Abstract
Food quality is a relevant characteristic to be transferred within eusocial insect colonies because its evaluation improves the collective foraging efficiency. In honeybees, colony mates could directly acquire this resource characteristic during trophallactic encounters with nectar foragers. In the present study, we focused on the gustatory responsiveness of bees that have unloaded food from incoming foragers. The sugar sensitivity of receiver bees was assessed in the laboratory by using the proboscis extension response paradigm. After unloading, hive bees were captured either from a colony that foraged freely in the environmental surroundings or from a colony that foraged at an artificial feeder with a known sucrose solution. In the first situation, the sugar sensitivity of the hive bees negatively correlated with the sugar concentration of the nectar crops brought back by forager mates. Similarly, in the controlled situation, the highest sucrose concentration the receivers accepted during trophallaxis corresponded to the highest thresholds to sucrose. The results indicate that first-order receivers modify their sugar sensitivity according to the quality of the food previously transferred through trophallaxis by the incoming foragers. In addition, trophallaxis is a mechanism capable of transferring gustatory information in honeybees. Its implications at a social scale might involve changes in the social information as well as in nectar distribution within the colony.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Information sharing is fundamental during the performance of collective tasks in animal species without a centralized control such as in eusocial insects (Wilson 1971; Seeley 1995). One of these tasks is resource exploitation that is mainly based on the coordination of different groups of individuals belonging to the same morphological caste, the workers (Wilson 1971; Michener 1974). The division of labor is supposed to improve the efficiency of this activity, which is constantly exposed to environmental changes. The age polyethism found in honeybees and other eusocial insects reflects this division of labor, in which workers perform different activities during their lifespan (Rösch 1925; Lindauer 1952; Seeley 1982). While young workers perform in-hive duties, the oldest ones forage outside (Rösch 1925; Oettingen-Spielberg 1949; Lindauer 1952; Seeley 1982).
One aspect considered fundamental in the division of labor in eusocial insects is related to behavioral response thresholds (Robinson 1992). For instance, the passage from in-hive tasks to foraging outside in honeybees is proposed to reflect changes in the responses to stimuli that elicit the performance of the new task. Different behavioral approaches have been performed to analyze this issue, from the study of thresholds for honeybee dancing (Lindauer 1948; Seeley 1989) to the proboscis extension response (PER) for bees of any age. Regarding this aspect, bees extend the proboscis as a reflex response to antennal stimulation with sugar solution (Kuwabara 1957), and their response threshold to sugar can be estimated by the lowest concentration that causes PER on antennal stimulation with an increasing series of sugar solutions (Page et al. 1998). Sucrose response thresholds (SRT) of young workers (pre-foraging bees) are associated with foraging choices later in their life suggesting that genetic factors affect this response (Pankiw and Page 2000; Pankiw et al. 2004). Young workers that become pollen foragers have low response thresholds, while bees that become nectar foragers have high response thresholds (Page et al. 1998; Pankiw and Page 1999). However, SRT are also modulated by the fed sugar solution. Bees fed with high-concentration sucrose solutions present higher thresholds to sucrose than those fed with low-concentration solutions (Pankiw et al. 2001, 2004).
Nectar foraging in honeybees represents an example of partitioning of related tasks, which as a consequence, conforms operational chains between colony members of different specialization, including the subcastes of foragers and those hive bees involved in food receiving and processing (Jeanne 1991; Ratnieks and Anderson 1999). Foragers transfer the collected nectar to processors in the delivery area close to the hive entrance via trophallaxis (Park 1925; Lindauer 1948, 1954; von Frisch 1967; Seeley 1995). Through these oral interactions, hive bees receive not only food but also information about the nectar collected. For instance, via trophallaxis, nectar receivers learn associatively the contingency between the liquid food and its odor (Gil and De Marco 2005; Farina et al. 2007). The acquired odor information biases later behavioral interactions between foragers and receivers within the hive (Goyret and Farina 2005).
