Abstract
The costs and benefits of dispersal that select for sex-biased dispersal are still poorly understood. Many studies examine fitness consequences of dispersal after first breeding, while dispersal costs are most likely paid before first breeding during the movement, settlement, and post-settlement stages. We studied survival correlates of dispersal between flock settlement and first breeding during the first winter of juvenile willow tits (Poecile montanus), a small passerine that has female-biased natal dispersal, but shows no dispersal-associated survival differences after first breeding. This resident food-hoarding species winters in small stable non-kin territorial flocks. We collected capture-recapture data by following flocks from autumn to the following spring. We compared monthly survival and return rates of juveniles that were born and recruited within the study area (philopatric juveniles) and juveniles that originated from outside the study area (immigrant juveniles). Among males, survival was highest for philopatric juveniles whereas survival of females was higher among immigrant juveniles, providing one explanation for the female-biased natal dispersal observed in the species. Philopatric males may benefit from prior residency either through increased site familiarity and knowledge of winter food resources and/or by gaining higher social ranks during flock establishment. However, rank data provided little support for the latter hypothesis. Other mechanisms such as increased ability to find high-quality flocks and mates may be important for female survival. Our results provide further evidence that dispersal costs are paid mainly before first breeding and that sex-specific costs of dispersal play a role in the evolution of sex-biased dispersal.
Significance statement
This paper shows that female-biased dispersal can be a consequence of sex-specific costs and benefits of dispersal during the post-settlement stage of the dispersal process which is a very poorly understood stage in dispersal theory. By examining correlates of dispersal before rather than after first breeding as it is usually done, our study aids in understanding the selection pressures modifying dispersal strategies. Our results have wide applicability because there are many resident taxa similar to the willow tit (our study species) that have a settlement stage and a prolonged non-reproductive phase before their first reproduction.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Dispersal is a fundamental part of life history among all taxa (Clobert et al. 2012). Dispersal and site fidelity form competing strategies that are often affected by environment-, condition-, and phenotype-related costs and benefits that may be sex-specific (Greenwood 1980; Gros et al. 2008; Tarwater and Beissinger 2012; Nevoux et al. 2013; Bonte et al. 2014). In addition, competition or cooperation between kin and inbreeding avoidance may create sex-specific selection pressures on dispersal (Bowler and Benton 2005; Lawson Handley and Perrin 2007; Gros et al. 2008). Therefore, sex-biased dispersal is a common pattern (Greenwood 1980). Different environmental factors define whether dispersal leads to increased reproductive success (Ekman et al. 2001; Gienapp and Merilä 2011; Tarwater and Beissinger 2012; Terraube et al. 2014; Ekman and Griesser 2016), whereas the cost is often realized as increased mortality during the different stages of dispersal (Pärn et al. 2009; Pakanen et al. 2010; Bonte et al. 2012). Most studies have focused on fitness consequences after first reproduction when mortality costs are actually rare (Doligez and Pärt 2008; Waser et al. 2013). Crucially, there are only few studies on the costs associated with the transfer, settlement, and post-settlement stages of dispersal before first breeding (Devillard and Bray 2009; Bonte et al. 2012).
In many species, dispersers spend their first non-breeding season in groups. Group living often involves a hierarchy and in many cases even specific kin structures that may influence resource access and ultimately survival (Lambrechts and Dhondt 1986; Koivula et al. 1996; Baker et al. 1998; Griesser et al. 2006). This means that philopatric and dispersing individuals may be in different hierarchical positions especially when kin forms the basis of groups (Ekman et al. 2000, 2001, 2002; Ekman and Griesser 2016). However, little is still known about different sex-specific costs and benefits in species with non-kin wintering groups during these important stages that eventually determine success of natal dispersal (but see Eden 1987a, b).
The willow tit (Poecile montanus) is a small resident passerine that winters in small and stable (usually four to six individuals) territorial non-kin flocks that have social hierarchies determined by sex and age but also size and prior residency (Ekman 1979, 1990; Hogstad 1987; Koivula et al. 1996; Lahti et al. 1996). Based on female-biased dispersal in this species (Orell et al. 1999), we hypothesize that males benefit from moving short distances. Previous studies have shown that there are no apparent fitness differences between immigrant and philopatric individuals after first breeding (Orell et al. 1999). This indicates that sex-specific costs or benefits of dispersal may occur before first breeding, i.e., during their first autumn and winter.
