We evaluated preferences among juveniles for partners in play, affiliative, and investigative interactions, as measured by the ratio of observed numbers of interactions to numbers expected by random association of juveniles within litters. Partner preferences in play behavior did not vary with the status of juveniles as full or half siblings or with ear tag color (Table 3). However, play interactions tended to occur more frequently between brothers and less frequently between sisters than would be expected by random pairing of juveniles in litters (Table 3, Fig. 2). Partner preferences in affiliative and investigative interactions did not vary with sibling status, sexes in dyads, or ear tag colors, and there were no significant interactions between fixed variables in any of the above analyses (Table 3).
Partner preferences in dyadic play interactions among juvenile U. beldingi littermates as indicated by ratios of observed numbers of interactions to numbers that would be expected by random partnering of juveniles within litters. Different lower case letters indicate significant differences between dyadic pairings based on sex. Samples sizes indicate the number of dyadic pairings within groups
To further assess social partner preferences, we evaluated the duration of play and affiliative interactions between juveniles. The duration of affiliative interactions did not vary overall with the status of a juvenile’s partner as a full or half sibling or with the sexes of juveniles in dyadic pairings, and there was no interaction between these variables (Table 4). The duration of play interactions did not vary significantly with status as full or half siblings but did vary with the sex of juveniles in interactions (Table 4). Moreover, there was a significant interaction between these variables (Table 4), suggesting that differences in the duration of play bouts with full versus half siblings were not uniform across dyadic parings based on sex. In particular, play bouts lasted longer between full brothers than half brothers (Fig. 3, F 1, 366 = 6.93, P = 0.009), but there was no difference in the duration of play bouts between full versus half sibling pairings of sisters (Fig. 3, F 1, 450 = 0.038, P = 0.845) or sisters and brothers (Fig. 3, F 1, 868 = 0.47, P = 0.492). For post hoc comparisons among pairing of juveniles based on sex, samples of full and half siblings were collapsed within categories. Play bouts varied in duration as a function of the sexes in dyadic interactions (Fig. 3, F 2, 1794 = 4.1, P = 0.017), and in particular lasted significantly longer in male-male pairings of juveniles than in female-female pairings (Fig. 3, 49.0 ± 3.5 s, n = 417 vs 39.6 ± 1.8 s, n = 512, respectively, t = 2.64, P = 0.023) or female-male pairings (Fig. 3, 49.9 + 3.5 s, n = 417 vs 41.1 + 1.6 s, n = 939, respectively, t = 2.57, P = 0.028), primarily due to longer duration of play interactions between full brothers (Fig. 3).
Duration of play interactions between juvenile U. beldingi littermates. Different lower case letters indicate significant differences between dyadic pairings based on sex, with data combined for full siblings and maternal half siblings within each group. The asterisk indicates a significant difference between full sibling and maternal half sibling dyads. Sample sizes indicate the number of interactions within groups
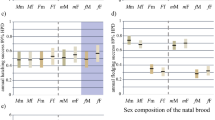
We evaluated the possibility that differences in the duration of play interactions between full and half brothers that we observed might be related to differences in body mass between full and half siblings rather than relatedness. A total of 493 dyadic pairings of littermates was included in this analysis. Body mass differences between littermates tended to be smaller among full siblings than half siblings (Fig. 4, 4.3 ± 0.3 g, n = 163 vs 5.5 ± 0.2 g, n = 330, respectively, F 1, 420 = 7.79, P = 0.005) but did not vary with the sex of juveniles in dyadic pairings (Fig. 4, F 2, 420 = 2.34, P = 0.097), and there was no interaction between these variables (Fig. 4, F 2, 420 = 0.47, P = 0.624). Estimates of variance for random effects in this analysis were as follows: litter 3.9, dyad <0.001, error 13.4. Mass differences were significantly smaller in full sibling pairings than half sibling pairings in sister-brother dyads (Fig. 4, F 1, 188 = 5.94, P = 0.016) and brother dyads (Fig. 4, F 1, 44 = 5.82, P = 0.017), but not in sister dyads (Fig. 4, F 1, 78 = 0.71, P = 0.403).
