Abstract
Lactation imposes substantial physiological costs on mothers and should therefore not be directed towards foreign offspring. Such allonursing, however, is common in mammal species that share roosts. Hypotheses to explain allonursing among such plural breeders include misdirected parental care, milk evacuation, brood parasitism, reciprocity, and kin selection. The necessary behavioral data, in combination with data on kinship and kin recognition, have rarely been available to distinguish among these explanations, however. In this study, we provide evidence for cooperative nursing and adoption by plural-breeding females in a nocturnal primate, the gray mouse lemur (Microcebus murinus), in which females forage solitarily during the night, but form day-time sleeping groups with one to two other females. We observed 34 resident females in an 8 ha study area in Kirindy Forest, Madagascar, over three consecutive annual breeding seasons and determined genetic relationships among all members of this population. Five sleeping groups of adult females were filmed inside their roosts during one breeding season after females gave birth. The composition of groups changed substantially across years, but they always consisted of close maternal relatives. All females within a group gave birth to one to three infants. They regularly transferred only their own offspring among roosting sites, demonstrating an ability to discriminate between their own and other’s offspring, but they regularly groomed and nursed related offspring other than their own and adopted related dependent young after their mother’s death. Kin selection may therefore be the main selective force behind cooperative breeding among these closely related females with a high mortality risk, providing each of them with family insurance.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Cooperative breeding, the care of offspring other than the own, occurs in several mammalian species (reviewed by Packer et al. 1992; see also, König 1993; Lunn et al. 2000). Most studies on cooperative breeding in mammals have focused on singular breeders where only a single female reproduces and where individuals that engage in care of foreign offspring are nonbreeding helpers (Solomon and French 1997; Koenig and Dickinson 2004). Cooperative plural breeding, when several females breed together and invest in foreign offspring, is rare and has been most frequently reported for species that are organized into family-structured societies (Emlen 1995; Solomon and French 1997). The differences in the causes and consequences of helping in singular breeders and cooperation in plural breeders are considerable: Helpers forfeit their own direct reproductive success but may increase indirect fitness, if the receiver of care is related, and/or may increase their chance of future successful reproduction. Cooperative plural breeders, in contrast, may increase their own direct fitness if cooperation is mutualistic and/or increase their indirect fitness when breeding with relatives (Pen and Weissing 2000; Kokko et al. 2001; Clutton-Brock 2002).
Explanations of cooperative breeding in plural breeders must reveal why they live and breed in groups, and why they help (König 1997; Hayes 2000). Whether grouping occurs due to ecological constraints (Emlen 1982; Getz et al. 1992), whether benefits of grouping apply primarily to adults in the group, or whether grouping is particularly advantageous to raising dependent offspring can be distinguished through: (1) Changes in group composition when additional suitable nests are provided, (2) group composition depending on the presence of dependent offspring, and (3) alloparental care depending on groups size and genetic relationship (Emlen 1995; Lewis and Pusey 1997). Hypotheses to explain allonursing among plural breeders include (1) misdirected parental care, when mothers cannot discriminate kin, (2) milk evacuation, where mothers allonurse to evacuate surplus milk that their own offspring did not consume, (3) brood parasitism, where offspring steal milk, (4) reciprocity, where two females achieve a higher fitness when nursing each other’s offspring to a similar extent than when they do not share milk, (5) kin selection, where a mother nurses alien offspring only if they share genes by common descent allowing her to spread those genes in her population, and (6) genetic imprinting, where males of polygynous species benefit from indiscriminate nursing of all their offspring, which may have selected for paternally expressed genes that suppress kin recognition during lactation (reviewed by Roulin 2002; see also, Roulin and Hager 2003). Information about mothers’ ability to discriminate kin (as defined by Nakagawa and Waas 2004) is crucial for distinguishing among these hypotheses.