The transfer stage of natal dispersal for willow tits occurs after gaining independence when juveniles leave their natal territories in search of vacancies in wintering flocks that are usually formed around territorial pairs (Ekman 1979, 1990; Hogstad 1987; Lahti et al. 1996). Juveniles from a brood disperse one by one about 14–16 days after fledging (Hogstad 1990). Juveniles disperse from their natal territories establishing themselves as flock members in the autumn. Some individuals disperse only locally while others disperse further. During the post-settlement stage, groups stay together through the wintering period before their first breeding. Membership in a group is important because it increases chances of surviving the non-breeding season, and the wintering territory may provide a future breeding territory (Ekman et al. 1981; Ekman 1990; Koivula et al. 1996). Different social or phenotypic factors within these wintering groups may create different sex-specific selective pressures for dispersed and philopatric individuals. Thus, the post-settlement stage may be a crucial determinant of dispersal success. We tested for sex-specific costs of natal dispersal during the first winter by examining correlates of dispersal. We used capture-recapture data collected by following willow tit flocks during four winters to compare sex-specific over-wintering survival of juvenile philopatric individuals that remained in the study area during the winter with that of immigrants which had originated from outside the study area.
Methods
Data collection
This study forms part of an individual-based population study in Oulu, Finland (N 65° 08′, E 25° 53′; Orell et al. 1999). The study area was 22 km2 during data collection. All chicks were ringed at the age of 13 days, which enabled classifying dispersal status (philopatric vs. immigrant) for all individuals. Philopatric juveniles were individuals that were born in the study area, while immigrants were unringed juveniles born outside the study area. Survival data were collected during four winters (1991–1992, 1992–1993, 1995–1996, 1997–1998). Wintering birds were caught and ringed with different color ring combinations in their territories from August to October. Birds were measured, sexed, and aged as yearlings and adults. We determined the dominance rank in 26 flocks (including N = 63 juveniles) based on social interactions (see Lahti et al. 1996 for methods on ranking individuals). Ranks varied from 1 to 7.
During the first three winters, data collection involved an intense capture-recapture scheme throughout winter (Lahti et al. 1998). The capture-recapture data spanned from September to May, thus including nine encounter occasions. Birds were habituated with food (pork fat) and conditioned to approach the observer in response to playback of willow tit song. The training lasted usually less than 1 week, and fat was no longer continuously provided after habituation. The capture-recapture data were collected by visiting flock territories 20–30 ha in size and their neighboring areas (at least the bordering flock territories) two to four times each month (Lahti et al. 1998). When flocks were attracted with playback, food was provided only for the time that enabled reading of color rings. See Lahti et al. (1998) for detailed descriptions of field methods. It was not possible to record data blind because our study involved focal animals in the field.
Data analysis
Capture-recapture data included yearling willow tits from 40 flocks that were either ringed as chicks in the study area (33 philopatric males [PM] and 16 philopatric females [PF]) or individuals which immigrated into the study population as unringed juveniles (16 immigrant males [IM] and 21 immigrant females [IF]). The capture-recapture data were analyzed in MARK (version 8.0) with variations of the CJS model that is developed for open population live-encounter data (Lebreton et al. 1992; White and Burnham 1999). The global model included effects of year (3 years; 1991–1992, 1992–1993, 1995–1996), sex and dispersal status (DS) on survival Φ(YEAR+SEX*DS), and recapture probabilities, p(YEAR+SEX*DS). Data did not allow including within-winter temporal variation. The global model fitted the data well (GOFBOOTSTRAP; p = 0.53; ĉ = 1.06). We used distance to the study area center as an individual covariate (see below for more information).
