Abstract
Multiple mating by females is widespread and generates sperm competition among the ejaculates of rival males over fertilization. One way in which males can avoid or reduce sperm competition is by displacing or removing previous males’ sperm from female sperm stores. An apparent example of this occurs in the bushcricket Metaplastes ornatus. Males perform a specialised sperm removal behaviour (SRB), using their highly-derived subgenital plate, with which they remove sperm from the female’s spermatheca during the early phases of mating before transferring a spermatophore of their own. Here we investigated whether males strategically invest in SRB according to the amount of previously stored sperm present in females. Each male was tested twice, once with a female containing sperm (‘filled’ condition) and once with a female from whom most previously deposited sperm had recently been removed by another male (‘emptied’ condition). For comparison, a separate group of males was paired with virgin females. Males did not discriminate between non-virgin females in the ‘emptied’ or ‘filled’ conditions in terms of their investment in SRB, suggesting they may not able to perceive the amount of sperm present in the female’s spermatheca. By contrast, male investment in SRB was significantly reduced in pairings with virgin females, indicating that males are sensitive to some aspect of a female’s mating status. Our results thus suggest that males modulate SRB in response to female-mediated cues, possibly chemical cues left by previous males, which would not be present on virgin but would be on non-virgin females.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
When females mate with multiple males, sperm competition arises between the ejaculates of rival males over fertilization (Parker 1970, 1998). Considering that promiscuity in females is quite common throughout the animal kingdom (Parker et al. 2013), sperm competition can be expected to have shaped diverse traits of males, including morphological, physiological and behavioural features (Parker 1970, 1998). Indeed, many adaptations to engage in or avoid sperm competition have by now been described throughout various taxa (Birkhead and Møller 1998, Birkhead et al. 2008), including insects (Shuker and Simmons 2014). Since such adaptations to sperm competition can be costly in terms of time and energy (Scharf et al. 2013; Dewsbury 1982; Lehmann 2012), we can expect that males might prudently adjust their investment in them according to relevant cues of sperm competition risk and intensity and hence to their expected fitness returns on investment (Wedell et al. 2002). Indeed, there is now firm evidence indicating that males often do adjust their investment according to the actual costs and benefits (Scharf et al. 2013). Male flour beetles (Gnatocerus cornutus), for instance, adjust courtship and sperm allocation according to perceived risk and intensity of sperm competition (Lane et al. 2015) and male cuttlefish (Sepia lycidas) adjust their ejaculate allocation as well as sperm removal duration according to the females’ mating history, increasing investment in sperm removal when females have recently mated with a different partner (Wada et al. 2010). An increased sperm removal duration and ejaculation number per mating when the male itself was not the last male a female mated with was observed. In this study, we also focus on adjustment in sperm removal behaviour as a likely defensive adaptation to sperm competition.
In order to be able to adjust their behaviour strategically according to factors such as female mating status, males must of course be able to detect relevant cues (Thomas 2011; Wedell et al. 2002) such as the volume of stored sperm (Andrés and Cordero Rivera 2000) or chemical cues left by other males (Thomas and Simmons 2009). One example of sperm removal behaviour (hereafter ‘SRB’) has been described in our study species, the bushcricket Metaplastes ornatus. Whilst holding the female with their cerci during the early phase of mating, males of this species use a specialised organ, the subgenital plate, to remove previously deposited sperm from the female reproductive tract (von Helversen and von Helversen 1991, for a video recording of the behaviour, see Supplementary Material). During these “phase-I couplings”, the male performs the SRB, thrusting the plate back and forth several times into the female genital chamber, with bouts of thrusting interspersed with pauses during which the male and the female separate completely. The process typically lasts from 10 to 60 min. Only then, in the phase-II coupling, does the male release and attach his spermatophore to the female’s genital opening (von Helversen and von Helversen 1991). The spermatophore itself comprises a water- and protein-rich nuptial gift, the spermatophylax (Heller et al. 1998), and an ampulla filled with sperm; in total the spermatophore weighs on average 22% of the male body weight (von Helversen and von Helversen 1991).
