Abstract
We investigated how morphological traits of territorial males in the polygynous bat Saccopteryx bilineata were related to their reproductive success. Because of the frequency of aerial courtship displays and defence manoeuvres, and the high energetic costs of flight, we expected small and symmetric males to be better able to court females on the wing and to monopolize copulations with females in their harems. We predicted that small and symmetric males would sire more offspring within the colony and a larger portion of the young born within their harem than large or asymmetric males. We measured size and fluctuating asymmetry of 21 territorial males and analysed their reproductive success in 6 offspring cohorts (n=209 juveniles) using 11 microsatellite loci. As predicted, small and symmetric males had, on average, a higher reproductive success in the colony than large and asymmetric males. The percentage of young sired by males within their harem increased as males decreased in size, but was not influenced by fluctuating asymmetry. As fluctuating asymmetry of males correlated with their reproductive success within the colony but not within their harems, we infer that fluctuating asymmetry is probably related to female choice, whereas male size is probably important for harem defence on the wing.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In mammals, polygyny is the most common mating system, and in polygynous mating systems, male reproductive success is often highly skewed, with a few successful males siring the majority of offspring (Say et al. 2003; Shuster and Wade 2003). This reproductive skew leads to intense male-male competition, which often results in large-sized males or males carrying large body weapons (review in Andersson 1994). In grey seals (Halichoerus grypus) for example, the largest male may sire 10 times more offspring than the smallest male (e.g. Anderson and Fedak 1985, but see Worthington-Wilmer et al. 2000). However, some studies reveal controversial results regarding large male size and breeding success in mammals (e.g. Rose et al. 1998; Schulte-Hostedde et al. 2003).
Most studies that focus on male morphology and reproductive success have been performed on terrestrial mammals, which encounter similar selective forces. In mating systems of airborne animals like bats, selection may act differently. In aerial species, locomotion on the wing constitutes a great portion of the daily energy budget, and natural and sexual selection may favour small size because it is advantageous in terms of energy expenditure and manoeuvrability (Stockwell 2001; Voigt 2000). Flight performance is also affected by the bilateral symmetry of an individual’s wings (Swaddle 1997). In addition, deviations from bilateral symmetry, or fluctuating asymmetry (FA), are also potentially important as indicators of male quality (see Møller and Swaddle 1997 for the concept of fluctuating asymmetry). To gain new insights into the consequences of selection on size and fluctuating asymmetry of males, we performed a study on morphology and reproductive success in polygynous male greater sac-winged bats.
Greater sac-winged bats (Saccopteryx bilineata; Emballonuridae) roost in cavities formed by buttress roots at the base of huge rainforest trees, in the well-lit portions of hollowed trees or in buildings. Colonies may contain up to 60 individuals and are divided into smaller social units, each containing a single adult male and several females, called harem hereafter (Bradbury and Vehrencamp 1977). Harem defence and courtship displays are performed on the wing (Bradbury and Emmons 1974; Bradbury and Vehrencamp 1976; Voigt and von Helversen 1999). Male-male contests occur either from one territory to the other by fanning the scent of specialized wing sacs to the neighbouring male or on the wing by chasing a male in the colony (Bradbury and Emmons 1974; Voigt and von Helversen 1999). Male territories are defended year round (Voigt and Streich 2003).
Recent genetic studies revealed that harem males do not monopolize paternity of offspring produced by females in their territory: males sire, on average, only 30% of the offspring within their territory (Heckel and von Helversen 2003). Nonetheless, males do benefit from defending a territory because the reproductive success of harem males is greater than that of non-harem males (Heckel and von Helversen 2002), and because the reproductive success of harem males increases with increasing harem size (Heckel and von Helversen 2003). In contrast to most similar-sized mammals, S. bilineata is a long-lived species, like most other bats. Colonies may persist over many years at the same site and the same male may occupy a territory for many years, in some cases in excess of 8 years (Voigt et al. 2003). Changes in harem ownership rarely occur, and non-harem males queue for access to a vacant harem territory instead of engaging in severe physical contests (Voigt and Streich 2003).