In 1992, an additional feeding experiment was carried out to examine the effects of food on survival. This experiment involved 21 flocks that were not followed throughout winter (Lahti et al. 1998). Flocks were either fed throughout the winter (n = 27 individuals, 10 groups) or control groups that were fed only when ringing them (n = 18 individuals, 9 groups). In addition, 29 flocks were identified in 1997 including 91 individuals. We analyzed survival of these birds together with those collected in the main capture-recapture study (90 flocks, n: PM = 64; PF = 34; IM = 67; IF = 61) using return rates to the following breeding season, i.e., whether or not an individual was known to be alive during the breeding season (May to July). We acknowledge that this analysis neglects recapture probabilities. However, Lahti et al. (1998) found that CJS models and return rates gave qualitatively similar results because the recapture probabilities were high (monthly averages varied between 0.76 and 0.94). Furthermore, differences in recapture probabilities between philopatric and immigrant individuals were tested in the capture-recapture modeling. A priori models included sex, dispersal status, feeding status, and distance to study area center (see below for more information) as fixed effects. Flock ID and year were included as random effects. We included all relevant interactions but restricted them to two per model due to limited sample size.
For the birds included in this study, mean natal dispersal distance of the recruiting philopatric males was 1576 m (SE 111, n = 64) similar to that of recruiting females 1659 m (SE 201, n = 34; t = 0.053, df = 64.89, p = 0.958) suggesting that the dispersal distances of the sexes do not differ so much within the study area. However, Orell et al. (1999) found longer dispersal distances for females when using more data. Females tended to comprise a smaller portion of the philopatric individuals compared to the immigrant individuals (χ 2 = 3.314, df = 1, p = 0.069), where females were more abundant than males. This may suggest that females more likely disperse away from the study area before flock settlement and do indeed have longer dispersal distances.
Because rank may affect winter survival (Ekman 1990; Koivula et al. 1996), we reanalyzed the effect of dispersal status with a subset of data that included the 63 individuals from 26 flocks that had been determined for dominance ranks. In this analysis, our a priori models included sex, dispersal status, dominance rank, and flock size as fixed effects, and year and flock ID as random effects. Dominance rank was only included with flock size. We considered dominance rank as a continuous variable because ranking indicates linear relationships such that a given rank always has the same number of individual that dominates it and its consequences may also be considered linear (Lahti et al. 1994). We considered only the interaction between sex and dispersal status. Supplementary food throughout winter was not given in 1991 and 1995 when dominance rank was determined for flocks.
We used generalized linear mixed effect models (GLMMs, binomial errors and logit link) with function “glmer” in R 3.1.3 (R Development Core Team 2015) to analyze return rates from autumn to the next breeding season. A priori models were fitted to the data and compared with the (Quasi-) Akaike information criterion with a small sample correction ((Q)AICc; Burnham and Anderson 2002). A difference of ∆(Q)AICc >2 was considered to infer a relevant difference in model support (Burnham and Anderson 2002), and conclusions were based on models within ∆(Q)AICc ≤2. Variables that resulted in ∆(Q)AICc ≤2 from the best model were considered to be uninformative (Arnold 2010).
Examining the likelihood of permanent emigration of immigrants
Correlates of dispersal can be biased by within-individual consistent behavior, such that immigrants are more prone to permanently emigrate from the study area (Doligez and Pärt 2008; Pakanen et al. 2011). We controlled for this in three ways. First, we used distance from the flock territory to the center of the study area as a proxy for permanent emigration in our models explaining survival/return rates. Permanent emigration should be more likely at the edge of the study area. If permanent emigration affects our results, birds located near the center of the study area should have a higher probability of apparent survival than those located near the edge of the study area. A negative association of distance to center of the study area with survival would thus indicate permanent emigration, and an interaction with dispersal status would indicate a stronger tendency of permanent emigration for either dispersal group. This variable only works if (1) the study area is uniform and round-shaped such as in our study and (2) if the dispersal distances are relatively short in relation to the study area. Movement from the site of capture and resighting to the breeding sites was only about 400 m (Lahti et al. 1998) suggesting that the distance from the center of the study area (22 km2) will show a difference in apparent survival if emigration exists. Second, we used a linear model to compare post-winter dispersal distances to their first breeding sites in order to assess possible differences in permanent emigration for individuals that were known to have bred after their first winter. Third, individuals may start to move to their breeding territories in the spring time and are not seen anymore. We therefore used the monthly capture-recapture data to calculate the average times (months) of disappearance to see when individuals disappeared. We used a non-parametric test to examine the effect of dispersal status within sexes.