Previously, von Helversen and von Helversen (1991) convincingly demonstrated that SRB in M. ornatus removes sperm from the female’s sperm storing organ, the spermatheca. They compared the amount of sperm present in spermathecae following one complete mating and following a coupling in which SRB was interrupted before the attachment of a new spermatophore. In the latter case, the amount of sperm was reduced by about 85%. The male behaviour probably induces the female to release stored sperm from the spermatheca. It can be concluded that, due to the sperm removal he performs, the male’s own relative proportion of sperm stored in the spermatheca is increased, presumably boosting his chance of fertilizing subsequent eggs. Given a filled spermatheca of the encountered female, performing SRB should therefore be advantageous for the male, especially keeping in mind the substantial costs of the nuptial gift, the spermatophore. On the other hand, possible costs of SRB itself also have to be considered, such as time and energy investment, e.g. a possibly higher risk of predation due to the prolonged duration of mating. Somewhat surprisingly, von Helversen and von Helversen (1991) found that males exhibit SRB even when encountering virgin females, but they did not investigate potential causes of variation in the amount of SRB performed with each female.
In this study, we asked whether M. ornatus males are able to detect the amount of stored sperm present in the spermathecae of females they encounter, and whether they strategically adjust the amount of SRB accordingly. We predicted that it should pay for males to invest more in SRB when the female spermatheca contains many stored sperm from previous matings, and tested this by measuring SRB in three different contexts: with field-caught adult females (‘filled’ sperm stores); with field-caught adult females that had recently had their amount of stored sperm substantially reduced by a different male (‘emptied’ sperm stores); and with virgin females guaranteed to have completely empty sperm stores.
Materials and Methods
Model Species and Animal Maintenance
M. ornatus is a bushcricket endemic to the southern Balkan Peninsula (Pavićević et al. 2014). There is no laboratory population established of this species, and all experiments were conducted from 31.05.2015 to 11.06.2015 at a field site in Paleokastro, central Greece. Adult male and female M. ornatus were collected from the field from an area of 100–200 m around N 38° 57.527, E 22° 1.257 throughout the experimental period. Female nymphs were collected on 31.05.2015, at higher elevation and therefore constituting a “younger” population, from an area 100–200 m around 38° N 59.269′, 21° E 54.193′.
During the experiment, a maximum of 10–14 individuals were kept together in transparent plastic cages (15 × 25 × 19 cm) before their assignment to the experimental conditions. Thereafter females were kept in groups according to their sperm storage status and males were kept individually in order to be able to track their identity. Testing was conducted in separate transparent testing cages (7 × 14 × 9 cm). Food in the form of local leaves (Judas tree, Cercis siliquastrum) was available ad libitum. Egg-laying substrate for females and enrichment in the form of branches was provided in the cages. During the day, the cages with the animals were placed outside in half-shade, and at night, the cages were placed inside a building. All experiments were conducted between 09:00 and 19:00 each day, and at the end of the experiment all animals were released back into the wild.
Experimental Design
In the first part of the experiment (Fig. 1a), adult males were paired to mate with field-caught adult females in one of two conditions: “F” females that had not mated since being collected (‘filled’ sperm stores) and “E” females that had been paired with a non-experimental male that was allowed to perform SRB prior to the experimental trial, but separated prior to spermatophore transfer (‘emptied’ sperm stores). We adopted a within-subjects experimental design, such that each focal male encountered females in both conditions (but each female was used only once), in a randomised order and with a gap of at least 3 days between trials (resulting in a total time in captivity for males of 8–10 days and for females 3–4 days). In order to identify individual focal males, they were kept isolated after the first test (in a 400 ml plastic cup, approximately 8 × 13 cm). Prepared females that had been paired with a non-experimental male were kept in cages (7 × 14 × 9 cm, maximum 3 animals per cage) and separated according to their condition (‘filled’ or ‘emptied’).