We further evaluated the duration of play interactions between brothers, factoring in body mass differences between play partners. This analysis included 434 play interactions among male U. beldingi. The duration of play bouts decreased as the magnitude of difference in body mass between partners increased (Fig. 5, F 5, 371 = 6.69, P < 0.001). The duration of play bouts, however, did not vary significantly overall with the status of a play partner as a full or half brother (Fig. 5, F 1, 371 = 0.21, P = 0.644), and there was not a significant interaction between independent variables in the analysis (Fig. 5, F 5, 371 = 0.55, P = 0.735). Estimates of variance for random effects in this analysis were as follows: litter 0.001, dyad <0.001, error 3,524.8. For post hoc comparisons between categories of mass difference, samples of full and half brothers were collapsed within categories. Play interactions lasted significantly longer in pairings of males whose body mass differed by ≤5 % than in pairings with larger mass differences (Fig. 5). The slope of the regression for mass differences versus duration of play bouts was −1.2 (F 1, 432 = 5.4, P = 0.020).
Variation in the duration of dyadic play interactions among juvenile male U. beldingi as a function of body mass differences between play partners. Body mass differences are expressed as a percentage of the smaller play partner’s body mass. Different lower case letters indicate significant differences between body mass groupings, with data for full brothers and maternal half brothers combined within each grouping. Sample sizes indicate the number of dyadic pairings within each body mass grouping
To assess the likelihood that sisters would interact beyond the juvenile summer, we evaluated overwinter survival of females. In the second year of the study, we recovered 19.7 % (13/66) of females trapped as juveniles in the first year of the study. These included nine females who did not have a surviving littermate sister and two pairs of sisters. Sisters in one of the pairs were full siblings and in the other pair were half siblings. Thus, 15.4 % (2/13) of yearling females in the second year of the study had a surviving full sister, which represents a decline from the 57.6 % (38/66) of juvenile females in the first year of the study who had at least one full sister. All four females in the yearling sister pairs in the second year of the study weaned a litter, and 33.3 % (3/9) of yearling females without a surviving littermate sister weaned a litter. The half sisters had maternal burrows separated by 18 m, and their mother was present in the study area during the second year of the study. The maternal burrows of the full sisters were separated by approximately 50 m due to a range shift by one of the sisters away from her natal area, and their mother was not present in the second year of the study. Among the nine yearling females without a surviving littermate sister, 66.7 % (2/3) of those who weaned a litter had their mother present in the second year of the study, but only 16.7 % (1/6) of those who did not wean a litter had their mother present.
Discussion
Juvenile U. beldingi in our study did not engage in play or other social interactions preferentially with full siblings over maternal half siblings. Moreover, play bouts and affilliative interactions between juvenile females and full siblings did not last any longer than those with half siblings. Nunes et al. (2004b) observed that juvenile U. beldingi have preferences among siblings for partners in play interactions. However, results of our study suggest that among juvenile females, these preferences are not based on sibling relatedness. We note that although kinship may influence social relationships in animals, a variety of other factors are important in maintaining amicable sociality (Hare and Murie 2007).
The lack of preference by juvenile female U. beldingi for full over maternal half sisters in play interactions may reflect an importance for young females to establish social bonds with both full and half sisters. Mortality tends to be high in juvenile U. beldingi during their first summer and overwinter period, with 29 % of juveniles on average surviving to the yearling summer (Sherman and Morton 1984). Low survival may make it unlikely that a female will have both full and half sisters available for interaction beyond the juvenile summer. In the second year of our study, only 15 % of yearling females had a surviving full sister, only 15 % had a surviving half sister, and no yearling females had both. High degrees of multiple paternity in U. beldingi (Hanken and Sherman 1981) might further reduce the likelihood that females will have full sisters available for cooperative relationships during their reproductive lifespan. In forming cooperative relationships with sisters, such as sharing in the defense of overlapping maternal territories (Sherman 1977), females might typically not have a choice between a full and half sister. However, even if females establish social bonds with both full and half sisters early in life, the nature of interactions between full and half sisters may still differ in adulthood with cooperative relationships being more extensive between full than half siblings (Holmes and Sherman 1982). Although we observed no preferences among juvenile females for full over half siblings in the frequency of play interactions, it remains possible that nuanced details in the expression of play behavior may vary with the degree of relatedness between young females and their siblings.