These hypotheses give rise to several predictions: If females prefer to raise their young alone (examples see, Lewis and Pusey 1997) but suitable nests for doing so are limited, female group size should decrease and more females should breed on their own when additional nesting opportunities become available. When females form groups for social reasons but cannot discriminate their kin, groups may contain nonrelatives. In this case, any cooperative behavior should be mutual, and allonursing can be due to either misdirected maternal care or brood parasitism. If females are able to discriminate kin, however, only closely related females should form groups in which efforts for costly care of foreign young depend on relatedness (Emlen 1995; Silk 2002).
We tested these predictions for a basal primate, the gray mouse lemur (Microcebus murinus) from Madagascar. Cooperative breeding is known to occur in several group living primate species and includes various allomothering behaviors, such as allogrooming and infant carrying, allonursing, and even adoption (e.g., Thierry and Anderson 1986; Stanford 1992; Gibson et al. 1993). It is not known, however, whether cooperative breeding also occurs among solitary foragers, which comprise more than a quarter of all primate species (Bearder 1999) [It should be emphasized that “solitary” denotes the opposite of “gregarious” with respect to foraging, but not of “social” (Charles-Dominique 1977; Bearder 1987).]. Several features of gray mouse lemur socioecology suggest that the conditions for cooperative breeding among plural breeders may exist in this species (socioecology of gray mouse lemurs reviewed by, Kappeler 2000; see also, Eberle and Kappeler 2002; Radespiel et al. 2003; Eberle and Kappeler 2004a,b). At night, individuals forage solitarily for insects, fruit, and gum, but their home ranges overlap extensively between and within sexes. During the day, they rest in hollow trees. During austral winter, they form mixed-sex sleeping groups, containing up to 15 animals, and most females hibernate for several months, whereas most males remain active. During the rest of the year, the sexes usually separate and females form sleeping groups of two to five individuals, whereas most males sleep alone. Reproduction is highly seasonal, with matings being limited annually to a 4-week period. After 2 months of gestation, females give birth to one to three altricial young. Females remain in the natal area, whereas most males disperse.
We compiled a precise genetic and demographic database of a wild population of M. murinus to determine genetic relationships among females that form sleeping groups, to discover whether they all reproduce, whether they raise their young together, and whether they cooperate in doing so. These data allow us to characterize cooperative breeding for the first time in a basal primate, as well as to distinguish among hypotheses that explain this phenomenon in mammals.
Materials and methods
Demographic and genetic data
Trapping
We regularly captured 505 mouse lemurs inhabiting a 30-ha study area in the dry deciduous Kirindy Forest in Western Madagascar between 1994 and 2005. The study area is equipped with a rectangular system of foot trails at 25-m intervals. To trap mouse lemurs, we baited Sherman live traps with small pieces of banana and set them near trail intersections in the late afternoon on three consecutive nights per month. Captures were conducted each month in a 9-ha central study area (160 trap locations) between March and December, and within the entire 30-ha area (492 trap locations) in April and November since 1999. “Central study area” does not derive from a certain population structure but merely from the history of the coverage of our study area.
Captured animals were collected in the early morning, individually marked with subdermal transponders (or reidentified in case of recaptures), subjected to standard morphometric measurements, and released at the site of capture in the following late afternoon. Tissue samples for genetic analyses were taken from all captured animals in the form of small (2–3 mm2) ear biopsies during brief anesthesia induced by applying 0.01 ml Ketanest 100 (Rensing 1999) subdermally. Zygomatic arch breadth was measured as a proxy for body size. We equipped lactating females with radio-collars, so that we could determine their sleeping sites during the day, and intensified trapping locally around these sleeping sites to mark as many juveniles per generation as possible.
