Abstract
Sperm competition models predict that males should adjust their sperm expenditure according to the risk and/or intensity of sperm competition. In this paper, we analysed copulatory behaviour of both sexes and sperm expenditure in relation to female mating status (virgin or mated) in the freshwater crayfish Austropotamobius italicus, a species where males have been reported to feed on and remove sperm laid by other males. The same females were allowed to be inseminated sequentially by two males, and we compared the sexual behaviours of partners between the first (virgin females) and the second mating (mated females). We found that female resistance did not differ between the first and the second mating, nor males refused or took more time to mount a mated female. However, when mating with a mated female, males reached an effective copulation position significantly later. This occurred because second-mating males removed, by eating, all or most spermatophores previously deposited by first males. As removal was often incomplete, this resulted in a larger amount of sperm being deposited on female ventral parts after the second mating, although second males did not allocate more sperm to mated females than first males did. Thus, the peculiar mode of sperm competition, where males remove previously deposited sperm, and the consequent predictable strong last male prevalence in paternity likely led to the observed lack of adjustment of sperm expenditure to female mating status in this species.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In polyandrous species, where both sexes mate multiply, male fertilization success may often depend on the ability of his sperm to successfully compete with that of his rivals (Simmons and Siva-Jothy 1998; Simmons 2001). Sperm competition, which is defined as the post-copulatory competition between the sperms of two or more males to fertilize female eggs during a breeding season (Birkhead and Møller 1992, 1998; Parker 1998), results inevitably in an evolutionary arms race (Dawkins and Krebs 1979) between mechanisms of pre-emptive advantage in competition for fertilizations and counter-mechanisms aimed at preventing preemption (Parker 1984). Such arms races have produced a variety of morphological, behavioural and physiological adaptations enhancing the competitive advantage of a male’s sperm or countering the sperm of competitors (Smith 1984). The adaptations that arise in particular cases depend on several factors, e.g. the morphology of the female reproductive tract, the existence and form of sperm storage organs and the opportunity for multiple mating (Birkhead and Møller 1992). Recent models suggest that males should increase their ejaculate expenditure when experiencing a high risk of sperm competition (see Wedell and Cook 1999; Wedell et al. 2002). For example, the mating status (virgin or mated) of a polyandrous female may alter her reproductive value to a male, and males should be able to assess it and vary their reproductive behaviour accordingly, e.g. by increasing sperm number or displacing rival sperm when mating with a mated female. In most instances, virgin females are preferred as mates, as they offer a reduced probability of sperm competition and thus the highest immediate returns for male investment, even when first-male sperm precedence is low (Bonduriansky 2001; Simmons 2001; Engqvist and Reinhold 2006). However, the benefit of mating with a virgin female depends on the variation in female mating frequency and on the time lag between mating and oviposition (Lewis and Jutkiewicz 1998; Arnaud et al. 2001). High female mating frequency and long lags between insemination and egg spawning/fertilization may shift male preference towards mated females (Engqvist and Reinhold 2006).
For a female, the costs and benefits associated with mating may also vary with her reproductive status. For virgin females, the benefits of copulation are relatively clear; they acquire sperm necessary for egg fertilization. Similarly, the immediate fertilization gains from polyandry are well documented (Arnqvist and Nilsson 2000). Polyandrous females may also accrue genetic benefits from their mates (Jennions and Petrie 2000; Arnqvist and Nilsson 2000; Zeh and Zeh 2003; Radwan 2003) but may be required to offset their gains with the potential costs of mating multiply (Chapman et al. 1995; Clutton-Brock and Parker 1995). This potential cost–benefit trade-off may be sufficient to promote variation in female receptiveness depending on her current reproductive status (Kelly et al. 1998; Mair and Blackwell 1998).
In this study, we conducted pairing experiments using the freshwater crayfish Austropotamobius italicus (Faxon 1914; Crustacea: Astacidae) to study the consequences of female mating status on male and female copulatory behaviour, sperm expenditure and sperm competition. Astacid freshwater crayfish offer a valuable opportunity to study the effects of female mating status on the copulatory behaviour of both sexes, sperm allocation and sperm competition due to their promiscuous breeding system, which includes multiple mating, the absence of any form of mate guarding, the occurrence of sexual coercion and a peculiar modality of external fertilization and sperm competition (see below).
