Abstract
To investigate the role of template plasticity in shaping nest-mate recognition processes in ants, we constructed experimental mixed-species groups of Manica rubida with either Myrmica rubra, Tetramorium bicarinatum or Formica selysi. Selecting Ma. rubida as the focal species, we observed the behaviour within mixed-species groups and the transfer rates of cuticular hydrocarbons (CHC) onto the focal ants, and we also tested the aggression of the focal species reared either alone or in association with each of the three different species. We show that Ma. rubida workers were always amicable towards their mixed group members, as towards members of the respective parental colonies, irrespective of the associated species. They did, however, express different levels of aggression towards single-species groups of the other species tested, depending on the species with which they were reared. The study suggests that similarity in CHC profiles in two species leads to a narrow template in mixed groups, while dissimilarity is followed by lower levels of aggression (a broader template), at least against species with similar CHC compound compositions (i.e. both a broader template in the focal ants and familiarity with the compound groups of the tested individuals operate together). This refutes the hypothesis that ants reared in mixed-species groups are systematically more tolerant. It also demonstrates that heterospecific information is not treated equally during development. We suggest that post-imaginal learning, template reforming and decision making are more precisely tuned when the two species' chemical complexes are similar.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
It is generally accepted that nest-mate recognition in ants, as in many other social insects, involves the matching of a chemical label present throughout the body surface to a neural template. The result of a mismatch is generally overt aggression between the counterparts (Lacy and Sherman 1983). While the nature of the label has been extensively studied, the nature of the template and its modus operandi are little known (Hölldobler and Wilson 1990). In ants, cuticular lipids are thought to play a major role as chemical mediators in recognition processes (Singer 1998; Lenoir et al. 1999, 2001a,b), and among these, cuticular hydrocarbons (CHC) are particularly notable (Bagnères et al. 1991a for termites; Lahav et al. 1999; Thomas et al. 1999; Wagner et al. 2000 for ants). Early in adult life, each colony member must learn these cues, which, when encoded as a neural template (memory), serve for determination of the colonial membership of other individuals encountered by the ants (Crozier and Pamilo 1996).
For large colonies, it was suggested that colonial identity is achieved by creating a uniform colony odour, the gestalt (Crozier and Dix 1979). It was later shown that this common odour blend is the result of a continuous transfer of recognition cues mediated by trophallaxis, allogrooming and physical contact between colony members (Soroker et al. 1994, 2003; Dahbi et al. 1999). It was further demonstrated that the postpharyngeal gland (PPG) serves as a “gestalt organ”, i.e. it is the site where recognition cues from nest-mates are admixed and subsequently delivered onto the body surface, thus maintaining the colony gestalt (Soroker et al. 1994, 1995; Meskali et al. 1995; Lenoir et al. 2001c).
Studies with mixed-species groups provided further insight regarding the role of the gestalt odour and recognition cue exchanges among colony members. Although naturally occurring mixed-species groups are rare (with the exception of slave-making or parasitic ants), we can take advantage of their pre-programmed reactions to isolate the different parameters affecting recognition, e.g. label and template formation and plasticity. The following are some examples that demonstrate the usefulness of mixed-species groups in deciphering nest-mate recognition systems. The fact that these groups can be formed only with callow ants (Fielde 1903; Plateaux 1960; Errard and Jaisson 1984; Jaisson 1991) has been corroborated by studies regarding the ontogeny of nest-mate recognition (Carlin and Hölldobler 1986; Morel et al. 1988; Stuart 1992; Lenoir et al. 1999) as well as the ontogeny of the presumed recognition signals (Hefetz et al. 1992; Soroker et al. 1995; Dahbi et al. 1999). In these mixed-species groups, associated individuals modified their mutual species-specific recognition odour by acquiring the heterospecific odour components and exhibiting a mixed profile on both the cuticle and the PPG (Bagnères et al. 1991b; Hefetz et al. 1992). This acquisition permits the two species to inhabit the same nest without displaying interspecific aggression (Vienne et al. 1990; Vienne 1993; Errard 1994a). Using radioactive tracers, it was also demonstrated that acquisition of the heterospecific odours is accomplished by mutual exchange rather than de novo synthesis (Vienne et al. 1995a) in accordance with the above-defined gestalt model. The findings of mutual acquisition of the heterospecific CHCs by members of the mixed-species group has shed light on the basis of individual integration within a colony and the importance of the gestalt in colony odour formation (Bagnères et al. 1991b; Errard and Jaisson 1991; Hefetz et al. 1992; Errard 1994a). Regarding the template, the use of mixed-species groups has enabled testing of the hypothesis that at least part of the template has to be learned (Vander Meer 1988; Vander Meer and Morel 1998), and the use of mixed-species groups has also provided a good assessment of the memory term of such a template (Errard 1994b). The relative tolerance of ants from mixed-species groups towards ants that are conspecific to their group-mates (Errard and Hefetz 1997) has led to the hypothesis that these ants acquired a broader template and consequently become more tolerant to alien ants. However, It was not possible to draw conclusions about the magnitude of template broadening in these experiments. It is possible that the template becomes very broad, to the point of becoming indiscriminate, and therefore, these ants become inherently tolerant to alien ants. An alternative is that template broadening is more explicit and relates to the learning of specific recognition cues emanating from their group-mates. Thus, through odour familiarization followed by odour generalization, the ants become tolerant to alien ants exhibiting these heterospecific label components. We further postulated that greater differences in label chemistry lead to better generalization.