Individual quality
We used linear models to examine for differences in body size measurements between philopatric and immigrant individuals within each sex. Body size measurements (wing length [maximal chord length], tarsus length, and mass) were used as proxies for individual quality. Larger birds (using tarsus length) have better fighting ability as shown earlier by Koivula et al. (1993). In addition, we used ordinal regression models to assess the effects of sex, dispersal status, and flock size on dominance ranks. We also calculated the probability of breeding for individuals known to be alive during the period from May to June and examined the effects of sex and dispersal status using generalized linear models (binomial distribution, logit link).
Results
Apparent monthly survival
The best model for apparent monthly winter survival included an interaction with dispersal status and sex (Online Resource 1, Tables S1 and S2). Philopatric males had higher survival than immigrant males while the immigrant females had higher survival than philopatric females (Fig. 1; see Online Resource 1, Table S2, for parameter coefficients from the best model). Distance to the center of the study area did not affect survival estimates alone or in interaction with dispersal status (Table S1). The recapture probabilities varied between years (1991, 0.80 ± 0.034; 1992, 0.87 ± 0.073; 1995, 0.91 ± 0.025) and tended to be higher for philopatrics, but the coefficient overlapped zero (βSTATUS 0.36, CI −0.321–1.037; Table S1).
Return rates
The interaction between sex and dispersal status was confirmed when analyzing return rates using a larger dataset (226 individuals; Online Resource 1, Table S3, Fig. 2). Supplemental food increased return rates (Online Resource 1, Tables S3 and S4; see also Lahti et al. 1998), and there was no evidence for an influence of interactions between food and dispersal status or between food and sex (Online Resource 1, Table S3; see Table S4 for parameter coefficients from the best model).
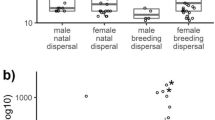
a Return rates (black columns = data, white columns = model predictions + SE) of juvenile willow tits from autumn to summer separately for philopatric males (PM), immigrant males (IM), philopatric females (PF), and immigrant females (IF). b The dispersal distances of surviving individuals from their wintering territory to first breeding site in the following breeding season
The interaction between sex and dispersal status remained strong also when considering dominance rank and flock size in the model (Online Resource 1, Tables S5 and S6). Flock size and dominance rank did not affect return rates (Online Resource 1, Table S5; see Table S6 for parameter coefficients from the best model). These models did not include distance to the study area center because it was previously found unimportant.
Body size and dominance ranks
Wing length, body mass, and tarsus lengths did not differ between philopatric and immigrant individuals in males or females (Online Resource 2, Tables S7 and S11). When controlled for flock size; dominance ranks were lower for females than males but roughly similar between immigrants and philopatrics in both males (mean PM 2.48, SE 0.148; IM 2.58, SE 0.313) and females (mean PF 4.3, SE 0.225; IF 4.3, SE 0.280, Online Resource 2, Tables S7 and S11).
Probability of breeding, post-winter dispersal, and time of disappearance
In both sexes, the probability of breeding did not differ between philopatrics and immigrants (males: PM 0.59, IM 0.73; females: PF 0.78, IF 0.91; Online Resource 2, Tables S7 and S12). Dispersal distances from winter flocks to summer breeding sites were short and did not differ between immigrants and philopatrics in either sex (Fig. 2, Online Resource 2, Tables S7 and S13). Dispersal distances from the original wintering flock territories of the juvenile year to the breeding sites in the second summer as adults (i.e., after their second winter) remained short also for those individuals that were not observed breeding in their first summer as adults (males 651 m, SE 163.0, n = 14; females 408 m, SE 43.4, n = 6). The average times of disappearance (month) were in mid-winter (November–December) and did not differ between philopatric and immigrant individuals in males (W = 65, p = 0.72) or females (W = 87, p = 0.63; Online Resource 2).