A. italicus is a long-lived (maximum life span of 10–13 years) crayfish native to Italy (Grandjean et al. 2000), which reproduces once a year in October–November (Matthews and Reynolds 1995). Males are larger, possess larger chelae than females and use them to secure and position females before and during copulation (Snedden 1990; Gherardi et al. 2000). Indeed, during sexual encounters, males, after brief exchanges of tactile and olfactory signals, suddenly grasp females using their claws, turn them on the back and release spermatophores, which are attached to the thoracic sternites of females, mainly on a specialized receptor, the spermatophoric plate (Acquistapace et al. 2002). Fertilization is therefore external, and spawning occurs within days to weeks from mating (Galeotti et al. 2006). Multiple mating by both sexes, and hence sperm competition, is documented either in the wild and in the laboratory in several crayfish genera including Austropotamobius (Berrill and Arsenault 1984; Woodlock and Reynolds 1988a,b; Hessen et al. 1989; Reynolds et al. 1992; Villanelli and Gherardi 1998; Reynolds 2002), but the consequences of such a behaviour on male and female fitness have rarely been investigated (Snedden 1990; Walker et al. 2002). In A. italicus (and in other Astacidae), the peculiar modality of sperm competition, with males feeding on spermatophores previously deposited by other males before releasing their own sperm (Reynolds et al. 1992; Villanelli and Gherardi 1998), may limit the potential benefits of multiple matings and mixed paternity on female fitness, likely leading to a strongly skewed last-male prevalence in paternity.
In particular, the aims of this study were: (1) to assess whether females mated sequentially to two males refuse or resist longer to the second than the first male’s advances (or, from a male point of view, if males take more time to seize mated females compared to virgin ones). If multiple mating is an adaptive female strategy and previous mating does not render females refractory to mating, we expected no differences in female resistance to mating between the first and the second pairing experiment; and (2) to assess if males vary their copulation behaviour and sperm expenditure when mating with a mated female. Concerning sperm expenditure, as males remove sperm of rivals, we could expect either no differences in sperm deposition between first and second males, or that males ejaculate more sperm when mating with a mated female, due to the perceived increased risk of sperm competition (Wedell et al. 2002) or the greater probability to sire most offspring in the resulting clutch.
Materials and methods
Subjects and housing conditions
Sexually mature A. italicus (carapace length (CL) >27 mm for females and >30 mm for males, Woodlock and Reynolds 1988b; Nardi et al. 2004) were collected from a stream in the northern Apennines (N. Italy) during September–October 2005 before the start of the breeding season (no females berrying spermatophores were ever collected at this time). Sexes were held separately under a natural light–dark cycle in opaque plastic jars (80 × 60 × 60 cm) filled with 150 l of recirculating filtered water (20 individuals/jar), provided with a gravel substratum and shelters, and fed with dry crayfish food twice a week. Water temperature ranged from 18°C in October to 14–12°C in November and December. Crayfish were uniquely marked with a colour number on the top of their carapace to allow individual identification. Carapace length and right and left chelae length were measured using a digital caliper (accuracy 0.01 mm); as a measure of chelae length, we used the maximum chela length. Individuals with one or both missing chelipeds were discarded. At the end of experiments, all crayfish were returned to the stream of origin.
Experimental design
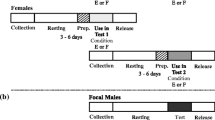
Starting from a stock of 80 males and 40 females, we collected behavioural and sperm data from 31 females mating twice in sequence with a different male (overall, 62 different males were thus used; nine females did not mate on their first trial and were discarded). Receptive females were randomly assigned to two virgin males of similar CL (absolute difference in CL <5% of the largest male) in sequence, with the only constraint that they had a CL similar or smaller than that of their male partners. In this way, we avoided pairing males with females that were larger than them to maximize mating probabilities (Woodlock and Reynolds 1988a). Males used in the experiment had therefore a mean CL of 37.8 mm (SD 4.24, range 30.3–44.7 mm, n = 62), with no differences between the first- and second-mating males (paired samples t test, t 30 = 0.34, P = 0.74). Females averaged 34.2 mm CL (SD 3.98, range 28.7–43.2 mm, n = 31), and the mean difference between male and female CL among copulating pairs was 3.6 mm (range −0.05–9.85 mm, n = 62). Receptive females were identified by the presence of whitish patches deriving from glair gland maturation along abdominal sternites.