Genetic analyses
We determined genetic relationships among all 313 individuals living in the 30-ha study area between 1999 and 2001, and 63 of the remaining 192 individuals living in the same area between 1994 and 1998. DNA was isolated from ear biopsies following standard protocols (Qiagen QIAmp DNA Mini Kit No. 51306). For microsatellite analyses, we used 17 loci with an average of 17 alleles (Wimmer 2000; Hapke et al. 2003). The probability of genotype identity using these loci was 4.3×10−21 for a pair of randomly drawn animals and 2.9×10−8 for full sibs (Paetkau and Strobeck 1994; Taberlet and Luikart 1999). Parentage analysis on the basis of combined mismatch and likelihood analysis was performed with Cervus 2.0 (Marshall et al. 1998). Candidate parents were excluded through at least two homozygous mismatches or one heterozygous mismatch. The likelihood analysis for nonexcluded candidates was based on detailed parentage simulation (100,000 runs, 70 candidate parents, assumptions: 0.95 sampling rate, 0.92 average loci typing rate, 0.01 error rate, one close relative of the true parent among the other candidate parents) to estimate the resolving power of all loci and to estimate critical values to evaluate the parentage analysis statistically. The mean probability of nonexclusion (first parent) was 2.2×10−6. We characterized relatedness within dyads according to matrilines, although most litters had mixed paternities and male mating partners changed across years (Eberle and Kappeler 2004a,b). Most siblings, for example, were therefore half siblings.
Observations
Twenty-six, 27, and 25 adult females were present in the central 9-ha observation area during three consecutive lactation seasons from January to March in 1999, 2000, and 2001, respectively. During these three seasons, we equipped 16, 19, and 18 of these females with radio collars and determined the composition of their daytime sleeping groups with the help of a transponder-reading device between four and seven times per week. Females were considered as forming a day-time group when they spent more than 50% of the days together during a given lactation season. During lactation season, between January and March 2000, we made 233 h of focal-animal observations of 17 radio-tagged individual females. Documentation of reproductive activity was based on genetic data and on observations of pregnancy or active teats during trapping and on observations of matings or mating plugs during mating seasons (for further details of mating behavior, see Eberle and Kappeler 2004a,b).
Between December 1999 and March 2000, we filmed groups of females and their dependent young in artificial nest boxes. We offered 15 of these nest boxes at 1–2 m height within the 9-ha observation area. These were prepared from hollow dead trees, 30–35 cm high, with an inner diameter of 10–15 cm, 5–10 cm of wall thickness, with a wooden lid and bottom and a small lateral entrance at the top. The lid could be equipped with an infrared video camera and a video system that recorded the scene only when an animal moved or, during periods without movements in the nest, every minute for 6 s. We filmed five groups of females, comprising 12 females in total, and including three pairs and two groups of three (Table 1), for a total of 410 h during 44 days (5–12 days, median 8). We filmed a single nest box at a time and switched among nest boxes at an interval of 2–5 days. The five groups were filmed approximately 3 h during daytime (15–36 h, median 24) and 6 h during the first half of the night (30–72 h, median 48).
During video analyses, we identified individuals with the help of marked radio collars (adults), the markings in their ears from taking small tissue biopsies for DNA-analyses (adults and pups), and, when litter age differed sufficiently, different body size (pups). We did not use size, color, or earmarks of the pups to determine age or kinship during observations, but only to identify and follow an individual when analyzing videotapes. Kinship was later determined genetically. We recorded the duration of the following behavioral elements: Nursing: pup’s mouth is in contact with a female’s teat for a minimum of 30 sec; allogrooming: licking a conspecific at least three times, first three times must occur within 3 s, recording reset at intervals longer than 5 s. To identify differences in the mothers’ grooming and nursing efforts for own and foreign offspring, we compared the time they spent for grooming and nursing own vs foreign offspring.
Results
Kinship, social history, reproductive skew, and mortality
We could perform precise parentage analysis for each year and each parent separately by exact mismatch analysis alone. Seventy-two of 74 females captured in the central study area since 1999 could be assigned unambiguously to one of 12 matrilineal pedigrees that trace back to 12 females born between 1994 and 1997. One of the 74 females came from a family in the surrounding trapping area; the mother could not be determined for the 74th female (for details, see Eberle and Kappeler 2004a,b).