The broad template hypothesis is supported by studies on nest-mate recognition in pest ants and highly polygynous species (Keller and Passera 1989). Comparison of the cues/template nest-mate recognition system in monogyne vs polygyne Solenopsis invicta populations suggested that differences in the two population types lie in the template. Individuals from a polygyne population have a broader template and accept intruders with a wider variety of recognition profiles, but they themselves are not accepted into monogyne colonies (Morel et al. 1990; Vander Meer and Morel 1998).
The research reported here was designed to test the following alternative hypotheses: (1) ants reared in mixed-species groups are inherently less aggressive towards intruders than ants reared in single-species groups, or (2) ants in mixed-species groups are less aggressive towards alien ants belonging to their group-mate species because they are already familiar with their major recognition cues. The acuteness of familiarization is a function of label similarity between the two species in question. A prediction consistent with hypothesis 1 is that, irrespective of the species encountered or its label chemistry, workers will be tolerant. In contrast, according to hypothesis 2, recognition-odour generalization should be affected by the chemical composition of the label; introduction of new classes of hydrocarbons into the label will facilitate the acquisition of a broad template, while similar recognition profiles will result in template fixation.
To test these hypotheses, we established mixed-species groups composed of Manica rubida with either Myrmica rubra, Tetramorium bicarinatum or Formica selysi, and we observed the behaviour within the mixed-species groups as well as determining the magnitude of CHC transfer onto the focal ants. We then tested the response of Ma. rubida workers (from either single- or mixed-species groups) towards all four species from either single- or mixed-species groups. Ma. rubida was selected as the focal species because it maintains high colony insularity. The selection of the other species was based on profile similarities or differences to that of the focal species.
Materials and methods
Ants
Colonies of Ma. rubida (Myrmicinae, oligogyne species; seven colonies), My. rubra (Myrmicinae, polygyne species; five colonies) and F. selysi (Formicinae, monogyne species; four colonies) were collected in June 2002 from the same biotope (French Alps, altitude 800 m). Tetramorium bicarinatum (Myrmicinae, polygyne species) was obtained from two laboratory stock colonies originally collected in September 1994 in Brazil from two different biotopes (Itabuna and Ilhéus sites, Bahia). All colonies included queens, brood and workers and constituted the colonies from which the mixed-species groups were prepared. In the laboratory, the colonies were reared in blackened nesting tubes (180×17 mm) placed in a plastic box (280×275×85 mm) that also served as a foraging arena. The colonies were reared at 20±3°C under natural photoperiod and were regularly fed with the same diet of honey and mealworms ad libitum.
Preparation of mixed groups
Mixed-species groups were composed of 10–15 workers of each species that were less than 5 h post-emergence when removed from their respective natal nests (number of groups 18 Ma. rubida/My. rubra, 15 Ma. rubida/T. bicarinatum and 20 Ma. rubida/F. selysi). Since it is impossible to create mixed-species groups that include the respective queens, we used queenless groups as our single-species control groups. These were composed of 20 workers that were removed at emergence from the same natal nest (ten Ma. rubida, ten My. rubra, ten T. bicarinatum and ten F. selysi groups). All groups were kept queenless for at least 2 months before conducting the aggression tests.
Within-nest interactions were followed using 2-month-old mixed-species groups (five Ma. rubida/My. rubra, two Ma. rubida/T. bicarinatum and two Ma. rubida/F. selysi groups) and supplemented with ten medium-size larvae from each species (Corbara and Errard 1991; Vienne et al. 1995b).
Behavioural observations
Discrimination tests
The bioassay comprised dyadic encounters between a Ma. rubida worker (resident ant), taken either from a single-species group or any of the mixed-species groups, and a target ant (intruder) that was freshly killed by freezing. Previous assays in which both ants were alive showed comparable results to live vs frozen ant. For simplicity, we therefore selected the live vs frozen ant bioassay (Roulston et al. 2003).
Encounters were conducted for 3 min in a Petri dish (90 mm diameter) that was thoroughly cleaned between tests so that no odours remained from other ants. Before each test, the test ant was allowed to settle by secluding it in a glass tube for 1 min in the Petri dish. Tests began by removing the glass tube and recording the reaction of the test ant towards the target ant by using an event recorder according to the following aggression index (AI): 0, inspection and antennal contact; 1, threat, as indicated by mandibular opening; 2, biting; and 3, curling of the abdomen in stinging attempts. The frequencies and duration of each behavioural component were recorded, and the overall aggression exhibited in each encounter was calculated using the following formula (Hefetz et al. 1996; Errard and Hefetz 1997),
,where AI i represents the index of aggression, t i, the duration of each act and T, the total interaction time defined as the sum of durations in which the ants were in physical contact.
The number of replicate experiments was 12–21. Individuals were only tested once in a given encounter to avoid possible effects of familiarization. The results were analysed using analysis of variance (ANOVA) (Statistica for Windows 95).