Mating trials were conducted in the afternoon between 14 and 25 November. Ten minutes before a trial started, pair members were placed separately in a 15-l plastic aquarium, provided with a gravel substratum and an opaque plastic divider forming two habituation chambers. The divider was then removed and the animals were allowed to freely interact for 30 min. Pairs were observed under dim red light, and their behaviour was video taped with a Sony DCR-TRV25E digital camera using the nightshot function. If females were successfully inseminated by the first males, they were presented to the second males 10 min after the first mating trial was ended and left to freely interact until a second mating and insemination occurred. If second copulation did not occur on the same day, the trial was repeated on the following days by using a different male. Sixty-seven percent of females re-mated within the same afternoon, 20% the day after and 13% 2 days later.
After each insemination, we took a picture of the ventral parts of each female to estimate the sperm area and its variation after the second mating; females were photographed with a reference centimetre scale as background under standardized conditions (light, exposure and distance of the subject set constant for all pictures) by using a Canon PowerShot A85 digital camera, with a resolution of 5 Mp (1,704 × 2,272 pixels output images). After the first mating, we also drew the surface covered by spermatophores on the female ventral side on a standard realistic ventral outline printed on white paper, as in a previous study (see Rubolini et al. 2006 for details), to check if the two methods provided comparable estimates of sperm areas (see below). The picture of the sperm of a single female after a second mating was corrupted, so sample size for comparisons concerning sperm area was based on 30 females.
Sperm expenditure measure
As a measure of sperm expenditure, we calculated the area covered by spermatophores on the female ventral parts. Digital images of inseminated females were transferred to a PC and processed by the Adobe Photoshop CS2 image processing software. The total area covered by spermatophores was outlined with the Brush Tool facility (5 pixels diameter) on a new layer and measured in pixels using the Magic Wand Tool (tolerance 32). The reference centimetre scale was measured with the Measure Tool (in pixels) and the sperm area in pixels was appropriately transformed in cm2. The sperm area measures were highly repeatable both within and between the pictures of the same female (r = 1.0, F 9,10 = 4,969.2, P < 0.0001, and r = 0.99, F 9,10 = 2,376, P < 0.0001, respectively) and were strongly correlated to the sperm area index calculated from printed outlines of female ventral parts we used in a previous study (r = 0.87, n = 30, P < 0.0001; see Rubolini et al. 2006). Spermatophores, consisting of vermicular white filaments of variable length, were mostly deposited horizontally as a single layer on the female ventral side (on or around the female spermatophoric plate), with limited overlap, so the sperm area should represent a reliable index of sperm expenditure (see Rubolini et al. 2006). By accurate visual comparison of the pictures of female ventral parts taken after each mating, it was possible to distinguish and measure the sperm area referring to each male after the second mating (Fig. 1). In fact, females did not groom or otherwise alter spermatophore shape or position (Woodlock and Reynolds 1988a, personal observation), and males were not seen messing-up or displacing spermatophores as well, but simply removed sperm or not. Thus, we are confident that our estimates of each male’s contribution to total sperm area were correct.
Modalities of sperm competition in A. italicus (on the left, spermatophores visible after first mating by a given male; on the right, spermatophores visible after a second mating by a different male; see Materials and methods). a Complete replacement of spermatophores; b partial replacement; c addition of spermatophores. In b and c, areas encircled by black line represent spermatophores deposited by second mating males
Mating behaviour variables
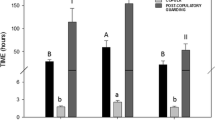
Video recordings of sexual interactions were analysed to measure the number of female flights, the latency to mounting, the interval between mounting and copulation, copulation duration and the number of ejaculations performed. For a detailed description of male and female copulatory behaviour, see Rubolini et al. (2006). Briefly, copulation is frontal; the male holds firmly the female chelipeds and usually stands over her with his gonopodia positioned over the female’s spermatophoric plate. Copulation does not start as soon as females are grasped and turned on their carapace by males, but some time elapses between mounting and the start of spermatophore deposition mainly because males are not in the correct position or females resist. We defined the start of copulation at the time when a male reached the correct copulating position with his copulating organs approximately coinciding with the female’s spermatophoric plate. Ejaculations (tail flips hereafter), consisting of rapid, sequential tail flips and simultaneous forward movements of the male’s body, could be clearly observed and counted on most video recordings. The number of tail flips and the duration of copulation both reliably predict the amount of spermatophores deposited by males during a mating (see Results and Rubolini et al. 2006). The same observer recorded all behavioural variables blind of mating treatments.
The latency to mounting and the number of female flights can both represent indexes of pre-mating female preference, as females may resist advances by undesired males by fleeing away; the time elapsed between female mounting and insemination by males could be considered a measure of difficulties faced by males in reaching the correct copulation position (including overcoming female resistance and removal of spermatophores previously deposited by another male).