Across years, the composition of breeding groups was modified by births, deaths, splits, and mergers but the 33 females monitored during lactation seasons from 1999–2002 formed a total of eight distinct breeding group clusters whereas individuals from different matrilineal clusters never formed groups (Fig. 1). During the rearing season, all females that had close maternal relatives in the area formed groups and they did so exclusively with these relatives. Females that bred alone (one female each during the breeding seasons in 1999 and 2001, Fig. 1) had no close maternal relatives in the area during these periods. Family groups mostly consisted of maternal first- or second-degree relatives: 76% of the 51 dyads in these groups were formed by a mother and her daughter or by sisters (n=28 mother daughter dyads, 11 sister dyads), 20% by grandmothers and granddaughters or aunts and nieces (n=7+3 dyads), and the remaining 4% by cousins (n=2 dyads). The eight clusters corresponded to eight of the 12 matrilineal pedigrees of the central study area, i.e., they represented distinct matrilineal families (Emlen 1995).
Social history of 33 adult females during three consecutive annual lactation seasons. Individual females are continued vertically, surviving female descendants are linked with their mothers by diagonal lines. Females that did not survive to the next breeding season are marked by a cross. Females that formed a sleeping group are framed. Females formed eight distinct sleeping clusters (I–VIII) that corresponded with families
The 33 monitored females had 64 opportunities to reproduce during the three breeding seasons. Females reproduced in 62 out of 64 cases; we lacked information concerning the reproductive activity of the remaining two females. All 12 filmed females gave birth, with an average of 2.0 young (range: 1–3) (Table 1). The pups spent the first 3 to 4 weeks of their life in the maternal nest and started foraging on their own at an age of 8 to 10 weeks. Nursing was observed until an age of 6 weeks; one infant was observed attempting to nurse at an age of 12 weeks. Three females of the 12 filmed in 2000 died during breeding and infant mortality before weaning age was 58% (14 out of 24) in the filmed groups in 2000.
Nest use
An individual female used an average of six different sleeping trees (median; range 1–18, from 1999 to 2001 during nonhibernation periods, n=26 females that could be localized in sleeping trees more than 10 times over a period of at least 6 months). Most females (24/26) temporarily used artificial nest boxes in 2000 and 2001 (median 2.0 nest boxes, range 1–4). The average size of groups of adult females did not change across years, from 1999, when no artificial nest boxes were provided, to 2000 and 2001, when artificial nest boxes were available (means 1999–2001 2.26/2.23/2.29 females, SD 0.16/0.32/0.21, n=27/41/29 days, ANOVA F=(2, 94), p=0.63). During the 2000 lactation season, 18 out of 25 females used artificial nest boxes for between 3 days and 16 weeks, 15 females utilized a single box, and three females utilized two boxes. When changing nests, the entire breeding group moved. Males also spent the day in artificial nest boxes but mostly alone and never with females.
Before weaning, two of the filmed families used only a single nest box while three families moved as a whole between two and three nests. Two of these families used two nest boxes; one used one nest box and two natural nests. Females with dependent young aged 1–6 weeks spent on average 1.1 h in the nest with their young during the first half of the night (median; range 0.0–4.1, n=12 filmed females). During most of this time, only one female was present in the nest. Analyses as to whether the length of the females’ foraging period, i.e., the time span of offspring starvation, depended on the number of mothers in a nest were not possible because of the small number of nests that could be filmed and the fact that no female bred on her own when we filmed nests in 2000. Males never entered a nest box with breeding families.
Infant care
Females carried dependent offspring up to the age of 6 weeks, either when they changed nests or when they parked offspring in the vegetation during foraging. We observed seven cases of infant carrying. We could identify the carried young in five cases, for five different females (Table 2). All these females carried their own young exclusively, with low probabilities of selecting exclusively own young by chance (Table 2).
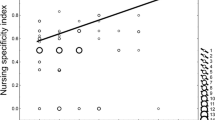
Females groomed and nursed foreign young (Fig. 2). Nine of 12 filmed females nursed and groomed at least one offspring from each foreign litter in the nest. Females expended more care towards their own young both during the night, when only a single female was in the nest, and during the day, when all females were present (Table 3). Although own young were always preferred, the proportion of nursing foreign young increased when only one mother was in the nest, compared to when all mothers were present (Table 3). Nursing was always initiated by the pups and their attempts to suckle were never repelled by either their own or a foreign mother. The proportion of grooming foreign vs own young did not change when only a single mother was in the nest, compared to when all mothers were present (Table 3).