Abbreviations used in the text are as follows: origin of the Ma. rubida ant utilized in the test of discrimination towards a target ant: Ma/Ma—single-species groups of Ma. rubida workers; Ma/My, Ma/T and Ma/F—mixed-species groups composed of Ma. rubida with either My. rubra, T. bicarinatum or F. selysi, respectively. Target ants that originated from single-species group are abbreviated as Ma, My, T and F for Ma. rubida (control), My. rubra, T. bicarinatum or F. selysi, respectively. Ma-p, My-p, T-p or F-p define target ants from the parent, single-species colonies of Ma. rubida, My. rubra, T. bicarinatum or F. selysi, respectively. Ma-d, My-d, T-d or F-d define conspecific target ants that were alien to the parent colonies of Ma. rubida, My. rubra, T. bicarinatum or F. selysi, respectively. Ma-s, My-s, T-s or F-s define target ants that were group-mates of the mixed-species groups of Ma. rubida, My. rubra, T. bicarinatum or F. selysi, respectively.
Within-nest interactions
Ethograms of individuals from the different mixed-species groups were obtained 2 months after their creation using time-lapse photography (Corbara et al. 1986) as well as direct observations. Spot observations or picture scanning were done every 30 min for three consecutive nights and 2 days (a total of 100 pictures or observations for each group). The pictures were viewed under a stereomicroscope, and a single behaviour was assigned to each individual per observation. Subsequently, the different items were grouped into different classes (Vienne et al. 1995b). For the present study, we focused on the heterospecific social interactions within a mixed-species group, including given and received trophallaxis, grooming and physical contacts (Vienne et al. 1995b). The results were analysed using a χ 2 test.
Chemical analysis
Identification of CHCs of Ma. rubida and F. selysi from single- and mixed-species groups were previously reported by Bagnères et al. (1991b) and Hefetz et al. (1992). Identification of CHCs of My. rubra in single-species groups was reported by Bagnères and Morgan (1990a,b), while the identification of My. rubra from mixed-species groups was by Vienne et al. (1990) and Vienne (1993), and the identification of T. bicarinatum from single-species group was by Astruc et al. (2001). For the present study, we ascertained that the CHC profiles of the workers from our laboratory-reared colonies qualitatively matched with the previously identified CHC composition by gas chromatography (GC)/mass spectroscopy (MS) analyses. Table 1 lists the identified compounds in the four species and their mean (±SEM) relative intensity based on gas chromatographic analyses.
For extraction, five to ten workers from each parent colony were killed by freezing and immersed individually in 2 ml of pentane for 10 min. Previous studies (Soroker et al. 1995) have shown that this period is sufficient for complete extraction of CHC with minimal contamination of internal HC. The extracts were then evaporated and redissolved in 50 μl of pentane containing eicosane (n-C20) as internal standard, of which 2 μl was injected into the GC (on-column Varian 3300) equipped with a capillary column (Chrompack CPSIL 5 WCOT, 25 m, 0.25 mm internal diameter) that was temperature-programmed from 100 to 280°C at 5°C/min. Compound quantification was obtained by peak integration using an Enica integrator.
The hydrocarbon profiles of Ma. rubida workers reared in mixed-species groups (Ma. rubida/My. rubra, Ma. rubida/T. bicarinatum and Ma. rubida/F. selysi) were identified by GC/MS analyses. Extracts were obtained through total-body washes of ten Ma. rubida workers in 1 ml of pentane for 10 min and processed as above. The extracts were run on a DB5 column that was temperature-programmed from 120 to 300°C at 5°C/min. The compounds were identified by their mass fragmentation pattern and retention time comparisons. For each cuticular profile, the relative value of each identified peak with respect to the total was calculated and expressed as a percentage. The total of relative amounts of heterospecific compounds found in different Ma. rubida extracts—those transferred from associated species to Ma. rubida reared in different mixed-species groups—were compared using a χ 2 test.
Results
Discrimination tests
In all the control encounters (nest-mates from single-species groups of Ma. rubida and group-mates from the mixed-species groups Ma/My (My-s), Ma/T (T-s) and Ma/F (F-s), Ma. rubida workers were not aggressive towards the ants they encountered [least significant difference (LSD) test and Newman–Keuls test, F 3,48=6.99; p=0.43]. Each time a resident ant met the introduced dead ant, it antennated it briefly and then continued walking (0.09±0.03>AI>0.04±0.02) (Table 2 row “nest” or “group-mates”).
The behaviour of Ma. rubida workers was different when encountering an alien dead ant, depending on the latter's origin. ANOVA analysis revealed that the reactions of single-species Ma. rubida workers towards alien heterospecific single-species dead ants were generally the most aggressive (LSD test, F 5,75=12.70, p<0.01 Table 2 column “Ma/Ma”).
The reaction of Ma. rubida from mixed-species groups with F. selysi (Ma/F) towards single-species-group My. rubra ants was not different from that expressed towards ants from an alien homospecific Ma. rubida (LSD test, p=0.13) but was significantly less aggressive towards both T. bicarinatum and F. selysi, irrespective of whether the latter came from the parent or from a different single-species colony (LSD test, F 5,85=18.15, p<0.01). In fact, aggression was as low as towards group-mates (Table 2 column “Ma/F”). Similar results were obtained when Ma. rubida from the mixed-species groups with T. bicarinatum were tested (LSD test, F 5,75=8.10, p<0.01). The ants were aggressive both to the single-species Ma. rubida from a colony different from the parent colony and to single-species My. rubra. In contrast, they were not more aggressive towards single-species F. selysi or single-species T. bicarinatum, irrespective of colony type, than towards their group-mates (Table 2 column “Ma/T”). The results with ants from mixed-species groups of Ma. rubida and My. rubra were quite different (LSD test, F 5,87=2.77, p=0.02). The ants were aggressive to all single-species group ants except for those coming from their parent colony. Aggression towards the latter was slightly higher than that expressed towards group-mates, but not significantly so (Table 2 column “Ma/My”).