Besides food odor information, the food source profitability is another relevant aspect to be acquired by the hive bees regarding the feeding place to adjust in-hive tasks. Although it is already known that the quality of the nectar circulating within the hive modulates the response thresholds to sucrose in young hive bees (Pankiw et al. 2004), there is no evidence that food quality information can be shared among honeybees by trophallaxis. As nectar receivers initiate nectar distribution within the bee colonies (Seeley 1995), assessing their response threshold in relation to the food collected would allow answering if trophallaxis is a communication mechanism involved in gustatory information transfer in honeybees. With this in mind, we first correlated the PER score (Page et al. 1998) of hive bees receiving food via trophallaxis in the delivery area with the sugar concentration of the nectar crops of the incoming foragers. While the crop contents of bees collecting natural sources in the surrounding environment were assessed, hive bees were captured simultaneously, and their PER scores (Page et al. 1998) were evaluated in the laboratory. Second, we measured the PER score of the first-order nectar receivers that interacted with a group of trained foragers collecting different sugar concentrations at an artificial feeding site.
Materials and methods
The experiments were performed between January and May 2004, during the summer–autumn season in this region, at the experimental field of the University of Buenos Aires (34°32′S, 58°26′W). We used two colonies, C1 and C2, of European Apis mellifera containing about 3,200 honeybees within two-frame observation hives that were placed inside the laboratory under controlled environmental conditions. Colonies had a queen, brood, and stored food.
Experimental procedure
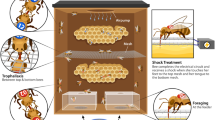
The aim of this study was to evaluate the gustatory responsiveness of honeybees involved in food unloading from nectar foragers within the hive. The proboscis extension reflex to sucrose of these bees was used to correlate this response with the food quality (i.e., sugar concentration) carried into the colony by active foragers. Two different scenarios were prepared to analyze gustatory responsiveness of in-hive nectar receivers: (1) a natural situation and (2) a controlled situation. In the natural situation, the sugar sensitivity of bees receiving food in the delivery area of the hive, where the most food unloadings occur (Seeley 1989), was measured and correlated with the sugar concentration of incoming forager crop contents. These measurements were performed during a period of 4 months in which the colony, C1, freely collected natural food sources. Before capture, hive bees that received food in the delivery area for at least 5 s from a colony mate were marked during 60–90 min. A 5-s period guarantees an effective passage of food during trophallaxis (Farina and Wainselboim 2001). This procedure was performed twice per experimental day, once in the morning and once in the afternoon. To mark the receiver bees, we used a transparent Perspex sliding door that was partly covered with a fine mesh through which the receivers’ thorax could be painted while they interacted with colony mates (Fig. 1). This device fitted into a sliding panel that could be moved horizontally, from side to side, allowing to scan the whole area of the exposed face of the hive. Afterwards, the mosquito-screen sliding door was replaced by a second Perspex panel (3 × 27 cm) with an opening in the center (2.5-cm diameter) that allowed the insertion of a suction tube to capture the marked receiver bees. This new sliding panel allowed us to move the opening in two dimensions. The capture of the marked hive bees lasted for 30–45 min. After capturing, focal bees were placed in a closed Perspex box (5 × 5 × 10 cm), and a gentle flow of CO2 was applied during 5 s through a small opening in the box. This ensured that all bees were anesthetized in the following 10 s and remained in this state for the next 120 s. While still anesthetized, bees were harnessed in plastic tubes that restrained the body movement but allowed free movement of the antennae and mouthparts (Bitterman et al. 1983). Bees were kept in the dark, within an incubator (25°C, 55% relative humidity) for 1 h before the responsiveness to sugar was measured.
Experimental device and procedure to capture nectar-receivers inside the hive to test their response thresholds to sucrose in a PER assay. The experimental hive with its sliding acrylic walls (a). The lower one was partly covered with a fine mesh that allowed painting the receivers’ thorax (black-marked bee) during the trophallaxis with either unknown donors (natural situation) or marked foragers (controlled situation; b). Afterwards, the mesh panel was replaced by an acrylic one with an opening in the center (as seen on the top half in a) that allowed the insertion of a suction tube to capture the marked receivers (c). The captured receiver bees can protrude their proboscis as a reflex response to antennal stimulation with a sugar solution in the PER paradigm (d). Modified from Farina et al. 2007
We also measured the sugar concentration of the crop contents of incoming forager mates. Before and after capturing receivers, one of two sliding doors (the closest to the entrance) of an acrylic box (5 × 5 × 10 cm) connected to the entrance was closed until a number of incoming foragers accumulated inside the box (5 min approximately). After this period, the other sliding door was also closed and the box with bees removed from the hive entrance. Approximately 20 bees were collected with this procedure. These bees were anesthetized with CO2, and their crop content was expelled by gently pressing the abdomen (Pankiw et al. 2004). The drop expelled was placed directly on a portable refractometer (Carl Zeiss, accuracy 0–85 ± 0.5%), which was used to determine the sucrose concentration. Only those bees with crop contents with a sugar concentration above 0 where considered as nectar foragers. Bees with crop contents equal to 0 were considered as water foragers, and those bees carrying pollen as pollen foragers. Water and pollen foragers were not taken into account. A daily average concentration was calculated using all these concentrations, and also a monthly average was calculated from the daily averages.