One female died in each of three filmed groups when the infants were 5–6 weeks old. One of these groups left the nest box shortly before the female’s death, and the remaining two groups did so shortly after the mothers’ death so that they could no longer be filmed. However, five of six of the young of these females survived to an age of at least 12 weeks.
Discussion
This study revealed that closely related female gray mouse lemurs form breeding groups and breed cooperatively. All females in a group reproduce and, despite their ability to discriminate kin, they engage in care of offspring other than their own. Below, we discuss predictions of the various hypotheses proposed to explain breeding in groups and of cooperation in this solitarily foraging primate.
First, breeding in groups may be necessitated by a shortage of suitable roosts. It is difficult to assess whether suitable sleeping roosts are limited for female gray mouse lemurs. Outside the breeding season, males use more sleeping sites than females (unpublished data). Sex-specific nest use with females using fewer but higher quality roosts than males has been described in other forests, indicating that suitable roosts are limited but monopolized by females (Radespiel et al. 1998). In our study, only a fraction of available natural and artificial nests were used at a time. The fact that females bred and moved in groups although additional suitable nests were available suggests that grouping during breeding is not the result of limited availability of suitable roost.
Second, advantages of communal nest defense could also drive resting and breeding in groups. Detailed data on nest defense are not available for gray mouse lemurs but hollow trees that were regularly used for daytime resting were temporarily occupied also by other species such as greater hedgehog tenrecs (Setifer setosus), collared iquanids (Oplurus cuvieri), and Malagasy tree boas (Sanzinia madagascariensis), suggesting that efficient nest defense is important and, thus, partly responsible for grouping.
Third, thermoregulatory benefits may explain why related and unrelated individuals of both sexes share the same nest in much larger groups during hibernation (Schmid 1998, Eberle and Kappeler unpublished data). However, roost sharing in small groups during the hot and humid breeding season exclusively with closely related females, despite shared range use among unrelated females, suggests that advantages related to rearing offspring with kin, rather than thermoregulatory benefits drive cooperative breeding in this species.
Fourth, kin discrimination is a crucial factor for distinguishing among the remaining hypotheses. Female gray mouse lemurs can discriminate kin, as indicated by their grouping pattern during breeding seasons and by the selective carrying of own offspring despite low probabilities of drawing own offspring from the nest only by chance. Moreover, mothers groomed own offspring more often, and pups suckled more often at their own mother. Finally, in four cases, sisters or an aunt and her niece formed a group, indicating that they can also recognize relatives other than mother and offspring. Misdirected parental care (Roulin 2002) is therefore unlikely to explain allonursing in this species. Despite their discrimination ability, mothers never repelled foreign offspring and exercised costly allonursing. It is improbable that repelling a few small foreign pups is more costly than allonursing (König et al. 1988; Clutton-Brock et al. 1989; Packer et al. 1992). Therefore, brood parasitism, i.e., milk theft by pups (Roulin 2002), can also be excluded as a viable explanation. Milk evacuation, when mothers allonurse to evacuate surplus milk that their own offspring did not consume (Roulin 2002), is also unlikely to play a role because allonursing was basically reciprocal. Furthermore, the genetic imprinting hypothesis (Roulin and Hager 2003) does not apply in this species because male mouse lemurs mate with several females (in captivity: Radespiel et al. 2002; Andrès et al. 2003; in the wild: Eberle and Kappeler 2004a,b), but females discriminate kin. Allonursing was therefore not due to misdirected parental care, brood parasitism, milk evacuation, or genetic imprinting.