We note that among yearling female U. beldingi, cooperative relationships between females and their mothers may be more important for improving reproductive success than relationships between females and their littermate sisters. Yearling female U. beldingi express lower intensities of aggressive and vigilant behaviors in defending maternal territories than do older and more experienced females (Nunes 2014a), and yearling females have greater success in weaning a litter when they have a surviving mother with a maternal territory nearby who assists in evicting intruders from the daughter’s territory (Nunes 2014b).
We observed an association between the proportion of females in litters and overall rates of social interactions within litters. As the proportion of females within litters increased, rates of social play tended to decrease. Moreover, play interactions between sisters tended to occur at lower frequencies than would be expected by random pairing of juveniles within litters, but play interactions between brothers tended to occur at higher than expected frequencies. Young males in various species, including U. beldingi, exhibit greater motivation to initiate play interactions than do young females (Pellis et al. 1997; Pasztor et al. 2001; Nunes et al. 2004b; Cameron et al. 2008). Moreover, play can be contagious. In rats (Rattus norvegicus), juveniles may respond to other juveniles who initiate social play behavior at high rates by initiating social play at higher rates themselves (Pellis and McKenna 1992). Thus, greater male motivation to play and the contagion effect might cause rates of play to be higher than average in male-biased litters in some species. We also observed that as the proportion of females within litters increased in our study, rates of affiliative and investigative interactions among littermates increased. These increases in nonplay social interactions may be a by-product of lower rates of play in female-biased litters, with social contact being more likely to result in nonplay interactions in female- than male-biased litters. Nonplay social interactions may also have a role in forming social bonds between young female U. beldingi. Nonplay social interactions have been suggested to contribute to development of long-term social bonds in yellow-bellied marmots (Smith et al. 2013) and ponies (Equus caballus; Rho et al. 2007).
Lower rates of social play in female- than male-biased litters in our study raise the possibility that lower rates of play behavior may be sufficient to establish social bonds between young female U. beldingi. Higher rates of social play among juvenile male U. beldingi may be necessary for functions that might be especially important for young males, such as improving motor skill and coordination (Nunes et al. 2004a, b). Natural selection may favor young animals who engage in social play at optimal levels that balance the benefits of play with potential risks and priorities for available energy. Engaging in play behavior can increase the risk of physical injury, make individuals conspicuous and more vulnerable to predation, or increase transmission of disease and mortality, and in some cases, young animals may alter their play behavior to minimize these risks (Fagen 1981; Biben et al. 1989; Harcourt 1991; de Olivera et al. 2003; Kuehl et al. 2008). Moreover, play requires energy, and engaging in play behavior may direct energy away from growth processes important in young animals (Miller and Byers 1991). Although play typically comprises only a small portion of a young animal’s energy budget, play behavior can fluctuate with the overall amount of energy available to individuals (Bekoff and Byers 1992; Caro 1995; Nunes et al. 1999; Sharpe et al. 2002).
Among juvenile U. beldingi in our study, play bouts tended to last longer between brothers than between sisters or between brothers and sisters. Moreover, among brothers, play bouts lasted significantly longer in full than half brother pairings. This difference between pairings of full compared to half brothers may reflect strengthening of social bonds between full brothers via play interactions; however, we believe this unlikely. Juvenile males in our study did not have preferences for full over half brothers in play or other social interactions, as might be expected if young males sought to strengthen social ties with full brothers. Moreover, U. beldingi littermate brothers do not have overlapping home ranges after emigrating from the natal area, and thus, interactions among these brothers in adulthood are likely to be very rare (Holekamp 1984, 1986).