In the controlled situation, we used a second colony (C2) to measure the gustatory responsiveness of bees receiving food directly from foragers collecting a sugar solution at an artificial feeder outside the hive. A group of foragers was trained to collect either a 15% or a 50% weight/weight (w/w) unscented sugar solution at a small plate feeder (about 8-cm diameter), placed at a distance of about 30 cm from the hive entrance for about 90 min. During the training period, foragers were marked with a color spot on the thorax. After this period, the hive bees that received these solutions for at least 5 s from the color-marked foragers were marked with a second color. To mark the receiver bees, we used the same sliding acrylic wall described above. The capture of the marked hive bees lasted for 30–45 min. After capture, bees were anesthetized with CO2 and harnessed as mentioned above. These bees were also kept in the dark under controlled environmental conditions for 1 h before their sugar responses were measured.
Gustatory responsiveness: sucrose-response threshold assays
The reflex of proboscis extension after contacting the antennae with a sucrose solution was used to investigate a bee’s sensitivity to varying concentrations of sucrose. This protocol had been previously used in honeybees (e.g., Page et al. 1998; Pankiw and Page 1999). The lowest concentration at which an individual bee reflexively responds by extending her proboscis is interpreted as her sugar sensitivity. Before performing the assay, to avoid confounding effects of thirst, bees were offered water by allowing them to drink from a drop held in the tip of a toothpick. Bees were assayed using a concentration series of 0.1, 0.3, 1, 3, 10, and 30% w/w sucrose solution. All bees were lined up, and each was tested once, in sequential order, at each of the concentrations, e.g., all were tested at 0.1% first, then all were tested at 0.3%, and so on (Page et al. 1998). Bees were always tested with an ascending order of sucrose concentrations to reduce potential sensitization that can occur with higher concentrations of sucrose. Between each sucrose solution trial, all bees were tested for their response to water, and in the rare case that a bee responded to water, it was allowed to drink until satiated, and this did not affect the score. If this happened a second time, the individual was discarded. The interstimulus interval varied between 2 and 3 min depending on the number of individuals tested at one time, usually 15–20. The water assay served as a control on the potential effects of repeated sucrose stimulation that could lead to increased sensitization or habituation affecting subsequent responses.
We recorded the proportion of bees that responded to at least the 30% sucrose solution. We also obtained a PER score for each bee. This score is the number of concentrations in the series for which the bee extended her proboscis and correlates with the response threshold because animals normally respond to all concentrations above their threshold (Pankiw et al. 2004). This response was quantified from 1 to 6: a score of 1 represented a bee that only responded to the contact with the 30% sucrose solution, while a score of 6 represented one that responded to all six concentrations. It should be noted that most bees that responded to a low concentration also extended their probosces to the subsequent higher concentrations. If a bee failed to extend her proboscis at one concentration but did elicit a response at the immediate lower and higher concentrations, this “gap” was disregarded and the higher concentration registered. But if there was a “gap” of two or more concentrations, then the lower one was registered. Only bees that responded to a minimum of one concentration were included in the analysis; those that did not respond to any sugar concentration were not included in the analysis because they might have been harmed during the harnessing process or they could be satiated.
Statistical analysis
Because the assumptions of homogeneity of variances were not met, nonparametric analyses were used. For the natural situation, Spearman’s correlation of response score and sugar concentration carried by foragers was performed. For the controlled situation, Mann–Whitney U test was used to compare the PER scores between groups exposed to different sucrose concentrations (Zar 1999). Four hundred twenty-seven receivers responded at least to one sucrose concentration during the PER test and 398 foragers’ crops were analyzed during 27 experimental days for the natural situation. For the controlled situation, 85 receiver bees responded at least to one sucrose concentration during the PER test during 5 experimental days.