Figure 1 depicts the results of some of the encounters, demonstrating how the composition of the mixed-species group affected template formation (LSD test, F 8,12=4.83, p<10−4). Ma. rubida workers that were taken from a single-species group were always aggressive, irrespective of whether they encountered homospecific but alien ants or heterospecific ants. This was also the case with Ma. rubida that were reared in a mixed-species group with My. rubra. On the other hand, when Ma. rubida were reared with either F. selysi or T. bicarinatum, they became tolerant of any ant of these species, but they remained aggressive towards My. rubra. Thus, Ma. rubida reared in mixed species do not become generally more tolerant, but they do exhibit specific template shifts.
Aggression (expressed as Aggression Index) of Manica rubida from various rearing groups towards dead ants of Ma. rubida, Myrmica rubra, Tetramorium bicarinatum or Formica selysi from various sources. Different letters represent the groups that differed significantly. ANOVA, LSD test—F 8,12=4.83, p<10−4. For abbreviations, see “Materials and methods”
Cuticular hydrocarbon analyses
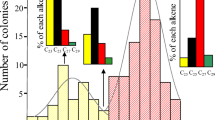
Previous GC/MS analyses of cuticular compounds of the four species studied have shown that they consist of complex blends of hydrocarbons (cited in the legend to Table 1). Table 1 gives the list of hydrocarbons with their relative intensity, and Fig. 2 depicts the general composition grouped according to classes of compounds. F. selysi and T. bicarinatum were outstanding in their composition, possessing similarly high amounts of alkenes (39.59 and 34.33%, respectively; p=0.13) and alkadienes (17.31 and 18.15%, respectively; p=0.15). Although My. rubra also possessed alkenes (but no alkadienes), they comprised only 7.10% of the secretion (alkenes F 3,41=11.44, p<10−4 and alkadienes F 3,11=20.72, p<10−4). Linear alkanes were common to all the species, although Ma. rubida and T. bicarinatum tended to have higher proportions of this class of compounds (F 3,22=2.71, p=0.01). Branched alkanes (mono-, di- and trimethylalkanes), on the other hand, were very abundant in the cuticular waxes of Ma. rubida and My. rubra but only minor in the cuticular waxes of F. selysi and absent in T. bicarinatum (F 3,41=7.97, p<10−4). Likewise, My. rubra and Ma. rubida contained comparable amounts of branched alkanes (p=0.12), while F. selysi contained lower amounts of these compounds compared to My. rubra or Ma. rubida (p=0.06 and 0.002, respectively), and T. bicarinatum had none of these chemicals. In general, it can be stated that the secretions of Ma. rubida and My. rubra bear more similarity (considering the classes of compounds) to each other than to those of F. selysi or T. bicarinatum.
Cumulative relative intensity (percentages) of compounds in each category of hydrocarbons of Manica rubida, Myrmica rubra, Tetramorium bicarinatum and Formica selysi workers reared in single-species colonies. Values indicate, for each category, the mean relative percentage (±SEM) for each species (one colony per species and n=5 extracts per colony). Numbers above each bar represent the number of compounds in each of the compound classes
Quantification of the similarity of profiles showed that Ma. rubida profiles possessed 2 (1.87%) specific linear alkanes and 5 (15.32%) specific branched alkanes, compared with F. selysi profiles which possessed 11 (41.12%) specific alkenes. Compared with T. bicarinatum profiles, which possessed 16 (48.72%) specific alkenes, Ma. rubida species profiles possessed 15 (54.26%) specific branched alkanes, with all (9) its linear alkanes (39.21%) being common with T. bicarinatum. Compared with My. rubra profiles, which possessed three (7.12%) alkenes, Ma. rubida possessed two (1.87%) specific linear alkanes and eight (13.04%) specific branched alkanes. We should note that more than 50% of the compounds are shared between Ma. rubida and My. rubra because of the high overlap in the branched alkanes class of compounds.
A hierarchical cluster analysis (Ward's method, Euclidean distances) based on GC analyses of different extracts revealed a significant divergence between the different species (Euclidean distances, Ma. rubida–T. bicarinatum 591.00; Ma. rubida–F. selysi 278.00; and Ma. rubida–My. rubra 97.00). The first node (linkage distance 863.91) separated T. bicarinatum from the three other species, while the second node (linkage distance 340.64) divided F. selysi from My. rubra and Ma. rubida. The third node (linkage distance 97.17) separated My. rubra from Ma. rubida.