Another possibility is that longer play bouts between full than maternal half brothers are related to greater phenotypic similarity between full brothers. Body mass differences between full brothers in our study were significantly smaller than between half brothers, which may indicate greater similarity in phenotype among full brothers. Phenotypically similar males may be evenly matched and provide the greatest challenge in competitive play interactions such as wrestling (Thompson 1996; Nunes et al. 2004b), prompting males to favor play interactions with full brothers and to engage in longer play bouts with full brothers. The duration of play bouts between male U. beldingi in our study in fact decreased as the magnitude of differences in body mass increased in both full and half brother pairings, supporting the idea that males have preferences for partners with similar body mass in play interactions. Nunes et al. (2004a, b) observed that play improved motor skill and coordination in juvenile U. beldingi. Motor skill improved most when juveniles played with a variety of partners and were presumably exposed to a range of motor challenges. However, juveniles also tended to initiate play bouts preferentially with some partners more than others, and among males, the most preferred partner tended to be another male evenly matched in body mass. Improved motor skill and coordination accompanying play behavior might be especially important for juvenile male U. beldingi to help them prepare for potential challenges associated with emigrating from the natal area during the juvenile summer (Nunes et al. 2004a).
Our results failed to show an association between play partner preferences and sibling relatedness in juvenile U. beldingi; however, we cannot rule out the possibility that play behavior contributes to the shaping of social relationships between females in this species. Future studies examining nuanced details of play interactions might further elucidate the role of play behavior in social bonding and social development. However, results of our study suggest that if social bonding is an important function of play behavior in U. beldingi, it is likely not the main adaptive benefit individuals gain from play. Rather, establishment of social relationships might be part of a suite of benefits that includes advantages such as improved motor coordination and motor development (Nunes et al. 2004a, b).
References
Altmann J (1974) Observational study of behaviour: sampling methods. Behaviour 49:227–267
Baker RJ, Makova KD, Chesser RK (1999) Microsatellites indicate a high frequency of multiple paternity in Apodemus (Rodentia). Mol Ecol 8:107–111
Bekoff M (1972) The development of social interaction, play, and metacommunication in mammals: an ethological perspective. Q Rev Biol 47:412–434
Bekoff M (1988) Motor-training and physical fitness: possible short- and long term influences on the development of individual differences in behavior. Dev Psychobiol 21:601–612
Bekoff M, Byers JA (1992) Time, energy, and play. Anim Behav 44:981–982
Bekoff M, Byers JA (1998) Animal play: evolutionary, comparative, and ecological processes. Cambridge University Press, Cambridge
Biben M, Symmes D, Bernhards D (1989) Vigilance during play in squirrel monkeys. Am J Primatol 17:41–49
Blumstein DT, Chung LK, Smith JE (2013) Early play may predict later dominance relationships in yellow-bellied marmots (Marmots flaviventris). Proc R Soc Lond B 280:1759
Burghardt GM (2005) The genesis of animal play: testing the limits. MIT Press, Cambridge
Cameron EZ, Linklater WL, Stafford KJ, Minot EO (2008) Maternal investment results in better foal condition through increased play behavior in horses. Anim Behav 76:1511–1518
Carling MD, Avsharian Wiseman P, Byers JA (2003) Microsatellite analysis reveals multiple paternity in a population of wild pronghorn antelopes (Antilocapra Americana). J Mammal 84:1237–1243
Caro TM (1988) Adaptive significance of play: are we getting closer? Trends Ecol Evol 3:50–54