Male Saccopteryx spend considerable effort in defending their territory and in hovering courtship flight (Voigt and von Helversen 1999), and the field metabolic rate of males increases with the number of females per harem (Voigt et al. 2001). According to aerodynamic theory and experimental studies, small individuals have lower power requirements for flight than large individuals (Norberg 1995; Norberg and Rayner 1987; Voigt 2000; Voigt and Winter 1999 ). Small size could be beneficial for male S. bilineata for two reasons: (1) with the same amount of energy, a small male can perform more flights than a large male, and (2) small males may be better at tight manoeuvres than large males. A second feature that may be important for males defending and courting females on the wing is the symmetry of wings. According to aerodynamic theory, asymmetric wing morphology is associated with elevated flight costs and decreased manoeuvrability (Swaddle et al. 1996; Thomas 1993; Witter et al. 1994). In addition to these direct costs, fluctuating asymmetry could also be an indicator of male quality (Møller and Swaddle 1997). Therefore, we hypothesized that small and symmetric males should have, on average, a higher reproductive success within the colony than large and asymmetric males. If small size and symmetry is beneficial for aerial manoeuvrability and defence, small or symmetric males should be better able to monopolize a harem than large or asymmetric males. Therefore, we also tested whether the percentage of harem offspring sired by the harem defender increases with decreasing male size and fluctuating asymmetry.
Methods
Study population and census data
This study was performed over a 6-year period from 1995 to 2001 in a colony of sac-winged bats near the biological station “La Selva” (Organization for Tropical Studies, Costa Rica, Province Heredia, N 10°25′/W 84°00′). We visited the field site twice a year (except for 1998, for which census data for the mating season are missing; see below): prior and during the mating season from November through early January, and during lactation from July to August. The period of fieldwork included September to November 1995, June to August 1996, October 1996 to February 1997, June to August 1997, June to August 1998, December 1998 to February 1999, June to August 1999, November to December 1999, June to August 2000, December 2000 to January 2001, and June to August 2001. The study colony was located in an abandoned cottage surrounded by secondary rainforest. At dusk, we captured the bats with mistnets (6 m length, 2 m height) when they emerged from the roost to their foraging habitat. For genetic analysis, we removed a small piece of wing tissue from the plagiopatagium of each captured bat with a biopsy punch (3 mm diameter) (Worthington-Wilmer and Barrat 1996). For individual identification in the daytime roost, we placed coloured or numbered plastic bands (AC Hughes, Hampton Hill, UK) on the bats’ forearms. Females were marked on the left and males on the right forearm.
One week before collection of data on harem sizes (census data), we habituated the bats to the presence of human observers in the colony. We determined the sites of encounters between males to locate the territorial boundaries (Bradbury and Emmons 1974; Voigt and von Helversen 1999). During the mating season, we performed censuses on a daily basis or every 2nd to 5th day. Usually, censuses were conducted between 0800 hours and 1000 hours. To estimate harem sizes (HS), we counted the females in each territory for each census event and calculated an average HS for each territory and mating period. As we lack data for the mating season 1997/1998, we performed censuses during the parturition period 1998 and included these data in the analysis. A comparison of HS between the lactation period prior to and after this mating season revealed no significant changes (Heckel and von Helversen 2003). Hereafter, we use harem and territory, as well as harem holder and territory owner, as synonymous words.
Morphological measurements
During the mating seasons in 1999, 2000 and 2001, we measured the length (FL) and asymmetry (FA) of males. We restricted our analysis to males defending a territory, as males without a territory follow a different tactic, e.g. queuing for a territory (Voigt and Streich 2003). We laid the bat on a plane surface with the tail pointing towards the person performing the measurements. We protruded the right forearm at an angle of 30° from the corpus (Fig. 1). We performed similarly with the digits so that the angle between forearm and digit bones equalled 30° as well. The right FL was then measured with a calliper to an accuracy of 0.1 mm. For the left FL, we switched the bat to the other hand and performed the measurements in a mirror-like manner. Afterwards, we rotated the bat’s body by 180° with the ventral parts still touching the plane surface, but this time with the tail pointing away from the measurer. Afterwards, we repeated the measurements on both sides. Thus, we obtained four measurements per capturing event, two for each side and two performed by each of the experimenter’s hands. The aim of this routine was to control for any effects of side or handedness in the measurements. As we found a highly significant correlation between log-transformed FL and log-transformed body mass (Student t-test, t145=4.3, P<0.001), we use body size and mass as synonymous terms hereafter.
Schematic drawing of how morphological measurements of the right forearm were taken from bats. The forearm was protruded at an angle of α=30° from the corpus and the digits at the same angle from the forearm. The calliper was oriented at an angle of β=90° to the forearm. For the left FL, we switched the bat to the right hand and performed the measurements as described with the left hand. Afterwards, we rotated the bat’s body by 180° so that the legs were located at the place where the bat’s head was before and repeated the measurement routine with the right and left hand again.