Statistical analyses
We compared male and female mating behaviour variables and male sperm expenditure between the first and the second mating by using non-parametric (Wilcoxon) or parametric (t test) statistics for paired samples, depending on data distribution. Effect size estimates (Cohen’s d) for each test are also reported (Cohen 1992). Means are provided together with their associated standard errors, unless otherwise stated.
Results
The latency to female mounting did not differ between the first and second matings as well as the number of female flights, i.e. mated females did not resist longer nor were less attractive to second males in the mating sequence (Table 1). On the other hand, second males spent significantly more time between mounting and achieving an effective copulation than first males because the former were manipulating and feeding on spermatophores deposited by first males.
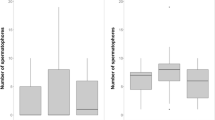
The sperm area laid by first males in the mating sequence did not differ from the sperm area deposited by second-mating males (Table 1), which is consistent with the similar number of tail flips and copulation duration of each male (Table 1). Actually, copulation duration and tail flips were both correlated with ejaculate size during either the first and the second mating (first mating: r s = 0.52, P = 0.002 and 0.51, P = 0.003, respectively; second mating: r s = 0.52, P = 0.004 and 0.75, P < 0.0001, n = 30 in all cases, see also Rubolini et al. 2006). The sperm area after the second mating increased by on average 30%, from 0.34 ± 0.02 to 0.44 ± 0.03 cm2 (t 29 = 3.34, P = 0.002, d = 1.24); therefore, given nearly equivalent ejaculate sizes, second males removed on average 77.2% of first male’s sperm (Fig. 2a), i.e. about 0.27 cm2. As a consequence, on average 84.7% of the total sperm area measured in the second picture derived from the second male in mating sequence (range 45.5–100%, Fig. 2b), thus suggesting a strong last-male skewed paternity. This pattern of sperm competition resulted in 63.3% of second males leaving intact some previously deposited spermatophores, 33.3% completely removing sperm by first males, and 3.3% simply adding their sperm without removing any previous spermatophore (see representative examples in Fig. 1). Apparently, there are no positional advantages to the placement of first male sperm (see also Fig. 1).
Discussion
Our experiment showed that male A. italicus were ready to mate with mated females as well as with virgin ones, and that mated females did not refuse or resist longer to re-mate than virgin ones. In addition, males modified their copulation behaviour when mating with a mated female by increasing the time needed to achieve an effective copulation position because they were feeding on sperm deposited by other males. However, although females mated twice carried 30% more sperm than females mated once, males did not increase their sperm expenditure (as well as copulation duration) when mating with mated females, as could have been expected based on sperm competition theory (see Wedell et al. 2002 for a review).
Therefore, a clearly perceived greater risk of sperm competition did not translate in a greater ejaculate investment by second males in the mating sequence, but both males produced similar ejaculates. This finding is not surprising, given the sperm removal behaviour of this species. Indeed, the case of equivalent sperm release by two males competing for fertilizing a female has been modelled theoretically by Parker (1998). This outcome is predicted to occur when: (1) there is a loaded raffle (i.e. a paternity bias towards one male due to sperm removal by second-mating males); (2) males have perfect information about their roles (first to mate, second to mate); and (3) males are randomly either in the favoured or disfavoured role. Mating patterns in this species and in our experimental setup appear to match all these conditions. Firstly, the peculiar mechanism of sperm competition in this species, where second mating resulted in about 77% removal of previously deposited spermatophores, predictably leads to a large last-male prevalence in realized paternity of resulting clutches. In fact, 84.7% of the sperm area measured on females after the second mating derived from the second male in the mating sequence, which monopolized females longer and also obtained the extra benefit of a proteic meal by feeding on the spermatophores of its rival. Secondly, male crayfish do obviously assess the mating status of females. Thirdly, males may randomly play the favoured or disfavoured role, at least in our experimental setting. As roles are random, the costs or benefits of deviations in sperm expenditure become exactly equal in each role, despite the loading in the raffle (Parker 1998). Thus, from the male point of view, it could be predicted that the best mating strategy to win sperm competition should be not to copulate with a virgin female or increase sperm allocation when mating with a mated female, but rather to copulate with a mated female as soon as possible before egg spawning, as males do not attempt to mate with spawning females anymore (Reynolds et al. 1992), and remove as much previously deposited sperm as possible.