Caro TM (1995) Short-term costs and correlates of play in cheetahs. Anim Behav 49:333–345
Clutton-Brock TH, Lukas D (2012) The evolution of social philopatry and dispersal in female mammals. Mol Ecol 21:472–492
Coulon J, Graziani L, Allainé D, Bel MC, Pouderoux S (1995) Infanticide in the Alpine marmot (Marmota marmota). Ethol Ecol Evol 7:191–194
de Olivera CR, Ruiz-Miranda CR, Kleiman DG, Beck BB (2003) Play behavior in juvenile golden lion tamarins (Callitrichidae: Primates): organization in relation to costs. Ethology 109:593–612
DeYoung RW, Demarais S, Gonzales RA, Honeycutt RL, Gee KL (2002) Multiple paternity in white-tailed deer (Odocoileus virginianus) revealed by DNA microsatellites. J Mammal 83:884–892
Fagen R (1981) Animal play behavior. Oxford University Press, Oxford
Fagen R, Fagen J (2004) Juvenile survival and benefits of play behavior in brown bears, Ursus arctos. J Evol Ecol Res 6:89–102
Fagen R, Fagen J (2009) Play behavior and multi-year survival in free-ranging brown bears, Ursus arctos. J Evol Ecol Res 11:1053–1067
Glen AS, Cardoso MJ, Dickman CR, Firestore KB (2009) Who’s your daddy? Paternity testing reveals promiscuity and multiple paternity in the carnivorous marsupial Dasyurus maculatus (Marsupialia: Dasyuridae). Biol J Linn Soc 96:1–7
Gomendio M (1988) The development of different types of play in gazelles: implications for the nature and functions of play. Anim Behav 36:825–836
Goossens B, Graziani L, Waits LP, Farand E, Magnolon S, Coulon J, Bel M, Taberlet P, Allaniné D (1998) Extra-pair paternity in the monogamous Alpine marmot revealed by nuclear DNA microsatellite analysis. Behav Ecol Sociobiol 43:281–288
Govindarajulu P, Hunte W, Vermeer LA, Horrocks JA (1993) The ontogeny of social play in a feral troop of vervet monkeys (Cercopithecus aethiops sabaeus): the function of early play. Int J Primatol 14:701–719
Greenwood PJ (1980) Mating systems, philopatry and dispersal in birds and mammals. Anim Behav 28:1140–1162
Hanken J, Sherman PW (1981) Multiple Paternity in Belding’s ground squirrel litters. Science 212:351–353
Harcourt R (1991) Survivorship costs of play in the South American fur seal. Anim Behav 42:509–511
Hare JF, Murie JO (2007) Ecology, kinship, and ground squirrel sociality: insights from comparative analysis. In: Wolff JO, Sherman PW (eds) Rodent societies: an ecological and evolutionary perspective. University of Chicago Press, Chicago, pp 345–355
Haynie ML, Van Den Bussche RA, Hoogland JL, Gilbert DA (2003) Parentage, multiple paternity, and breeding success in Gunnison’s and Utah Prairie dogs. J Mammal 84:1244–1253
Held SDE, Špinka M (2011) Animal play and animal welfare. Anim Behav 81:891–899
Helgen KM, Cole FR, Helgen LE, Wilson DE (2009) Generic revision in the holarctic ground squirrel genus Spermophilus. J Mammal 90:270–305
Holekamp KE (1984) Natal dispersal in Belding’s ground squirrels (Spermophilus beldingi). Behav Ecol Sociobiol 16:21–30
Holekamp KE (1986) Proximal causes of natal dispersal in Belding’s ground squirrels (Spermophilus beldingi). Ecol Monogr 56:365–391
Holekamp KE, Smale L (1991) Dominance acquisition during mammalian development: the “inheritance” of maternal rank. Integr Comp Biol 31:306–317
Holekamp KE, Smale L, Simpson HB, Holekamp NA (1984) Hormonal influences on natal dispersal in free-living Belding’s ground squirrels (Spermophilus beldingi). Horm Behav 18:465–483
Holmes WG (1994) The development of littermate preferences in juvenile Belding’s ground squirrels. Anim Behav 48:1071–1084
Holmes WG, Sherman PW (1982) The ontogeny of kin recognition in two species of ground squirrels. Am Zool 22:491–517
Jenkens SH, Eshelman BD (1984) Spermophilus beldingi. Mamm Species 221:1–8
Jennions MD, Macdonald DW (1994) Cooperative breeding in mammals. Trends Ecol Evol 9:89–93
Kalinowski ST, Taper ML, Marshall TC (2007) Revising how the computer program CERVUS accommodates genotyping error increases success in paternity assignment. Mol Ecol 16:1099–1106