Summary of the experimental design. a Condition E = ‘emptied’ female, condition F = ‘filled’ female (see Methods for explanation); collection of females and males in the field, resting in plastic cages, preparation of females either testing for mating willingness (for condition F) or phase-I coupling (von Helversen and von Helversen 1991) from non-experimental males (for condition E); Randomised testing order of conditions. b Virgin females; collection of females and males in the field, resting and maturation in plastic cages
In the second part of the experiment (Fig. 1b), a second group of adult males was paired under otherwise identical conditions with a virgin female that had been separated from other males prior to sexual maturity and kept in captivity for 12 days, and was thus certain to contain no stored sperm at the time of the trial.
Preparation of Females with ‘Emptied’ and ‘Filled’ Sperm Stores
In preparation for the experimental mating, each female was paired together with a non-experimental male in a testing cage in order to generate one of the two female test conditions, ‘emptied’ (E) or ‘filled’ (F). For the ‘emptied’ condition males performed SRB (“phase-I coupling” according to von Helversen and von Helversen 1991), wherein females were allowed to mount the male, the males could grasp the female with their cerci and insert the subgenital plate into the female’s genital chamber, moving it back and forth. As soon as the male folded down its subgenital plate while interlocking with the female and started to produce the spermatophore (i.e., the beginning of “phase-II coupling”, according to von Helversen and von Helversen 1991), the animals were separated. It was shown previously that the phase-I coupling leads to a very substantially reduced amount of sperm in the females’ spermathecae (von Helversen and von Helversen 1991), hence the females in the ‘emptied’ condition could be considered as having only few sperm in their spermathecae. For the contrasting ‘filled’ condition, only the females’ disposition to mate was checked for by pairing them first with a non-experimental male; females were considered as willing to mate if they mounted the male, and the animals were separated immediately after mounting, i.e. before any phase-I couplings involving the insertion of the male’s sub-genital plate could be performed. To ascertain whether our assumption that these field-caught females would thus have previously stored sperm when paired with the focal male was justified, we dissected a sub-sample of eleven females collected at the same time and at the same location. In order to determine whether sperm were present in each female’s spermatheca, the spermathecae were removed and investigated under a microscope (Olympus BHB, 200× magnification) and all eleven were indeed found to contain stored sperm. The amount of sperm was not quantified as it is expected that once filled, the number of sperm in a spermatheca will not vary too much. Before new sperm are added, most of the previous sperm will be removed by SRB, and the amount of sperm actually used for fertilisation is probably negligible.
Observation of Sperm Removal Behaviour
During each experimental pairing, the focal male and the female were put together in a testing cage outdoors, but in half-shade to prevent overheating. During occasional heavy rain, experiments were conducted indoors. The animals were free to approach each other and the males were allowed to perform the phase-I and phase-II coupling under continuous observation. The exhibited SRB was quantified by recording three parameters: the ‘total number of bouts’, ‘duration of bouts’ and the ‘total number of thrusts’ (for definitions of behavioural traits see the ethogram, Supplementary Material Table 1). If the animals showed no coupling after the scheduled resting time of 3 days, the resting phase was extended for another day and the animals were tested again the next day. If necessary, the resting phase was extended by one additional day. In total 10 males were tested twice, with a female from the “emptied” and “filled” condition, in a counterbalanced order. A further 10 males were tested once with a virgin female.
Data Analysis
The recorded parameters for the ‘emptied’ and ‘filled’ tests were analysed using a paired t-test. The recorded parameters from the 10 ‘virgin’ tests were compared with the control group, which we defined as the first test of the focal males that were paired with non-virgins (consisting of 5 ‘emptied’ condition, and 5 ‘filled’ condition matings, to avoid pseudoreplication), using an unpaired t-test, except for the number of bouts which were compared with a Mann-Whitney Rank-sum test as they were not normally distributed. Due to time constraints in the field, and as explained above, the part of the experiment involving virgin females was conducted after the first part with non-virgins (i.e., once virgin females had matured), meaning data from the two groups being compared here were not collected at precisely the same time; the resulting unbalanced design for this specific comparison should thus be interpreted with appropriate caution. Influence of the time of day of the mating on the behavioural parameters was investigated in preliminary linear regression analyses and revealed no significant effects; time of day was therefore excluded from subsequent analysis. Plots were generated and analysis was conducted using SigmaPlot 10.0 (Systat Software, Inc.).