Analyses of cuticular profiles of Ma. rubida kept in a mixed-species group revealed that, although in all cases, these ants had acquired the heterospecific compounds, the magnitude of acquisition in each case differed. They acquired about 23.0±3.2% of heterospecific hydrocarbon from their F. selysi nest-mates and 17.6±3.3% from their T. bicarinatum nest-mates, but they acquired only 3.8±1.5% from their My. rubra nest-mates. The heterospecific compound acquired by Ma. rubida from mixed-species groups with F. selysi (Ma/F) was not different from that observed for Ma. rubida from mixed-species groups with T. bicarinatum (Ma/T) (χ 2=18.6, p>0.05), but the acquired heterospecific compound was significantly lower when Ma. rubida came from mixed-species groups with My. rubra (Ma/F vs Ma/My, χ 2=50.7, p<0.001; Ma/T vs Ma/My, χ 2=37.8, p<0.001) (Fig. 3A). Within-nest observations corroborated the chemical data. The rate of heterospecific social interactions, including trophallaxis, allogrooming and physical contacts (percentages of total behavioural acts), received by Ma. rubida workers reared in mixed groups with F. selysi was 32.5±5.8%, and the rate of heterospecific social interactions was 23.5±3.9% when Ma. rubida workers were reared in mixed-species groups with T. bicarinatum (Ma/F vs Ma/T, χ 2=23.1, p>0.05). On the other hand, in mixed-species groups of Ma. rubida and My. rubra, the level of heterospecific interaction was only 11.1±1.2% of the total activities. In this mixed-species group, the heterospecific interactions were significantly lower than in mixed-groups with F. selysi (Ma/F) or with T. bicarinatum (Ma/T) (Ma/F vs Ma/My, χ 2=126.50, p<0.001; Ma/T vs Ma/My, χ 2=81.08, p<0.001) (Fig. 3B).
A Relative intensity (percentages) of the heterospecific hydrocarbons transferred to Ma. rubida workers reared in mixed-species groups with My. rubra (Ma/My), T. bicarinatum (Ma/T) or F. selysi (Ma/F) workers. Different letters represent groups that differed significantly (χ2 test, see text for more details). B Social interactions (trophallaxis, allogrooming and physical contacts—percentages of total behavioural acts) received by Ma. rubida workers reared in mixed groups with My. rubra (Ma/My), T. bicarinatum (Ma/T) or F. selysi (Ma/F) workers. Different letters represent groups that differed significantly (χ2 test, see text for more details)
Discussion
The experimental paradigm of using mixed-species groups of ants provides a useful tool for understanding the proximate mechanisms underlying nest-mate recognition. The fact that these groups are necessarily queenless does not detract from the effectiveness because short-term queenlessness does not affect the aggressive tendencies of the ants (Boulay et al. 2003). In a previous study (Errard and Hefetz 1997), we tested the discriminatory ability of the ant Ma. rubida reared in mixed-species groups with F. selysi, taking advantage of the fact that F. selysi possesses a series of n-alkenes that are completely absent from the profile of Ma. rubida. We concluded that familiarity with the F. selysi-specific compounds may reduce the aggressive reaction of the ants reared in mixed-species group, and that deciphering the signal in the recognition process may be hierarchical, and the resulting reaction is inverse to the familiarity of the signal. Using this system, however, we cannot exclude the possibility that ants reared in mixed-species groups become less aggressive than their conspecifics reared in single-species groups, irrespective of the identity of the group-mate species. A possible explanation was that the template of Ma. rubida reared in mixed-species groups becomes broader and thus, less discriminative than that of their single-species groups. Regarding the template, it was shown that workers reared in mixed-species groups learn and memorize the homo- and heterospecific chemical cues (i.e. mixed colonial odour) during their early social experience and incorporate them into their template (Errard 1994b). By expanding the above experiments to two additional species participating in a mixed-species group, our aim was to test between these two hypotheses.
The differential reaction of Ma. rubida workers reared in various mixed-species groups towards the different species rules out the hypothesis that such workers become inherently more tolerant and supports the alternative hypothesis. When associated as callow workers with either F. selysi or T. bicarinatum, workers of Ma. rubida exhibited amicable behaviour to all F. selysi and T. bicarinatum workers, irrespective of their colony origin. In contrast, when callow Ma. rubida were associated with My. rubra, the mixed-species ants remained aggressive to all three of the species to which they were exposed, including alien My. rubra. This suggests that their association with F. selysi or T. bicarinatum, but not with My. rubra, resulted in the acquisition of a broader template by the Ma. rubida workers that included the heterospecific compounds. While it is possible that the ants could have sensed specific compounds that they share in common with the other species without necessarily broadening the template, this seems unlikely. Accumulating evidence points to the fact that effective nest-mate recognition necessitates complex blends to achieve the subtle variations essential for discrimination between colonies within a population. It further supports the prediction that the acuteness of odour generalization depends on the degree of odour similarity. We conclude that greater differences between the labels of members of a mixed species group lead to better generalization.