Discussion
Our results show that the survival of juvenile willow tits during their first winter was dependent on an interaction between dispersal status and sex. Immigrant males had lower survival than philopatric males, while immigrant females had higher survival than philopatric females. This pattern of costs and benefits offers an explanation for the female-biased dispersal in willow tit (Orell et al. 1999). Given that individuals compete for vacancies in flocks of restricted size, it seems possible that prior residence, which determines settlement success, also plays an important role in determining the costs and benefits of dispersal in wintering male willow tits.
In Parids, particularly the social rank of young males affects their survival (Ekman et al. 1981; Ekman 1990; Koivula et al. 1996). The dominant willow tit pair forage higher up in the canopy safer from predation, while the subordinate juveniles forage further down and are more vulnerable to Pygmy owls (Claucidium passerinum; Kullberg 1998; Kullberg and Ekman 2000). Social rank of an individual may depend on their fighting ability or resource value they have acquired. These may be correlated with body size, sex, or prior residency, so that the earlier established males will have higher ranks (Nilsson and Smith 1988; Sandell and Smith 1991; Koivula et al. 1993). Assuming that movement from natal sites and locating and settling into a suitable flock consumes time, it can be hypothesized that philopatric males that disperse short distances from their natal sites should on average have a prior residency advantage over those dispersing longer distances (Eden 1987a; Massot et al. 1994). Thus, the costs of dispersal for males may be reinforced by social factors that interact with the dispersal process so that philopatric individuals hold an advantage caused by a better social position in the group (Eden 1987a). If dispersing males end up with lower social ranks, they may enjoy differential benefits from living in a group, with poorer foraging conditions causing increased stress and predation and consequently reduced survival (Eden 1987b; Nilsson and Smith 1988; Kullberg 1998; Kullberg and Ekman 2000).
However, dominance ranks (sample sizes: PM = 21, IM = 12) did not consistently differ between immigrant and philopatric individuals and dominance rank did not affect survival. Strong rank differences cannot be expected when the most common flock size is four individuals and when sex and age are strong predictors of dominance rank. However, in two cases where a flock included immigrant and philopatric males, the philopatric male was dominant. While tests showed no effect of rank on survival, immigrant males did experience significantly lower survival, suggesting that alternative mechanisms must be at work.
Philopatric individuals may have better knowledge about predators and resources (food and roosting sites; Pärt 1995) and their distribution during the winter. This information starts to accumulate after settlement and may increase survival. Intensive food hoarding in the fall amplifies this difference and the benefits of prior knowledge. Indeed, the feeding experiment showed that food may have been the main factor that drives the lower survival of subdominants (see also Lahti et al. 1998). Secondly, immigrants may also suffer from sustained higher stress levels due to possibly longer periods of floating during the movement stage of dispersal, which may have physiological or behavioral repercussions that reduce survival (Silverin 1997; Young and Monfort 2009; Clinchy et al. 2013). Why did the philopatric willow tit females not benefit from philopatry? While the same logic of prior residency basically applies to both sexes, the survival pattern of females suggests that mechanisms other than prior residency are more important for survival during the post-settlement stage of dispersal. Females are nearly always at the bottom of the hierarchy, and their survival may be more dependent on their ability to find a good mate and flock (Hogstad 1987; Ekman 1990; Nilsson 1990; Lahti et al. 1996). Indeed, for females a mate is a critical resource. Juveniles form pair bonds already in the autumn, and pairs are observed to form alliances within the flocks so that mates of dominant males benefit from access to safer micro-habitat (Ekman 1990).
Given prior residency effects, establishment becomes more difficult with time (Nilsson 1989). Hatching date may therefore affect the relative timing of an individual in terms of establishment and the need to disperse further. Thus, philopatry (short natal dispersal) may be an option for early hatching males, while late hatching birds are forced to disperse further. On the other hand, because timing of hatching may also correlate with fledgling body condition (Verhulst and Nilsson 2008), quality differences provide an alternative explanation for our results, i.e., males fledging from early nests in good condition disperse shortest distances. However, our proxies for quality did not differ between immigrants and philopatrics. Furthermore, studies on species that have territorial wintering groups such as willow tits (Koivula et al. 1993) and marsh tits (Poecile palustris; Nilsson and Smith 1988; Nilsson 1989, 1990) show that establishment success and social ranks are dictated by prior residency over individual body size.