Finally, cooperative breeding may be based on cooperation among breeding females. Our data on group composition and possible cases of adoption suggest that this hypothesis best explains cooperative breeding in gray mouse lemurs. Allonursing provides the necessary opportunity for adoption, and adoption of closely related orphans increases indirect fitness (Lyon and Eadie 2000; Griffin and West 2002; Clutton-Brock 2002; West et al. 2002). Moreover, adoption might be particularly beneficial in species with high mortality rates (e.g., Field et al. 2000). Infant and adult mortality is very high in gray mouse lemurs (Eberle, unpublished), most likely due to predation (Goodman et al. 1993; Rasoloarison et al. 1995). Weaning age is about 7 weeks and adult dentition is reached at an age of 12 weeks (Perret 1992). In each of the three filmed groups, where a female died during breeding, their infants were not yet weaned. While the possibility cannot be excluded that the older orphans weaned themselves early, the younger ones were not active outside the nest on their own when their mother disappeared. When females arrived at their nest at dawn they emitted characteristic calls, possibly to facilitate regrouping after solitary activity (Pagès-Feuillade 1988; for Microcebus ravelobensis, see Braune et al. 2005). Two families with orphans changed the nest after a mother’s death when dependent orphans were older than 6 weeks and already active outside the nest. These relatively old orphans may have followed their family on their own, guided by regrouping calls. It remains to be determined, however, whether adopting mothers carry younger related orphans to a new nest.
The fact that allonursing occurs among relatives is not in itself sufficient evidence that kin selection is responsible for cooperation (Griffin and West 2002). Moreover, competition among relatives can reduce benefits of altruism towards relatives (West et al. 2002). To determine the exact costs and benefits of cooperative breeding, data on lifetime reproductive success, combined with long-term behavioral data need to be analyzed using Hamilton’s rule (Hamilton 1964; Heinsohn and Legge 1999; West et al. 2002). In several species, pups of communal nests have a higher body mass (Clutton-Brock 1991), and in gray mouse lemurs, heavier offspring face lower mortality during their first year of life (Eberle and Kappeler 2004a). It remains to be determined whether body mass or survival of pups and mothers differ between mothers breeding alone or in groups. The main results of our study—exclusive breeding with close relatives, ability to discriminate kin, and allonursing—indicate that kin selection, rather than any of the other proposed processes, is the main evolutionary force behind cooperative breeding in this basal primate, providing each female with a family insurance in the face of high mortality risk.
References
Andrès M, Solignac M, Perret M (2003) Mating system in mouse lemurs: theories and facts, using analysis of paternity. Folia Primatol 74:355–366
Bearder SK (1987) Lorises, bushbabies, and tarsiers: diverse societies in solitary foragers. In: Smuts BB, Cheney DL, Seyfarth RM, Wrangham RW, Struhsaker TT (eds) Primate societies. University of Chicago Press, Chicago, pp 11–24
Bearder SK (1999) Physical and social diversity among nocturnal primates: a new view based on long term research. Primates 40:267–282
Braune P, Schmidt S, Zimmermann E (2005) Spacing and group coordination in a nocturnal primate, the golden brown mouse lemur (Microcebus ravelobensis): the role of olfactory and acoustic signals. Behav Ecol Sociobiol 58:587–596
Charles-Dominique P (1977) Ecology and behaviour of nocturnal primates. Columbia University Press, New York
Clutton-Brock TH (1991) The evolution of parental care. Princeton University Press, Princeton
Clutton-Brock TH (2002) Breeding together: kin selection and mutualism in cooperative vertebrates. Science 296:69–72