Results
During a period of almost 4 months, from late summer to the beginning of autumn, the mean PER score to sucrose of the honeybees receiving food in the delivery area as well as the mean sugar concentration carried by the incoming foragers fluctuated (Fig. 2). Both variables negatively correlated during the measured period, i.e., the correlation between the food receivers’ response score and the sugar concentration of incoming foragers was statistically significant (Spearman’s correlation test: ρ = −0.4848, n = 27, p = 0.010; Fig. 3). This suggests that the higher the sugar concentration carried to the hive, the higher the thresholds (i.e., lower scores) of the hive bees for extending their proboscis after contacting with a particular sugar solution.
Gustatory responsiveness of food-receiver hive bees and the sugar concentration of the incoming nectar to the hive. a PER score of hive bees receiving food at the delivery area (empty circles) and b sugar concentration of the foragers’ crop contents are daily represented during an experimental period of 4 months. Means (bold lines), medians, quartiles as vertical boxes, and ranges with bars are shown
In the natural situation, the bees were captured receiving food from the delivery area, a location where most of the bees offering are presumably foragers. However, it could be possible that not all the studied bees had received food from this kind of workers. Then, after the correlation was found, we performed an experiment to test directly the effect of the sugar solution received by trophallaxis over the PER scores of the hive bees. In this controlled situation, the PER scores of hive bees that actually received sugar solution from the incoming trained foragers decreased for the higher sugar concentration transferred (Mann–Whitney U test, U = 601.5, N = 85, p = 0.008; Fig. 4). Thus, a higher threshold for protruding probosces (lower PER scores) was found for bees receiving a higher sugar concentration from donor foragers.
PER score for the food-receiver bees tested when the donor foragers unloaded either a 15% or a 50% w/w sugar solution. Medians (filled circles), quartiles as vertical boxes, and ranges with bars are shown. Asterisks indicate statistical differences (p < 0.01; see “Results” for details). Median values: for 15%, 2, and for 50%, 2. Number of bees tested above bars
Discussion
Present results show that information regarding food quality modifies the response thresholds to sugar in those bees involved in nectar reception within the nest. Honeybees that received a low-concentrated sugar solution from incoming foragers showed higher PER scores (lower thresholds) compared to those that received high-concentrated solution (higher thresholds). These differences were found either when bee colonies collected food under natural conditions or under controlled ones. Regarding the natural situation, the correlation suggests that most of the bees that had offered food to the captured receivers were presumable foragers. Thus, after unloading food from incoming foragers, nectar receivers, which are those bees responsible for initiating the food distribution within the colony, adjust their response thresholds to sucrose according to the current incoming food quality. Then, this kind of information is indeed transferred via trophallaxis to first-order receivers because their behavior (i.e., their gustatory responsiveness within the PER paradigm) is modified. In this sense, this study complements previous related studies that showed the ability of honeybees to acquire food-odor information during trophallaxis by demonstrating that nectar receivers learned associatively the contingency between food and its odor through oral contacts (e.g., in experimental arenas: Gil and De Marco 2005; in hives: Farina et al. 2007). Thus, the role of trophallaxis as a mechanism to transfer chemosensory (olfactory and gustatory) information during foraging in honeybees is evident.
The strong link between the honeybees’ behavior and their ecology has been already described in several studies. The pioneering work of Lindauer (1948) showed how the threshold concentration of sucrose solution for dancing depends on changes in the bee’s environment. In addition, changes in sucrose response scores of young pre-foragers (3- to 6-day-old bees) have already been reported according to the sugar concentration offered to foragers (Pankiw et al. 2004). These young workers commonly perform nursing tasks within the hive (Rösch 1925; Lindauer 1952; Seeley 1982); they are not involved in foraging and have little direct contact with active foragers (Seeley 1995). The study of Pankiw et al. (2004) is, in this sense, one of the foraging-related examples suggesting a “global response” as a consequence of food-quality information propagation in honeybee colonies that potentially affects the behavior of most nestmates. Recently, global responses in relation to food-odor information have also been reported in honeybees, showing that the propagation of food-odor information affects later behaviors within the PER paradigm in hive bees of all ages (Grüter et al. 2006) as well as in a food-choice device outside the nest (Arenas et al. 2007). In the present paper, we focused on bees that were mostly middle-aged (i.e., approximately 14-day-old bees; Lindauer 1952; Seeley 1982) and performed nectar-processing within the hive, receiving food in the delivery area (Seeley 1989), and either unloading or concentrating it in areas more distant from the hive entrance (Park 1925; Lindauer 1954; Pírez and Farina 2004; Grüter and Farina 2007).