Our survival and return rate estimates were not biased by a higher probability of permanent emigration of immigrants (Doligez and Pärt 2008; Pakanen et al. 2011). Survival was not higher close to the center of the study area compared to the edge of the study area indicating that permanent emigration was relatively rare. Long distance dispersal behavior is unlikely during or after the winter because gaining a position in a wintering flock functions not only to increase survival through the winter but also to secure breeding in the next season within this territory (Orell et al. 1994). Such advantages of the wintering territory should not be different for philopatric and immigrant individuals. In line with this, post-winter dispersal distances and the times of disappearance during the winter did not differ between dispersal classes. As in any study that investigates correlates of dispersal in open populations, some immigrant individuals originate from near the edge of our study area and are therefore actually philopatric individuals. However, it makes our comparison conservative, because the “immigrant” group includes philopatric individuals of higher survival, but not the other way around. In fact, the strongest differences in survival between philopatrics and immigrants are usually seen in closed “island” populations whereas those study populations that are open (such as our study population) rarely show differences in survival (Appendix 3 in Pakanen et al. 2010).
To conclude, we found strong mortality costs of dispersal during the post-settlement stage for willow tit males only, supporting the view that female-biased dispersal in this and many other species is partially maintained by sex-specific costs to males (e.g., Pärn et al. 2009; Gienapp and Merilä 2011). Because we did not follow individuals during dispersal, we were unable to quantify establishment success in a wintering flock. The presence of floaters suggests that not all individuals gain positions in flocks (Hogstad 2014), and this may be one potential cost of dispersal. Costs of dispersal in the willow tit and other resident species thus probably accrue mostly before first breeding (Orell et al. 1999; Devillard and Bray 2009; Bonte et al. 2012). In the willow tit, these mortality costs of dispersal are sex-specific. They are probably linked to the wintering strategy of willow tits, which involves territorial non-kin flocks with social hierarchies. Whatever the mechanism, it may be a general phenomenon affecting sex-specific costs of dispersal in species that live in stable or ephemeral groups and may therefore be a driving force of male philopatry. Given that many resident species spend the non-breeding season in groups, over-wintering post-dispersal survival should be a promising avenue for future research, especially in relation to social status and consequent costs of dispersal.
References
Arnold TW (2010) Uninformative parameters and model selection using Akaike’s information criterion. J Wildlife Manage 74:1175–1178
Baker PJ, Robertson CPJ, Funk SM, Harris S (1998) Potential fitness benefits of group living in the red fox, Vulpes vulpes. Anim Behav 56:1411–1424
Bonte D, Van Dyck H, Bullock JM et al (2012) Costs of dispersal. Biol Rev 87:290–312
Bonte D, De Roissart A, Wybouw N, Van Leeuwen T (2014) Fitness maximization by dispersal: evidence from an invasion experiment. Ecology 95:3104–3111
Bowler DE, Benton TG (2005) Causes and consequences of animal dispersal strategies: relating individual behaviour to spatial dynamics. Biol Rev 80:205–225
Burnham KP, Anderson DR (2002) Model selection and multimodel inference: a practical information-theoretic approach. Springer, New York
Clinchy M, Sheriff MJ, Zanette LY (2013) Predator-induced stress and the ecology of fear. Funct Ecol 27:56–65
Clobert J, Baguette M, Benton TG (2012) Dispersal ecology and evolution. Oxford University Press, Oxford
Devillard S, Bray Y (2009) Assessing the effect on survival of natal dispersal using multistate capture–recapture models. Ecology 90:2902–2912