Results
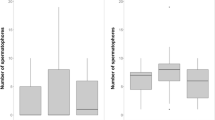
To test for strategic differential investment in ‘emptied’ versus ‘filled’ condition females, we measured three different aspects of male SRB: the number of bouts each focal male performed during phase I-coupling, the total number of thrusts in these bouts as well as the total duration of these bouts. There was considerable inter-male and inter-trial variability, but no consistent pattern of males increasing investment in SRB when paired with ‘filled’ females as we would have predicted: the mean of the total number of thrusts, the total duration of the bouts and the total number of bouts were all, on average, marginally higher in the ‘emptied’ condition, but none of these differences was significant (Fig. 2).
Individual performances for testing conditions F = ‘filled’ and E = ‘emptied’; Performances of each male are represented as single dots and joined by a line; N = 10 for both conditions. a Duration of bouts; ‘filled’ mean ± standard deviation (SD) = 19.8 ± 10.9 min; ‘emptied’ mean ± SD = 23.7 ± 12.9; ‘filled’ compared to ‘emptied’, paired t-test, t = −0.75, P = 0.471. b Number of bouts; ‘filled’ mean ± standard deviation (SD) = 10.0 ± 8.5; ‘emptied’ mean ± SD = 11.2 ± 7.1; ‘filled’ compared to ‘emptied’, paired t-test, t = −0.34, P = 0.735. c Number of thrusts; ‘filled’ mean ± standard deviation (SD) = 685 ± 484; ‘emptied’ mean ± SD = 834 ± 600; ‘filled’ compared to ‘emptied’, paired t-test, t = −0.5, P = 0.571
By contrast, there was clear evidence for differential investment in SRB when comparing male pairings with virgin versus non-virgin females. Compared to non-virgin controls, males paired to virgin females performed significantly fewer thrusts spread over significantly more bouts but of an overall significantly shorter total duration (Fig. 3).
Mean of performance for testing conditions control (data of first matings, 5 males tested with condition ‘filled’ and five males tested with condition ‘emptied’) and virgin; N = 10 for both conditions; Error bars are indicating the standard deviation (SD); a Duration of bouts: Control mean ± SD = 19.4 ± 9.8 min; Virgin mean ± SD = 8.9 ± 6.9 min; Control compared to virgin, unpaired t-test, t = 2.78, P = 0.012*. b Number of bouts: Control mean ± SD = 10.5 ± 8.7; Virgin mean ± SD = 18.9 ± 10.7; Control compared to virgin, unpaired t-test, U = 77.5 (Mann-Whitney Rank Sum Test), P = 0.041*. c Number of thrusts: Control mean ± SD = 654 ± 346; Virgin mean ± SD = 262 ± 185; Control compared to virgin, unpaired t-test, t = 3.1, P = 0.006**
Discussion
When investing in either avoidance of or engagement in sperm competition, males are expected to adjust their investment strategically according to the actual costs and benefits of that particular mating (Scharf et al. 2013). However, we found that male bushcrickets of the species M. ornatus do not respond differently towards “emptied” and “filled” non-virgin females, suggesting either that they cannot detect a difference between these females or that even after detecting a difference they do not discriminate between females on that basis. This is somewhat surprising, since the two conditions should clearly differ in the benefits of performing sperm removal. Considering that the sub-sample of collected adult females that were dissected all contained sperm and previous work has shown that SRB substantially reduces the amount of sperm present (von Helversen and von Helversen 1991), we can be confident that these two conditions clearly represent very different sperm competition scenarios. How then to reconcile our predictions and experimental results?