On the basis of behavioural tests, we can exclude the possibility that the differences between the two reactions were due to differential acquisition of the label rather than changes in the template. Both behavioural and chemical analyses indicated that Ma. rubida acquires smaller amounts of heterospecific hydrocarbons from My. rubra than from either F. selysi or T. bicarinatum. However, the fact that Ma. rubida workers were still able to discriminate between My. rubra group-mates and an alien, conspecific, single-species group indicates that despite the lower intragroup interactions, these mixed-species groups still created a group-specific label. We suggest that the differences lie in differential learning of the label and, accordingly, differential template shaping. Label differences between Ma. rubida and F. selysi or T. bicarinatum are far greater than between Ma. rubida and My. rubra, mostly due to the large amounts of unsaturated hydrocarbons that both F. selysi and T. bicarinatum possess. Exposure of the ants to a completely different type of signal as young imagos (exposure of Ma. rubida to massive amounts of F. selysi or T. bicarinatum alkenes) may lead to the creation of a rather heterogeneous template. In this case, it will be enough to expose the ants to these alkenes to achieve recognition and generate amicable interactions. Conversely, in workers exposed to a bouquet of chemicals (that of My. rubra) that is highly similar to their innate bouquet, the template can undergo minor but more accurate changes that may fine-tune recognition.
Several studies have pointed out that among CHCs, alkanes are the least informative, while branched alkanes can convey better information regarding specificity (Gamboa et al. 1996). The findings in ants that methyl-branched hydrocarbons are more colony-specific relative to linear alkanes (Bonavita-Cougourdan et al. 1987; Provost et al. 1992; Astruc et al. 2001) provided indirect support for this hypothesis, while more direct evidence was obtained in Polistes dominulus (Dani et al. 2001). Cuticular lipids of My. rubra and Ma. rubida are especially rich in branched alkanes, which raise the possibility that these compounds are important for the acuteness of learning and template fixation. We further suggest that the Manica–Myrmica case may be similar to the natural homospecific nest situation where, throughout the ant's lifetime, temporal changes in the chemical signal take place and, consequently, the template can change to attune to changes at the level of colony label. Such intrinsic shifts in CHCs or those caused by introducing environmental cues were demonstrated for several ant species (Wallis 1963; Jutsum et al. 1979; Obin 1986; Vander Meer 1988; Hölldobler and Wilson 1990; Heinze et al. 1996; Dahbi and Lenoir 1998; Vander Meer and Morel 1998; Boulay et al. 2000; Liang and Silverman 2000), and in the case of mixed-species groups, these shifts included both the hetero- and homospecific odours (Errard 1994a). Whether this broad imprinting is limited to the foundation period of the mixed-species group, or this broad imprinting can be attained throughout these groups' existence, is an open question.
It still remains unclear as to whether the exact process of cue learning and template formation is innate or is acquired through familiarization with nest-mates during a sensitive period (Jaisson 1987). Although we cannot exclude the existence of pre-imaginal learning (Isingrini et al. 1985), we suggest that environmental labels that are learned directly from the interactions with nest-mate workers during the first hours or days of adult life are determinants for the template formation. In addition, we should not underestimate the proximate mechanism involving the decision rules employed during nest-mate recognition and the ease with which it can be operated in such discriminative processes. The subtle chemical differences between Ma. rubida and their alien nest mates can activate a decision rule to reject any different label, whereas a quite distinct chemical difference between both species can activate a decision rule to accept all individuals bearing the same strange label.
While we cannot generalize this study to all ants due to the limited number of species studied, and because there might still be specific effects related to the nature of the species selected, it does provide new perspectives regarding template formation. Further experiments are needed to properly assign a function to each of the above-proposed mechanisms, for which the use of mixed-species groups may be useful. This has a large advantage over simply introducing a novel odour into a colony because each of the ant species exhibits its pre-programmed behaviour as if reared in a single-species colony, yet each of the ant species is exposed in an interactive way to another species that displays a complex and different recognition signal. Therefore, by selecting the appropriate species, one can probe into each of these mechanisms in a specific manner.
References
Astruc C, Malosse C, Errard C (2001) Lack of intraspecific aggression in the ant Tetramorium bicarinatum: a chemical hypothesis. J Chem Ecol 27:1229–1248