Doligez B, Pärt T (2008) Estimating fitness consequences of dispersal: a road to ‘know-where’? Non-random dispersal and the underestimation of dispersers’ fitness. J Anim Ecol 77:1199–1211
Eden SF (1987a) Dispersal and competitive ability in the magpie: an experimental study. Anim Behav 35:764–772
Eden SF (1987b) Natal philopatry of the magpie Pica pica. Ibis 129:477–490
Ekman J (1979) Coherence, composition and territories of winter social groups of the willow tit Parus montanus and the Crested Tit P. cristatus. Ornis Scand 10:56–68
Ekman J (1990) Alliances in winter flocks of willow tits—effects of rank on survival and reproductive success in male–female associations. Behav Ecol Sociobiol 26:239–245
Ekman J, Griesser M (2016) Siberian jays: delayed dispersal in absence of cooperative breeding. In: Koenig WD, Dickinson J (eds) Cooperative breeding in vertebrates: studies of ecology, evolution, and behavior. Cambridge University Press, Cambridge, pp 6–18
Ekman J, Cederholm G, Askenmo C (1981) Spacing and survival in winter groups of willow tit Parus montanus and crested tit P. cristatus—a removal study. J Anim Ecol 50:1–9
Ekman J, Bylin A, Tegelström H (2000) Parental nepotism enhances survival of retained offspring in the Siberian jay. Behav Ecol 11:416–420
Ekman J, Eggers S, Griesser M, Tegelström H (2001) Queuing for preferred territories: delayed dispersal of Siberian jays. J Anim Ecol 70:317–324
Ekman J, Eggers S, Griesser M (2002) Fighting to stay; the role of sibling rivalry for delayed dispersal. Anim Behav 64:453–459
Gienapp P, Merilä J (2011) Sex-specific fitness consequences of dispersal in Siberian Jays. Behav Ecol Sociobiol 65:131–140
Greenwood PJ (1980) Mating systems, philopatry and dispersal in birds and mammals. Anim Behav 28:1140–1162
Griesser M, Nystrand M, Ekman J (2006) Reduced mortality selects for family cohesion in a social species. Proc R Soc Lond B 273:1881–1886
Gros A, Hovestadt T, Poethke HJ (2008) Evolution of sex-biased dispersal: the role of sex-specific dispersal costs, demographic stochasticity and inbreeding. Ecol Model 219:226–233
Hogstad O (1987) Social rank in winter flocks of willow tits Parus montanus. Ibis 129:1–9
Hogstad O (1990) Dispersal date and settlement of juvenile willow tits Parus montanus in winter flocks. Fauna Norv Ser C Cinclus 3:49–55
Hogstad O (2014) Ecology and behaviour of winter floaters in a subalpine population of Willow Tits, Poecile montanus. Ornis Fennica 91:29–38
Koivula K, Lahti K, Orell M, Rytkönen S (1993) Prior residency as a key determinant of social dominance in the willow tit (Parus montanus). Behav Ecol Sociobiol 33:283–287
Koivula K, Orell M, Rytkönen S (1996) Winter survival and breeding success of dominant and subordinate Willow Tits Parus montanus. Ibis 138:624–629
Kullberg C (1998) Spatial niche dynamics under predation risk in the willow tit Parus montanus. J Avian Biol 29:235–240
Kullberg C, Ekman J (2000) Does predation maintain tit community diversity? Oikos 89:41–45
Lahti K, Koivula K, Orell M (1994) Is the social hierarchy always linear in tits? J Avian Biol 25:347–348
Lahti K, Koivula K, Orell M, Rytkonen S (1996) Social dominance in free-living willow tits Parus montanus: determinants and some implications of hierarchy. Ibis 138:539–544
Lahti K, Orell M, Rytkönen S, Koivula K (1998) Time and food dependence in willow tit winter survival. Ecology 79:2904–2916
Lambrechts M, Dhondt AA (1986) Male quality, reproduction and survival in the great tit (Parus major). Behav Ecol Sociobiol 19:57–63
Lawson Handley LJ, Perrin N (2007) Advances in our understanding of mammalian sex-biased dispersal. Mol Ecol 16:1559–1578
Lebreton J-D, Burnham KP, Clobert J, Anderson DR (1992) Modelling survival and testing biological hypothesis using marked animals: a unified approach with case studies. Ecol Monogr 62:67–118
Massot M, Clobert J, Lecomte J, Barbault R (1994) Incumbent advantage in common lizards and their colonizing ability. J Anim Ecol 63:431–440