Firstly, it is possible that as sperm removal is not 100% effective (on average ca 85% reduction, von Helversen and von Helversen 1991), it may still pay for males to invest in this behaviour even if a previous male has already removed most sperm from the females’ spermathecae.
Secondly, males may not actually assess directly the amount of sperm present in the spermathecae of females, but instead rely on additional cues that were not manipulated by our treatment, as indeed was found in other insects. For example, male Australian field crickets (Teleogryllus oceanicus) are known to use information in cuticular hydrocarbons deposited on the female by previous mates to adjust sperm allocation (Thomas and Simmons 2009). Male flour beetles (Gnatocerus cornutus) (Lane et al. 2015) and male fruit flies (Drosophila melanogaster) (Friberg 2006) also assess the level of sperm competition indirectly by detecting cuticular hydrocarbons of other males on the female, not the actual mating status of the female itself. Something similar could be happening here: both ‘emptied’ and ‘filled’ females had been in recent contact with other males, and so both conditions might have been perceived as a high sperm competition situation irrespective of the actual numbers of sperm in storage. Given the high amount of body contact during the bouts of SRB, and the males often being seen to groom their subgenital plate between bouts, there is scope for the males to sense cuticular hydrocarbons or sperm with e.g. their antennae, mouthparts or sensilla on other body parts. Such an explanation – that males use chemical cues to judge sperm competition risk – would also be in line with our second experimental finding, i.e. the reduced investment by males in SRB with virgin compared to non-virgin females. Since the virgin females were collected as nymphs and had never had any contact to males in their adult life before the experimental mating, they would not be expected to exhibit any cues from previous males. Alternatively, if males actually assess the presence of previously deposited sperm directly during the early phase of mating, it may simply be that the detection of any sperm is sufficient to stimulate maximal investment in SRB, as would likely still have been the case in the “emptied” condition. By contrast, this behaviour can be quickly curtailed if no sperm at all are detected, as would have been the case in the virgin condition.
But why do males show SRB at all when paired with a virgin female? One potential explanation could be that SRB has one or several additional functions other than removing sperm. Possibilities here for example include assessing the condition of the female, assessing the number of sperm present in the female, or some form of mechanical stimulation of egg-production (e.g. Andrés and Cordero Rivera 2000). The increased number of bouts found in virgin pairings, compared to the non-virgin control pairings, might hint at an additional assessment function of SRB. In each bout males could gather information about the females’ condition until they get a reliable perception about competitors’ cues being present or not before attaching the spermatophore. Alternatively, or in addition to these courtship or assessment functions, a further likely reason for males to exhibit SRB even towards virgins could simply be the substantial paternity costs likely to be associated with a ‘wrong’ decision (i.e., not removing sperm of a rival that are actually present), which we could then think of as a ‘play it safe’ strategy by the male. It may be relevant here that males of this species are likely to be strongly constrained in their mating rate, when we bear in mind the substantial cost of spermatophore production (weighing on average 22% of the male’s body mass, meaning males must rest for about 3 to 4 days between matings; von Helversen and von Helversen 1991). So, even if it is not necessary in some cases, the occasional performance of ‘needless’ SRB may be preferable to the occasional inadvertent transfer without prior SRB of a spermatophore to a female containing competitor sperm. An analogous ‘play it safe’ strategy could similarly also help account for the lack of discrimination between ‘emptied’ and ‘filled’ females: if males are not able to accurately assess how many competitor sperm are present, better to err on the side of caution and perform thorough sperm removal anyway, since the potential paternity gains from doing so could on average outweigh the short-term time and energy costs.
Our combined results suggest that male M. ornatus bushcrickets can indeed modulate SRB in response to female-mediated cues. However, they only discriminate between virgin and non-virgin females, and not between the two non-virgin conditions. They might do this by detecting the presence, but not the amount, of sperm, or by using other indirect cues, such as chemical cues left by other males.