Bagnères AG, Morgan ED (1990a) A simple method for the analysis of insect cuticular hydrocarbons. J Chem Ecol 16:3263–3276
Bagnères AG, Morgan ED (1990b) The post-pharyngeal gland and the cuticle of Formicidae contain the same characteristic hydrocarbons. Experientia 47:106–111
Bagnères AG, Killian A, Clément JL, Lange C (1991a) Interspecific recognition among termites of the genus Reticulitermes: evidence for a role of the cuticular hydrocarbons. J Chem Ecol 17:2397–2419
Bagnères AG, Errard C, Mulheim C, Joulie C, Lange C (1991b) Induced mimicry of colony odours in ants. J Chem Ecol 17:1641–1664
Bonavita-Cougourdan A, Clément JL, Lange C (1987) Nestmate recognition: the role of cuticular hydrocarbons in the ant Camponotus vagus Scop. J Entomol Sci 22:1–10
Boulay R, Hefetz A, Soroker V, Lenoir A (2000) Camponotus fellah colony integration: worker individuality necessitates frequent hydrocarbons exchanges. Anim Behav 59:1127–1133
Boulay R, Katzav-Gozansky T, Vander Meer RK, Hefetz A (2003) Colony insularity through queen control on worker social motivation in ants. Proc R Soc Lond, B Biol Sci 270:971–977
Carlin NF, Hölldobler B (1986) The kin recognition system of carpenter ants (Camponotus spp.). I. Hierarchical cues in small colonies. Behav Ecol Sociobiol 19:123–134
Corbara B, Errard C (1991) The organization of artificial heterospecific ant colonies. The case of the Manica rubida/Formica selysi association: mixed colony or parallel colonies? Behav Proc 13:237–249
Corbara B, Fresneau D, Lachaud JP, Leclerc Y, Goodall G (1986) An automated photographic technique for behavioural investigations of social insects. Behav Proc 13:237–249
Crozier RH, Dix MW (1979) Analysis of two genetic models for the innate components of colony odor in social Hymenoptera. Behav Ecol Sociobiol 4:217–224
Crozier RH, Pamilo P (1996) Evolution of social insect colonies. Oxford University Press, Oxford
Dahbi A, Lenoir A (1998) Nest separation and the dynamics of the gestalt odor in the polydomous ant Cataglyphis iberica (Hymenoptera, Formicidae). Behav Ecol Sociobiol 42:349–355
Dahbi A, Hefetz A, Cerda X, Lenoir A (1999) Trophallaxis mediates uniformity of colony odor in Cataglyphis iberica ants (Hymenoptera, Formicidae). J Insect Behav 12:559–567
Dani FR, Jones GR, Destri S, Spencer SH, Turillazzi S (2001) Deciphering the recognition signature within the cuticular chemical profile of paper wasps. Anim Behav 62:165–171
Errard C (1994a) Development of interpecific recognition behavior in the ants Manica rubida and Formica selysi (Hymenoptera: Formicidae) reared in mixed-species groups. J Insect Behav 7:83–99
Errard C (1994b) Long-term memory involved in nestmate recognition in ants. Anim Behav 48:263–271
Errard C, Hefetz A (1997) Label familiarity and discriminatory ability of ants reared in mixed groups. Insectes Soc 44:189–198
Errard C, Jaisson P (1984) Etudes des relations sociales dans les colonies mixtes hétérospécifiques chez les fourmis. Folia Entomol Mex 61:135–146
Errard C, Jaisson P (1991) Les premières étapes de la reconnaissance interspécifique chez les fourmis Manica rubida et Formica selysi (Hymenoptera: Formicidae) élevées en colonies mixtes. CR Acad Sci Paris 313:73–80
Fielde A (1903) Artificial mixed nests of ants. Biol Bull 5:320–325
Gamboa GJ, Grudzien TA, Espelie KA, Bura EA (1996) Kin recognition pheromones in social wasps: combining chemical and behavioural evidence. Anim Behav 34:685–695
Hefetz A, Errard C, Cojocaru M (1992) The occurrence of heterospecific substances in the postpharyngeal gland secretion of ants reared in mixed species colonies (Hymenoptera: Formicidae). Naturwissenschaften 79:417–420
Hefetz A, Errard C, Chambris A, Le Négrate A (1996) Postpharyngeal gland secretion as a modifier of aggressive behaviour in the myrmicine ant Manica rubida. J Insect Behav 9:709–717
Heinze J, Foitzik S, Hippert A, Hölldobler B (1996) Apparent dear–enemy phenomenon and environment-based recognition cues in the ant Leptothorax nylanderi. Ethology 102:510–522
Hölldobler B, Wilson EO (1990) The ants. Belknap Press of Harvard University Press, Cambridge
Isingrini M, Lenoir A, Jaisson P (1985) Preimaginal learning as a basis of colony-brood recognition in ant Cataglyphis cursor. Proc Nat Acad Sci U S A 82:8545–8547
Jaisson P (1987) The construction of fellowship between nestmates in social hymenoptera. In: Pasteels JM, Deneubourg JL (eds) Experientia supplementum 54. From individual to collective behaviour in social insects. Birkhauser Verlag, Basel, pp 313–331
Jaisson P (1991) Kinship and fellowship in ants and social wasps. In: Hepper PG (ed) Kin recognition. Cambridge University Press, Cambridge, pp 60–93
Jutsum AT, Saunders T, Cherrett J (1979) Intraspecific aggression in the leaf-cutting ant Acromyrmex octospinosus. Anim Behav 27:839–844
Keller L, Passera L (1989) Influence of the number of queens on nestmate recognition and attractiveness of queens to workers in the Argentine ant, Iridomyrmex humilis (Mayr). Anim Behav 37:733–740
Lacy RC, Sherman PW (1983) Kin recognition by phenotype matching. Am Nat 121:489–512
Lahav S, Soroker V, Hefetz A, Vander Meer RK (1999) Direct behavioral evidence for hydrocarbons as ant recognition discriminators. Naturwissenschaften 86:246–249