A key organizational principle of the honeybee food processing is that most hive bees are involved in only a few trophallaxes (e.g., recently emerged workers and nurse-aged bees), while some others are involved in many oral interactions (e.g., active and inactive foragers, nectar processors; Grüter and Farina 2007). This means that a rather small proportion of hive bees, the nectar receivers, have very high trophallactic rates and are particularly important for the flow of food and information within the colony (Pírez and Farina 2004; Goyret and Farina 2005; Grüter and Farina 2007). Certainly, the degree of this heterogeneity is dynamic and depends on the given foraging conditions (Naug and Smith 2007). The quality of food previously received by hive mates modulates their later appetitive response (i.e., whether or not the gustatory stimulus triggers the PER in hive bees). This fact, on the one hand, could affect the occurrence of food passage to the second-order receivers (Pírez and Farina 2004; Grüter and Farina 2007). On the other hand, it might affect the receivers’ decision making once they return to the delivery area to unload new nectar samples from incoming foragers.
The relationship between the incoming food quality and receiver sensitivity might work in a way that receivers with high thresholds refuse to unload foragers with low quality food but accept a broader range of food qualities with low thresholds. As foragers only collect food within a certain range of (high) concentrations when nectar sources are abundant in the hive surroundings (Lindauer 1948; Seeley 1995); receivers would only unload food that matches this narrow range of sugar concentrations. Contrarily, in low profitable food conditions, the range of sugar solutions processed inside the hive will be broader, facilitating the distribution of nectars of varying quality (Pankiw et al. 2004).
The captured hive bees most likely received solution during one interaction (although trophallaxes involving already marked receivers were observed occasionally). This possibility raises the question of how fast hive bees adjust their response thresholds. A rapid change in the response thresholds in these bees would lead to promote rapid social feedbacks affecting the food distribution dynamics at every moment.
We observed that nectar receivers did not present a sustained low response threshold during the 4-month observation period. Contrarily, they presented higher response thresholds when forager mates collected high-concentrated nectar sources, tuning their response threshold with the incoming food quality. Because gustatory responsiveness of pre-foraging bees are associated with foraging choices later in the bee’s life (Pankiw and Page 2000; Pankiw et al. 2004), it would be expected that hive bees that become pollen foragers should maintain low-response thresholds through their whole in-hive life. The lack of this bias in our focus bees opens the question whether the nectar receivers become nonpollen foragers later in their foraging life instead of pollen collectors.
In summary, honeybees can change their gustatory responsiveness after trophallaxis because their later behaviors are modified. Then, the occurrence of trophallaxis in the delivery area could not only be affected by prior olfactory experiences of the hive bees involved in food unloading, as described in previous studies, but also by gustatory ones. The food receivers play a significant role in regulating nectar distribution within honeybee colonies because the refusal or acceptance of nectar at this level of processing would have important consequences in foraging-related activities. This fact will be crucial both for the propagation of food information within the colony and for the operational coordination during foraging.