Nevoux M, Arlt D, Nicoll M, Jones C, Norris K (2013) The short- and long-term fitness consequences of natal dispersal in a wild bird population. Ecol Lett 16:438–445
Nilsson J-Å (1989) Causes and consequences of natal dispersal in the marsh tit, Parus palustris. J Anim Ecol 58:619–63
Nilsson J-Å (1990) Establishment success of experimentally delayed juvenile Marsh tits Parus palustris. Ethology 85:73–79
Nilsson J-Å, Smith HG (1988) Effects of dispersal date on winter flock establishment and social dominance in marsh tits, Parus palustris. J Anim Ecol 57:917–928
Orell M, Rytkönen S, Koivula K (1994) Causes of divorce in the monogamous willow tit, Parus montanus, and consequences for reproductive success. Anim Behav 48:1143–1154
Orell M, Lahti K, Koivula K, Rytkönen S, Welling P (1999) Immigration and gene flow in a northern willow tit (Parus montanus) population. J Evol Biol 12:283–295
Pakanen V-M, Rönkä A, Belda E-J, Luukkonen A, Kvist L, Koivula K (2010) Impact of dispersal status on estimates of local population growth rates in a Temminck’s stint (Calidris temminckii) population. Oikos 119:1493–1503
Pakanen V-M, Hildén O, Rönkä A, Belda E-J, Luukkonen A, Kvist L, Koivula K (2011) Breeding dispersal strategies following reproductive failure explain low apparent survival of immigrant Temminck’s stints. Oikos 120:615–622
Pärn H, Jensen H, Ringsby TH, Sæther B-E (2009) Sex-specific fitness correlates of dispersal in a house sparrow metapopulation. J Anim Ecol 78:1216–1225
Pärt T (1995) The importance of local familiarity and search costs for age-biased and sex-biased philopatry in the collared flycatcher. Anim Behav 49:1029–1038
R Development Core Team (2015) R: A language and environment for statistical computing. Version 3.1.3. R Foundation for statistical computing, Vienna, Austria. Available at: http://www.r-project.org/
Sandell M, Smith HG (1991) Dominance, prior occupancy, and winter residency in the great tit (Parus major). Behav Ecol Sociobiol 29:147–152
Silverin B (1997) The stress response and autumn dispersal behaviour in willow tits. Anim Behav 53:451–459
Tarwater CE, Beissinger SR (2012) Dispersal polymorphisms from natal phenotype–environment interactions have carry-over effects on lifetime reproductive success of a tropical parrot. Ecol Lett 15:1218–1229
Terraube J, Vasko V, Korpimäki E (2014) Mechanisms and reproductive consequences of breeding dispersal in a specialist predator under temporally varying food conditions. Oikos 124:762–771
Verhulst S, Nilsson J-Å (2008) The timing of birds’ breeding seasons: a review of experiments that manipulated timing of breeding. Philos T Roy Soc B 363:399–410
Waser PM, Nichols KM, Hadfield JD (2013) Fitness consequences of dispersal: is leaving home the best of a bad lot? Ecology 94:1287–1295
White GC, Burnham KP (1999) Program MARK: survival estimation from populations of marked animals. Bird Study 46:120–139
Young AJ, Monfort SL (2009) Stress and the costs of extra-territorial movement in a social carnivore. Biol Lett 5:439–441
Acknowledgments
We thank all field workers, especially Ari-Pekka Auvinen. We thank Robert L. Thomson for checking the text for English and three anonymous referees for valuable comments on the manuscript. This study was funded by the Academy of Finland for VMP (grant no. 278759) and MO (grant nos. 3548, 1051114, 1051369).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed. This article does not contain any studies with human participants performed by any of the authors.
Funding
This study was funded by the Academy of Finland (grant numbers 278759, 3548, 1051114, and 1051369).
Additional information
Communicated by D. Rubenstein
Rights and permissions
About this article
Cite this article
Pakanen, VM., Koivula, K., Orell, M. et al. Sex-specific mortality costs of dispersal during the post-settlement stage promote male philopatry in a resident passerine. Behav Ecol Sociobiol 70, 1727–1733 (2016). https://doi.org/10.1007/s00265-016-2178-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00265-016-2178-z