Lenoir A, Fresneau D, Errard C, Hefetz A (1999) The individuality and the colonial identity in ants: the emergence of the social representation concept. In: Detrain C, Deneubourg JL, Pasteels J (eds) Information processing in social insects. Birkhäuser, Basel, pp 219–237
Lenoir A, D'Ettorre P, Errard C, Hefetz A (2001a) Chemical ecology and social parasitism in ants. Annu Rev Entomol 46:573–599
Lenoir A, Cuisset D, Hefetz A (2001b) Effects of social isolation on pattern and nestmate recognition in the ant Aphaenogaster senilis (Hymenoptera, Formicidae). Insectes Soc 48:101–109
Lenoir A, Hefetz A, Simon T, Soroker V (2001c) Comparative dynamics of gestalt odour formation in two ant species Camponotus fellah and Aphaenogaster senilis (Hymenoptera: Formicidae). Physiol Entomol 26:275–283
Liang D, Silverman J (2000) “You are what you eat”: diet modifies cuticular hydrocarbons and nestmate recognition in the Argentine ant, Linepithema humile. Naturwissenschaften 87:412–416
Meskali M, Bonavita-Cougourdan A, Provost E, Bagnères AG, Dusticier G, Clément JL (1995) Mechanism underlying cuticular hydrocarbon homogeneity in the ant Camponotus vagus (Scop.) (Hymenoptera: Formicidae): role of postpharyngeal glands. J Chem Ecol 8:127–1148
Morel L, Vander Meer RK, Lavine BK (1988) Ontogeny of nestmate recognition cues in the red carpenter ant (Camponotus floridanus). Behav Ecol Sociobiol 22:175–183
Morel L, Vander Meer RK, Lofgren CS (1990) Comparison of nestmate recognition between monogyne and polygyne populations of Solenopsis invicta (Hymenoptera: Formicidae). Ann Entomol Soc Am 83:642–647
Obin MS (1986) Nestmate recognition cues in laboratory and field colonies of Solenopsis invicta Buren (Hymenoptera: Formicidae). J Chem Ecol 12:1965–1975
Plateaux L (1960) Adoptions expérimentales de larves entre des fourmis de genres différents: Leptothorax nylanderi Först. et Solenopsis fugax Latr. Insectes Soc 7:163–170
Provost E, Cerdan P, Bagnères AG, Morgan ED, Rivière G (1992) Role of the queen in Messor barbarus colony signature. In: Billen J (ed) Biology and evolution of social insects. Leuven University Press, Leuven, pp 195–202
Roulston TH, Buczkowski G, Silverman J (2003) Nest discrimination in ants: effect of bioassay on aggressive behavior. Insectes Soc 50:151–159
Singer TL (1998) Roles of hydrocarbons in the recognition systems of insects. Am Zool 38:394–405
Soroker V, Vienne C, Hefetz A, Nowbahari E (1994) The postpharyngeal gland as a “gestalt” organ for nestmate recognition in the ant Cataglyphis niger. Naturwissenschaften 81:510–513
Soroker V, Vienne C, Hefetz A (1995) Hydrocarbon dynamics within and between nestmates in Cataglyphis niger (Hymenoptera, Formicidae). J Chem Ecol 21:365–378
Soroker V, Lucas C, Simon T, Fresneau D, Durand JL, Hefetz A (2003) Hydrocarbon distribution and colony odour homogenisation in Pachycondyla Apicalis. Insectes Soc 50:212–217
Stuart R (1992) Nestmate recognition and the ontogeny of acceptability in the ant, Leptothorax curvispinosus. Behav Ecol Sociobiol 30:403–408
Thomas ML, Parry LJ, Allan RA, Elgar MA (1999) Geographic affinity, cuticular hydrocarbons and colony recognition in the Australian meat ant Iridomyrmex purpureus. Naturwissenschaften 86:87–92
Vander Meer RK (1988) Behavior and biochemical variation in the fire ant, Solenopsis invicta. In: Jeanne RL (ed) Interindividual behavior variability in social insects. Westview, Boulder, pp 223–255
Vander Meer RK, Morel L (1998) Nestmate recognition in ants. In: Vander Meer RK, Breed M, Winston M, Espelie KE (eds) Pheromone communication in social insects. Westview, Boulder, pp 79–103
Vienne C (1993) Organisation sociale et reconnaissance interindividuelle dans les colonies mixtes artificielles de fourmis. PhD thesis, University Paris Nord, Villetaneuse
Vienne C, Bagnères AG, Lange C, Errard C (1990) Etude chimique de la reconnaissance interindividuelle chez Myrmica rubra et Manica rubida (Formicidae, Myrmicinae) élevées en colonies mixtes artificielles. Actes Colloq Insectes Soc 6:261–265
Vienne C, Soroker V, Hefetz A (1995a) Congruency of hydrocarbon patterns in heterospecific groups of ants: transfer and/or biosynthesis? Insectes Soc 42:267–277
Vienne C, Errard C, Lenoir A (1995b) Species polyethism in heterospecific groups of Myrmicinae ants. Ethol Ecol Evol 7:133–146
Wagner D, Tissot M, Cuevas W, Gordon DM (2000) Harvester ants utilize cuticular hydrocarbons in nestmate recognition. J Chem Ecol 26:2245–2256
Wallis DI (1963) A comparison of the response to aggressive behavior in two species of ants, Formica fusca and Formica sanguinea. Anim Behav 11:164–171
Acknowledgements
We wish to thank Thibaud Monnin and Alain Lenoir for providing the ants used in this study. We thank three anonymous referees for constructive comments on this manuscript. We thank Raymond Jegat for technical help and Naomi Paz for editorial assistance.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by J. Heinze
Rights and permissions
About this article
Cite this article
Errard, C., Hefetz, A. & Jaisson, P. Social discrimination tuning in ants: template formation and chemical similarity. Behav Ecol Sociobiol 59, 353–363 (2006). https://doi.org/10.1007/s00265-005-0058-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00265-005-0058-z