Clutton-Brock TH, Albon SD, Guiness FE (1989) Fitness costs of gestation and lactation in wild mammals. Nature 337:260–262
Eberle M, Kappeler PM (2002) Mouse lemurs in space and time: a test of the socioecological model. Behav Ecol Sociobiol 51:131–139
Eberle M, Kappeler PM (2004a) Selected polyandry: female choice and inter-sexual conflict in a small nocturnal solitary primate (Microcebus murinus). Behav Ecol Sociobiol 27:91–100
Eberle M, Kappeler PM (2004b) Sex in the dark: determinants and consequences of mixed male mating tactics in Microcebus murinus, a small solitary nocturnal primate. Behav Ecol Sociobiol 27:77–90
Emlen ST (1982) The evolution of helping. I. An ecological constraints model. Am Nat 119:29–39
Emlen ST (1995) An evolutionary theory of the family. Proc Natl Acad Sci USA 92:8092–8099
Field J, Shreeves G, Sumner S, Casiraghi M (2000) Insurance-based advantage to helpers in a tropical hoverwasp. Nature 404:869–871
Getz LL, Gutermuth DF, Benson SM (1992) Pattern of nest occupancy of the prairie vole, Microtus ochrogaster, in different habitats. Am Midl Nat 128:197–202
Gibson S, Williams L, McDaniel M, Brazzel J, Abee C (1993) Allo-maternal lactation and nursing in squirrel monkeys (Saimiri boliviensis boliviensis). Am J Primatol 30:314
Goodman SM, O’Connor S, Langrand O (1993) A review of predation on lemurs: implications for the evolution of social behavior in small, nocturnal primates. In: Kappeler PM, Ganzhorn JU (eds) Lemur social systems and their ecological basis. Plenum Press, New York, pp 51–66
Griffin AS, West SA (2002) Kin selection: fact and fiction. TREE 17:15–21
Hamilton WD (1964) The genetical evolution of social behavior. J Theor Biol 7:1–52
Hapke A, Eberle M, Zischler H (2003) Isolation of new microsatellite markers and application in four species of mouse lemurs (Microcebus sp.). Mol Ecol Notes 3:205–208
Hayes LD (2000) To nest communally or not to nest communally: a review of rodent communal nesting and nursing. Anim Behav 59:677–688
Heinsohn R, Legge S (1999) The cost of helping. TREE 14:53–57
Kappeler PM (2000) Ecologie des microcèbes. Primatologie 3:145–171
Koenig WD, Dickinson JL (2004) Ecology and evolution of cooperative breeding in birds. Cambridge University Press, Cambridge
Kokko H, Johnstone RA, Clutton-Brock TH (2001) The evolution of cooperative breeding during group augmentation. Proc R Soc Lond B 268:187–196
König B (1993) Maternal investment of communally nursing female house mice (Mus musculus domesticus). Behav Processes 30:61–74
König B (1997) Cooperative care of young in mammals. Naturwiss 84:95–104
König B, Riester J, Markl H (1988) Maternal care in house mice (Mus musculus): II. The energy cost of lactation as a function of litter size. J Zool (London) 216:196–210
Lewis SE, Pusey AE (1997) Factors influencing the occurrence of communal care in plural breeding mammals. In: Solomon NG, French JA (eds) Communal breeding in mammals. Cambridge University Press, Cambridge, pp 335–363
Lunn NJ, Paetkau D, Calvert W, Atkinson S, Taylor M, Strobeck C (2000) Cub adoption by polar bears (Ursus maritimus): determining relatedness with microsatellite markers. J Zool (London) 251:23–30
Lyon BE, Eadie JMA (2000) Family matters: kin selection and the evolution of conspecific brood parasitism. Proc Natl Acad Sci 97:12942–12944
Marshall TC, Slate J, Kruuk LEB, Pemperton JM (1998) Statistical confidence for likelihood-based paternity inference in natural populations. Mol Ecol 7:639–655
Nakagawa S, Waas JR (2004) ‘O sibling, where art thou?’—a review of avian sibling recognition with respect to the mammalian literature. Biol Rev 79:101–119
Packer C, Lewis S, Pusey A (1992) A comparative analysis of non-offspring nursing. Anim Behav 43:265–281
Paetkau D, Strobeck C (1994) Microsatellite analysis of genetic variation in black bear populations. Mol Ecol 3:489–495