References
Andrés J, Cordero Rivera A (2000) Copulation duration and fertilization success in a damselfly: an example of cryptic female choice? Anim Behav 59:695–703. doi:10.1006/anbe.1999.1372
Birkhead TR, Møller AP (1998) Sperm competition and sexual selection. Academic Press
Birkhead TR, Hosken DJ, Pitnick SS (2008) Sperm biology: an evolutionary perspective. Academic Press
Dewsbury DA (1982) Ejaculate cost and male choice. Am Nat 119(5):601–610
Friberg U (2006) Male perception of female mating status: its effect on copulation duration, sperm defence and female fitness. Anim Behav 72(6):1259–1268
Heller KG, Faltin S, Fleischmann P, von Helversen O (1998) The chemical composition of the spermatophore in some species of phaneropterid bushcrickets (Orthoptera: Tettigonioidea). J Insect Physiol 44:1001–1008. doi:10.1016/S0022-1910(97)00171-6
Lane SM, Solino JH, Mitchell C, Blount JD, Okada K, Hunt J, House CM (2015) Rival male chemical cues evoke changes in male pre- and post-copulatory investment in a flour beetle. Behav Ecol 26:1021–1029. doi:10.1093/beheco/arv047
Lehmann GU (2012) Weighing costs and benefits of mating in bushcrickets (Insecta: Orthoptera: Tettigoniidae), with an emphasis on nuptial gifts, protandry and mate density. Front Zool 9(1):19. doi:10.1186/1742-9994-9-19
Parker GA (1970) Sperm competition and its evolutionary consequences in the insects. Biol Rev 45:525–567. doi:10.1111/j.1469-185X.1970.tb01176.x
Parker GA (1998) Sperm competition and the evolution of ejaculates: towards a theory base. In: Birkhead TR, Møller AP (eds) Sperm competition and sexual selection. Academic Press, London, p 3–54
Parker GA, Birkhead TR (2013). Polyandry: the history of a revolution. Phil Trans R Soc B 368(1613). doi:10.1098/rstb.2012.0335
Pavićević D, Ivković S, Horvat L (2014) New and rare species of orthopteroid insects in the fauna of Serbia. Fauna Balk 3:103–122
Scharf I, Peter F, Martin OY (2013) Reproductive trade-offs and direct costs for males in arthropods. Evol Biol 40:169–184. doi:10.1007/s11692-012-9213-4
Shuker DM, Simmons LW (2014) The evolution of insect mating systems. Oxford University Press
Thomas ML (2011) Detection of female mating status using chemical signals and cues. Biol Rev 86:1–13. doi:10.1111/j.1469-185X.2010.00130.x
Thomas ML, Simmons LW (2009) Male-derived cuticular hydrocarbons signal sperm competition intensity and affect ejaculate expenditure in crickets. Proc R Soc Lond [Biol] 276:383–388. doi:10.1098/rspb.2008.1206
von Helversen D, von Helversen O (1991) Pre-mating sperm removal in the bushcricket Metaplastes ornatus Ramme 1931 (Orthoptera, Tettigonoidea, Phaneropteridae). Behav Ecol Sociobiol 28:391–396. doi:10.1007/BF00164120
Wada T, Takegaki T, Mori T, Natsukari Y (2010) Sperm removal, ejaculation and their 391 behavioural interaction in male cuttlefish in response to female mating history. Anim Behav 79:613–619. doi:10.1016/j.anbehav.2009.12.004
Wedell N, Gage MJG, Parker GA (2002) Sperm competition, male prudence and sperm limited females. Trends Ecol Evol 17:313–320. doi:10.1016/S0169-5347(02)02533-8
Acknowledgements
We would like to thank Ralf Jochmann for providing video material of the animals and three anonymous reviewers for helpful comments on previous versions of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
ESM 1
Supplementary Material Table 1 (DOCX 27 kb)
(MP4 201147 kb)
Rights and permissions
About this article
Cite this article
Foraita, M., Lehfeldt, S., Reinhold, K. et al. Strategic Investment in Sperm Removal Behaviour in a Bushcricket. J Insect Behav 30, 170–179 (2017). https://doi.org/10.1007/s10905-017-9608-2
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10905-017-9608-2