An interesting and important finding emerging from our study is that females mated twice had a 30% greater sperm load than females mated once. This occurred because most second-mating males were unable to remove all the sperm deposited by previous males. If female crayfish are sperm limited (see Wedell et al. 2002), that is to say their egg fertilization success is limited by the amount of sperm released by their partners (see, e.g. MacDiarmid and Butler 1999), polyandrous females may greatly benefit in terms of egg fertilization success, provided that sperms of both males are equally fertilizing. This would add to the other potential benefits deriving from polyandry (see Jennions and Petrie 2000 for a review and other references in Introduction).
However, it should be considered that females also face some potential costs for polyandry in this mating system: for example, they may incur significant direct costs of re-mating due to sexual harassment by males, which may lead to limb losses or even killing of the female by carapace crushing particularly when males are much larger than females (personal observation; see also Woodlock and Reynolds 1988a).
In conclusion, our study indicates that A. italicus males are able to perceive female mating status and adjust their copulation behaviour accordingly by removing sperm previously deposited by other males. However, removal was incomplete in most instances, leading to the possibility of multiple paternity in this species, as observed in other freshwater crayfish (Walker et al. 2002), and leading to increased sperm loads in polyandrous females compared to monandrous ones. This modality of sperm competition, which should predictably result in a last-male biased paternity pattern in most instances, coupled with the male ability to assess female mating status, can lead to the observed similar sperm expenditure of first- and second-mating males.
References
Acquistapace P, Aquiloni L, Hazlett BA, Gherardi F (2002) Multimodal communication in crayfish: sex recognition during mate search by male Austropotamobius pallipes. Can J Zool 80:2041–2045
Arnaud L, Gage MJG, Haubruge E (2001) The dynamics of second and third male fertilization precedence in Tribolium castaneum. Entomol Exp Appl 99:55–64
Arnqvist G, Nilsson T (2000) The evolution of polyandry: multiple mating and female fitness in insects. Anim Behav 60:145–164
Berrill M, Arsenault MR (1984) The breeding behaviour of a northern temperate orconectid crayfish, Orconectes rusticus. Anim Behav 32:333–339
Birkhead TR, Møller AP (1992) Sperm competition in birds: evolutionary causes and consequences. Academic, London
Birkhead TR, Møller AP (eds) (1998) Sperm competition and sexual selection. Academic, London
Bonduriansky R (2001) The evolution of male mate choice in insects: a synthesis of ideas and evidence. Biol Rev 76:305–339
Chapman T, Liddle LF, Kalb JM, Wolfner MF, Partridge L (1995) Cost of mating in Drosophila melanogaster females is mediated by male accessory gland products. Nature 373:241–244
Clutton-Brock TH, Parker GA (1995) Sexual coercion in animal societies. Anim Behav 49:1345–1365
Cohen J (1992) A power primer. Psychol Bull 112:155–159
Dawkins R, Krebs JR (1979) Arms races between and within species. Proc R Soc Lond B 205:498–511
Engqvist L, Reinhold K (2006) Theoretical influence of female mating status and remating propensity on male sperm allocation patterns. J Evol Biol 19:1448–1458
Galeotti P, Rubolini D, Fea G, Ghia D, Nardi P, Gherardi F, Fasola M (2006) Female freshwater crayfish adjust egg and clutch size in relation to multiple male traits. Proc R Soc Lond B 273:1105–1110
Gherardi F, Acquistapace P, Barbaresi S (2000) The significance of chelae in the agonistic behaviour of the white-clawed crayfish Austropotamobius pallipes. Mar Freshw Behav Physiol 33:187–200
Grandjean F, Harris DJ, Souty-Grosset C, Crandall K (2000) Systematics of the European endangered crayfish species Austropotamobius pallipes (Decapoda: Astacidae). J Crustac Biol 20:522–529
Hessen DO, Agerberg A, Kjellberg G, Odelstrøm T, Westman K (1989) Food, nutrition, growth, reproduction and genetics. In: Skurdal J, Westman K, Bergan PI (eds) Crayfish culture in Europe. Report from the workshop on crayfish culture. Trondheim, Norway, pp 39–48
Jennions MD, Petrie M (2000) Why do females mate multiply? A review of the genetic benefits. Biol Rev 75:21–64