Pagès-Feuillade E (1988) Modalites de l’occupation de l’espace et relations interindividuelles chez un prosimien nocturne malgache (Microcebus murinus). Folia Primatol 50:204–220
Pen I, Weissing FJ (2000) Towards a unified theory of cooperative breeding: the role of ecology and life history re-examined. Proc R Soc Lond B 267:2411–2418
Perret M (1992) Environmental and social determinants of sexual function in the male lesser mouse lemur (Microcebus murinus). Folia Primatol 59:1–25
Radespiel U, Cepok S, Zietemann V, Zimmermann E (1998) Sex-specific usage patterns of sleeping sites in gray mouse lemurs (Microcebus murinus) in northwestern Madagascar. Am J Primatol 46:77–84
Radespiel U, Dal Secco V, Drögemüller C, Braune P, Labes E, Zimmermann E (2002) Sexual selection, multiple mating and paternity in grey mouse lemurs, Microcebus murinus. Anim Behav 63:259–268
Radespiel U, Lutermann H, Schmelting B, Bruford MW, Zimmermann E (2003) Patterns and dynamics of sex-biased dispersal in a nocturnal primate, the grey mouse lemur, Microcebus murinus. Anim Behav 65:709–719
Rasoloarison RM, Rasolonandrasana BPN, Ganzhorn JU, Goodman SM (1995) Predation on vertebrates in the Kirindy forest, Western Madagascar. Ecotropica 1:59–65
Rensing S (1999) Immobilization and anesthesia of nonhuman primates. Primate Rep 55:33–38
Roulin A (2002) Why do lactating females nurse alien offspring? A review of hypotheses and empirical evidence. Anim Behav 63:201–208
Roulin A, Hager R (2003) Indiscriminate nursing in communal breeders: a role for genomic imprinting. Ecol Lett 6:165–166
Schmid J (1998) Tree holes used for resting by gray mouse lemurs (Microcebus murinus) in Madagascar: insulation capacities and energetic consequences. Int J Primatol 19:797–809
Silk JB (2002) Kin selection in primate groups. Int J Primatol 23:849–976
Solomon NG, French JA (1997) Cooperative breeding in mammals. Cambridge University Press, Cambridge
Stanford CB (1992) Costs and benefits of allomothering in wild capped langurs (Presbytis pileata). Behav Ecol Sociobiol 30:29–34
Taberlet P, Luikart G (1999) Non-invasive genetic sampling and individual identification. Biol J Linn Soc 68:41–55
Thierry B, Anderson JR (1986) Adoption in anthropoid primates. Int J Primatol 7:191–216
West SA, Pen I, Griffin AS (2002) Cooperation and competition between relatives. Science 296:72–75
Wimmer B (2000) Untersuchung der Paarungssysteme und Populationsstruktur von Lemuren an Coquerel’s Zwergmaki (Mirza coquereli), dem grauen Mauslemur (Microcebus murinus), dem Rotstirnmaki (Eulemur fulvus rufus) und dem Larvensifaka (Propithecus verreauxi verreauxi). Ph.D. thesis, Universität München
Acknowledgements
We thank the late Prof. Berthe Rakotosamimanana (Département de Paléontologie et d’Anthropologie Biologique de l’Université d’Antananarivo), Prof. Olga Ramilijaona and Dr. Daniel Rakotondravony (Département de Biologie Animale, Université d’Antananarivo), Dr. Lucien Rakotozafy (Parc Botanique et Zoologique Tsimbazaza Antananarivo), the Commission Tripartite and the CAFF of the Direction des Eaux et Forêts, the CFPF Morondava, Prof. Jörg U. Ganzhorn, Prof. Hans Zischler, and Andreas Hapke for their authorization or support of this study. Thanks to Tiana Andrianjanahary for assistance in the field, and to Joanna Setchell and anonymous reviewers for helpful comments on an earlier version of the manuscript. This work was financially supported by the Deutsches Primatenzentrum (DPZ) and the Deutsche Forschungsgemeinschaft (DFG, Ka 1082/5-1, 2).
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by J. Setchell
Rights and permissions
About this article
Cite this article
Eberle, M., Kappeler, P.M. Family insurance: kin selection and cooperative breeding in a solitary primate (Microcebus murinus). Behav Ecol Sociobiol 60, 582–588 (2006). https://doi.org/10.1007/s00265-006-0203-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00265-006-0203-3