König B (1997) Cooperative care of young in mammals. Naturwissenschaften 84:95–104
Kraaijeveld-Smit FJL, Ward SJ, Temple-Smith PD (2002) Multiple paternity in a field population of a small carnivorous marsupial, the agile antechinus, Antechinus agilis. Behav Ecol Sociobiol 52:84–91
Kuehl HS, Elzner C, Moebius Y, Boesch C, Walsh PD (2008) The price of play: self organized infant mortality cycles in chimpanzees. PLoS ONE 3:e2440
Lacy RC (1997) Importance of genetic variation to the viability of mammalian populations. J Mammal 78:320–335
Lawson Hadley LJ, Perrin N (2007) Advances in our understanding of mammalian sex-biased dispersal. Mol Ecol 16:1559–1578
Lewis KP (2000) A comparative study of primate play behavior: implications for the study of cognition. Folia Primatol 71:417–421
Mancini G, Palagi E (2009) Play and social dynamics in a captive herd of gelada baboons (Theropithecus gelada). Behav Process 82:286–292
Marshall TC, Slate J, Kruuk LEB, Pemberton JM (1998) Statistical confidence for likelihood-based paternity inference in natural populations. Mol Ecol 7:639–655
Martin P, Bateson P (2007) Measuring behavior: an introductory guide, 3rd edn. Cambridge University Press, Cambridge
Mateo JM (2003) Kin recognition in ground squirrels and other rodents. J Mammal 84:1163–1181
Mateo JM (2010) Self-referent phenotype matching and long-term maintenance of kin recognition. Anim Behav 80:929–935
Mateo JM, Johnston RE (2000) Retention of social recognition after hibernation in Belding’s ground squirrels. Anim Behav 59:491–499
Miller MN, Byers JA (1991) Energetic costs of play in pronghorn fawns. Anim Behav 41:1007–1013
Morton ML, Gallup JS (1975) Reproductive cycle of the Belding ground squirrel (Spermophilus beldingi beldingi). Great Basin Nat 34:121–134
Nunes S (2007) Dispersal and philopatry. In: Wolff JO, Sherman PW (eds) Rodent societies: an ecological and evolutionary perspective. University of Chicago Press, Chicago, pp 150–162
Nunes S (2014) Maternal experience and territorial behavior in ground squirrels. J Mammal 95:491–502
Nunes S (2014b) Juvenile social play and yearling behavior and reproductive success in female Belding’s ground squirrels. J Ethol 32:145–153. doi:10.1007/s10164-0403-7
Nunes S, Muecke E-M, Anthony JA, Batterbee AS (1999) Endocrine and energetic mediation of play behavior in free-living ground squirrels. Horm Behav 36:153–165
Nunes S, Muecke E-M, Lancaster LT, Miller NA, Mueller MA, Muelhaus J, Castro L (2004a) Functions and consequences of play behavior in juvenile Belding’s ground squirrels. Anim Behav 68:27–37
Nunes S, Muecke E-M, Sanchez Z, Hoffmeier RR, Lancaster LT (2004b) Play behavior and motor development in juvenile Belding’s ground squirrels (Spermophilus beldingi). Behav Ecol Sociobiol 56:97–105
Pasztor TJ, Smith LK, MacDonald NK, Michener GR, Pellis SM (2001) Sexual and aggressive play fighting of sibling Richardson’s ground squirrels. Aggress Behav 27:323–337
Paukner A, Suomi SJ (2008) Sex differences in play behavior in juvenile tufted capuchin monkeys (Cebus apella). Primates 49:288–291
Pellis SM, McKenna MM (1992) Intrinsic and extrinsic influences on play fighting in rats: effects of dominance, partner’s playfulness, temperament and neonatal exposure to testosterone proprionate. Behav Brain Res 50:135–145
Pellis S, Pellis V (2009) The playful brain. Oneworld Press, London
Pellis SM, Field EF, Smith LK, Pellis VC (1997) Multiple differences in the play fighting of male and female rats. Implications for the causes and functions of play. Neurosci Biobehav Rev 21:105–120
Pellis SM, Pellis VC, Bell HC (2010) The function of play in the development of the social brain. Am J Play 2:278–296
Poirier FE, Smith EO (1974) Socializing functions of primate play. Integr Comp Biol 14:275–287
Rho JR, Srygley RB, Choe JC (2007) Sex preferences in Jeju pony foals (Equus caballus) for mutual grooming and play-fighting behaviors. Zool Sci 24:769–773
Rice WR (1989) Analyzing tables of statistical tests. Evolution 43:223–225