Kelly LS, Snell TW, Lonsdale DJ (1998) Chemical communication during mating of the harpacticoid Tigriopus japonicus. Philos Trans R Soc Lond B 353:737–744
Lewis SM, Jutkiewicz E (1998) Sperm precedence and sperm storage in multiply mated red flour beetles. Behav Ecol Sociobiol 43:365–369
MacDiarmid AB, Butler MJ (1999) Sperm economy and sperm limitation in spiny lobsters. Behav Ecol Sociobiol 64:14–24
Mair J, Blackwell A (1998) Effect of age and multiple mating on the mating behavior of Culicoides nubeculosus (Diptera: Ceratopogonidae). J Med Entomol 35:996–1001
Matthews MA, Reynolds JD (1995) A population study of the white-clawed crayfish Austropotamobius pallipes (Lereboullet) in an Irish reservoir. Biol Environ 95B:99–109
Nardi PA, Bernini F, Bo T, Bonardi A, Fea G, Ferrari S, Ghia D, Negri A, Razzetti E, Rossi S (2004) Il gambero di fiume nella Provincia di Alessandria. PI-ME Editrice, Pavia
Parker GA (1984) Sperm competition and the evolution of animal mating strategies. In: Smith RL (ed) Sperm competition and the evolution of animal mating systems. Academic, New York, pp 1–60
Parker GA (1998) Sperm competition and the evolution of ejaculates: towards a theory base. In: Birkhead TR, Møller AP (eds) Sperm competition and sexual selection. Academic, London, pp 307–337
Radwan J (2003) Male age, germline mutations and the benefits of polyandry. Ecol Lett 6:581–586
Reynolds JD (2002) Growth and reproduction. In: Holdich DM (ed) Biology of freshwater crayfish. Blackwell Science, Oxford, pp 152–191
Reynolds JD, Celada JD, Carral JM, Matthews MA (1992) Reproduction of astacid crayfish in captivity—current developments and implications for culture with special reference to Ireland and Spain. Invertbr Reprod Dev 22:253–266
Rubolini D, Galeotti P, Ferrari G, Spairani M, Bernini F, Fasola M (2006) Sperm allocation in relation to male traits, female size, and copulation behaviour in a freshwater crayfish species. Behav Ecol Sociobiol 60:212–219
Simmons LW (2001) Sperm competition and its evolutionary consequences in insects. Princeton University Press, Princeton
Simmons LW, Siva-Jothy MT (1998) Sperm competition in insects: mechanisms and the potential for sexual selection. In: Birkhead TR, Møller AP (eds) Sperm competition and sexual selection. Academic, London, pp 341–434
Smith RL (ed) (1984) Sperm competition and the evolution of animal mating systems. Academic, New York
Snedden WA (1990) Determinants of male mating success in the temperate crayfish Orconectes rusticus: chela size and sperm competition. Behaviour 115:100–113
Villanelli F, Gherardi F (1998) Breeding in the crayfish, Austropotamobius pallipes: mating patterns, mate choice and intermale competition. Freshw Biol 40:305–315
Walker D, Porter BA, Avise C (2002) Genetic parentage assessment in the crayfish Orconectes placidus, a high fecundity invertebrate with extended maternal brood care. Mol Ecol 11:2115–2122
Wedell N, Cook PA (1999) Butterflies tailor their ejaculate in response to sperm competition risk and intensity. Proc R Soc Lond B 266:1033–1039
Wedell N, Gage MJG, Parker GA (2002) Sperm competition, male prudence and sperm-limited females. Trends Ecol Evol 17:313–320
Woodlock B, Reynolds JD (1988a) Laboratory breeding studies of freshwater crayfish, Austropotamobius pallipes (Lereboullet). Freshw Biol 19:71–78
Woodlock B, Reynolds JD (1988b) Reproduction in an Irish lake population of the crayfish, Austropotamobius pallipes (Lereboullet). Freshw Biol 19:79–86
Zeh JA, Zeh DW (2003) Toward a new sexual selection paradigm: polyandry, conflict and incompatibility. Ethology 109:929–950
Acknowledgment
We are very grateful to S. Zaim, N. Mottini, M. Spairani, D. Ghia, G. Fea and F. Bernini for helping with the field and laboratory works. We also thank P. Backwell, M. Jennions and an anonymous referee for their constructive criticisms.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by P. Backwell
Rights and permissions
About this article
Cite this article
Galeotti, P., Pupin, F., Rubolini, D. et al. Effects of female mating status on copulation behaviour and sperm expenditure in the freshwater crayfish Austropotamobius italicus . Behav Ecol Sociobiol 61, 711–718 (2007). https://doi.org/10.1007/s00265-006-0301-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00265-006-0301-2