References
Arenas A, Fernández VM, Farina WM (2007) Floral odor learning within the hive affects honeybees' foraging decisions. Naturwissenschaften 94:218–222
Bitterman ME, Menzel R, Fietz A, Schafer S (1983) Classical-conditioning of proboscis extension in honeybees (Apis mellifera). J Comp Psychol 97:107–119
Farina WM, Wainselboim A (2001) Thermographic recordings show that honeybees may receive nectar from foragers even during short trophallactic contacts. Ins Sociaux 48:360–362
Farina WM, Grüter C, Acosta LE, Mc Cabe S (2007) Honeybees learn floral odors while receiving nectar from foragers within the hive. Naturwissenschaften 94:55–60
Gil M, De Marco RJ (2005) Olfactory learning by means of trophallaxis in Apis mellifera. J Exp Biol 208:671–680
Goyret J, Farina WM (2005) Non-random nectar unloading interactions between foragers and their receivers in the honeybee hive. Naturwissenschaften 92:440–443
Grüter C, Acosta LE, Farina WM (2006) Propagation of olfactory information within the honeybee hive. Behav Ecol Sociobiol 60:707–715
Grüter C, Farina WM (2007) Nectar distribution and its relation to food quality in honeybee (Apis mellifera) colonies. Ins Sociaux 54:87–94
Jeanne RL (1991) Polyethism. In: Ross KG, Matthews RW (eds) The social biology of wasps. Cornell University Press, Ithaca, New York, pp 389–425
Kuwabara M (1957) Bildung des bedingten Reflexes von Pavlovs Typus bei der Honigbiene, Apis mellifica. J Fac Science, Hokkaido University. Zoology 13:458–464
Lindauer M (1948) Über die Einwirkung von Durf-und Geschmacksstoffen sowie anderer Faktoren auf die Tänze der Bienen. Z vergl Physiol 31:348–412
Lindauer M (1952) Ein Beitrag zur Frage der Arbeitsteinlung im Bienenstaat. Z vergl Physiol 34:299–345
Lindauer M (1954) Temperaturregulierung und Wasserhaushalt im Bienenstaat. Z vergl Physiol 36:391–432
Michener CD (1974) The social behavior of bees. Harvard University Press, Cambridge (404 pp)
Naug D, Smith B (2007) Experimentally induced change in infectious period affects transmission dynamics in a social group. Proc R Soc B 274:61–65
Oettingen-Spielberg T (1949) Über das Wesen der Suchbiene. Z vergl Physiol 31:454–489
Page RE, Erber J, Fondrk MK (1998) The effect of genotype on response thresholds to sucrose and foraging behavior of honey bees (Apis mellifera L.). J Comp Physiol A 182:489–500
Pankiw T, Page RE (1999) The effect of genotype, age, and caste on response thresholds to sucrose and foraging behavior of honey bees. J Comp Physiol A 185:207–213
Pankiw T, Page RE (2000) Response thresholds to sucrose predict foraging division of labor in honey bees. Behav Ecol Sociobiol 47:265–267
Pankiw T, Waddington KD, Page RE (2001) Modulation of sucrose response thresholds in honey bees (Apis mellifera): influence of genotype, feeding and foraging experience. J Comp Physiol A 187:293–301
Pankiw T, Nelson M, Page RE, Fondrk MK (2004) The communal crop: modulation of sucrose response thresholds of pre-foraging honey bees with incoming nectar quality. Behav Ecol Sociobiol 55:286–292
Park W (1925) The storing and ripening of honey by honeybees. J Econ Entomol 18:405–410
Pirez N, Farina WM (2004) Nectar-receiver behavior in relation to the reward rate experienced by foraging honeybees. Behav Ecol Sociobiol 55:574–582
Ratnieks WLF, Anderson C (1999) Task partitioning in insect societies. Ins Sociaux 46:95–108
Robinson G (1992) Regulation of division of labor in insect societies. Annu Rev Entomol 37:637–665
Rösch GA (1925) Untersuchungen über die Arbeitsteilung im Bienenstaat. Z vergl Physiol 2:571–631
Seeley TD (1982) Adaptive significance of the age polyethism schedule in honeybee colonies. Behav Ecol Sociobiol 11:287–293
Seeley TD (1989) Social foraging in honey bees: how nectar foragers assess their colony's nutritional status. Behav Ecol Sociobiol 24:181–199
Seeley TD (1995) The wisdom of the hive: The social physiology of honey bee colonies. Harvard University Press, Cambridge, Massachusetts
von Frisch K (1967) The dance language and orientation of bees. Harvard University Press, Cambridge, Massachusetts
Wilson EO (1971) The insect societies. Belknap Press of Harvard University Press, Cambridge, Massachusetts
Zar JH (1999) Biostatistical analysis, 4th edn. Prentice Hall, New Jersey
Acknowledgment
We are indebted to M. Spivak (University of Minnesota) for suggesting to us to combine trophallaxis and SRT experiments. We are also indebted to C. Grüter for his valuable comments and suggestions on an early version of this manuscript. This study was supported by funds from CONICET, ANPCYT (01-12319) and University of Buenos Aires (X 036) to WMF. The present study complies with the current laws of the state country in which experiments were performed.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by M. Giurfa
Rights and permissions
About this article
Cite this article
Martinez, A., Farina, W.M. Honeybees modify gustatory responsiveness after receiving nectar from foragers within the hive. Behav Ecol Sociobiol 62, 529–535 (2008). https://doi.org/10.1007/s00265-007-0477-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00265-007-0477-0