Rousset F (2008) Genepop’007: a complete reimplementation of the Genepop software for Windows and Linux. Mol Ecol Resour 8:103–106
Sale MG, Kraaijevald-Smit FJL, Arnould JPY (2013) Multiple paternity in the swamp antechinus (Antechinus minimus). Aust Mammal 35:227–230
Say L, Pontier D, Natoli E (1999) High variation in multiple paternity of domestic cats (Felis catus L.) in relation to environmental conditions. Proc R Soc Lond B 266:2071–2074
Sharpe LL, Clutton-Brock TH, Brotherton PNM, Cameron EZ, Cherry MI (2002) Experimental provisioning increases play in free-ranging meerkats. Anim Behav 64:113–121
Sherman PW (1977) Nepotism and the evolution of alarm calls. Science 197:1246–1253
Sherman PW (1980) The Limits of ground squirrel nepotism. In: Barlow GW, Silverberg J (eds) Sociobiology: beyond nature/nurture? Westview Press, Boulder, pp 505–544
Sherman PW, Morton ML (1984) Demography of Belding’s ground squirrels. Ecology 65:1617–1628
Sikes RS, Gannon WL, The Animal Care and Use Committee of the American Society of Mammalogists (2011) Guidelines of the American Society of Mammalogists for the use of wild mammals in research. J Mammal 92:235–253
Smith JE, Chung LK, Blumstein DT (2013) Ontogeny and symmetry of social partner choice among free-living yellow-bellied marmots. Anim Behav 85:715–725
Solomon NG, French JA (1997) Cooperative breeding in mammals. Cambridge University Press, New York
Špinka M, Newberry RC, Bekoff M (2001) Mammalian play: training for the unexpected. Q Rev Biol 76:141–168
Sterck EHM, Watts DP, van Schaik CP (1997) The evolution of female social relationships in non-human primates. Behav Ecol Sociobiol 41:291–309
Thompson KV (1996) Play-partner preferences and the function of social play in infant sable antelope, Hippotragus niger. Anim Behav 52:1143–1155
Wahaj SA, Van Horn RC, Van Horn TL, Dreyer R, Hilgris R, Schwarz J, Holekamp KE (2004) Kin discrimination in the spotted hyena (Crocuta crocuta): nepotism among siblings. Behav Ecol Sociobiol 56:237–247
Ward C, Bauer EB, Smuts BB (2008) Partner preferences and asymmetries in social play among domestic dog, Canis lupus familiaris, littermates. Anim Behav 76:1187–1199
Waser PM, De Woody A (2006) Multiple paternity in a philopatric rodent: the interaction of competition and choice. Behav Ecol 17:971–978
Wilson S (1974) Juvenile play of the common seal Phoca vitulina vitulina with comparative notes on the grey seal Halichoerus grypus. Behaviour 48:37–60
Wolff JO, Sherman PW (2007) Rodent societies: an ecological and evolutionary perspective. University of Chicago Press, Chicago
Yasui Y (1998) The ‘genetic benefits’ of female multiple mating reconsidered. Trends Ecol Evol 13:246–250
Yasui Y (2001) Female multiple mating as a genetic bet-hedging strategy when mate choice criteria are unreliable. Ecol Res 16:605–616
Acknowledgments
We thank Jon Woo for assistance with genotyping microsatellites and Allison Luengen for assistance with statistical analyses. Alan Chan-Alvarado, Roxxana Beltran Valencia, Chelsea Harmon, Adelisa Legaspi, and Jonie Nguyen provided excellent assistance with field work. James Hare, Pierre-Olivier Montiglio, and an anonymous reviewer provided insightful comments on an earlier version of this paper. This work was supported by grants from the Faculty Development Fund at the University of San Francisco to SN and JAD.
Ethical standards
This work was conducted under permits from the California Department of Fish and Wildlife and the United States Forest Service and complies with the laws and standards of the USA.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by A. I. Schulte-Hostedde
Rights and permissions
About this article
Cite this article
Nunes, S., Weidenbach, J.N., Lafler, M.R. et al. Sibling relatedness and social play in juvenile ground squirrels. Behav Ecol Sociobiol 69, 357–369 (2015). https://doi.org/10.1007/s00265-014-1848-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00265-014-1848-y