We calculated the absolute difference between the right and the left FL and defined fluctuating asymmetry as FA=|right FL−left FL|. On average, we caught each individual five times (min.=2, max.=11). Therefore, each forearm was measured on average 10 times and a total mean number of 20 measurements were performed for each bat. The average difference between the right and the left FL equalled 0.061±0.179 mm, which did not deviate significantly from zero according to a one-sample t-test (t20=1.57, P=0.13). The skewness of the distribution function of FA equalled 0.05 (±0.97 SE) and the kurtosis −0.49 (±0.50 SE). We tested for the repeatability of our measurements with a mixed-model ANOVA following Swaddle et al. (1994), estimating whether the difference between individuals is greater than the measurement error (details of the ANOVA are listed in Table 1). An F-value of F20,292=2.28 (P<0.025) confirmed that the measures of FA were repeatable.
Parentage assignments
In total, we attempted parentage assignments for 209 juveniles. To maximize the reliability of paternity assignments, the maternal descent of juveniles was determined first, followed by the determination of the paternal descent. Behavioural observation, e.g. nursing behaviour, was used initially to determine maternal descent. Individually marked juveniles were assigned to specific females as their respective mothers after they were observed at least three times suckling from the female bat (see Heckel et al. 1999). On the rare occasion that we lacked observations on nursing behaviour, we used genetic data alone to assign maternal descent. Behavioural and genetic data about maternity were analysed independently, and later combined for the determination of maternal descent. Only those females that were present at least once during the corresponding year of the cohort were included in the maternity analyses.
Paternal descent was determined by genetic analyses of a minimum of ten microsatellite loci (Heckel and von Helversen 2003; Heckel et al. 2000). We considered all adult males ever caught in the area of the colony as putative sires (total n=127 males). Males were excluded as possible fathers for a juvenile cohort if their death was recorded prior to the corresponding mating season. All other males were included in the analysis of paternal descent, whether they had been recently observed in the colony or not.
To obtain optimal reliability, we used two methods for assigning paternities: (1) paternity exclusion for genetically incompatible males, and (2) likelihood-based paternity assignment. In exclusion-based paternity assignments, a male was excluded as putative father if it did not share at least one microsatellite allele at two or more loci with the juvenile in question (see details in Heckel and von Helversen 2003). In likelihood-based paternity assignments, we used CERVUS 1.0 (Marshall et al. 1998) with 10.000 simulations, 0.01 typing error rate, and a proportion of 80% sampled candidate fathers to identify the most likely father (see details in Heckel and von Helversen 2003).
We estimated the uncertainty in assignments by calculating a relaxed and strict confidence level (80 vs 95%). Final assignment of paternity was done parsimoniously: in cases when the only male not excluded was also the most likely sire, paternity was assigned to that male. When two or more males were not excluded as father of a juvenile, the most likely male was assumed to be the father. No paternity was assigned in cases when two or more genotypic mismatches were detected between all males and a juvenile.
We did not find indications for inbreeding, because the genotype frequencies of males did not deviate from Hardy Weinberg expectations at all 11 microsatellite loci.
Statistical analysis
We performed a weighted stepwise multiple regression (Backhaus et al. 1996) using the variables mean HS, FL, and FA as the independent variables predicting the mean number of sired offspring per cohort, weighted by the number of reproductive seasons a male is present. We tested the assumptions underlying linear regression analysis and found no evidence of any violations. To assess a significant correlation between the dependent and independent variables, we tested whether the slopes of the regression lines deviated significantly from zero using Student t-tests, based on df=20 and α=5%. To assess the probability of detecting a significant increase in r2 by including an additional covariate in the regression model (adjusting r2 for the first), we performed a power analysis with nQuery Adviser (Elashof 1995).
A Kolmogorov-Smirnov test showed no significant deviation from a normal distribution for FL (Z=0.68, P=0.74), HS (Z=1.05, P=0.23), and the mean number of sired offspring (Z=0.85, P=0.46). As the non-signed FA presents a half-normal distribution, we performed a Box-Cox transformation as recommended in Møller and Swaddle (1997) with y2=(y1−0.02)0.3 (y2=transformed measure of FA; y1=FA). The distribution of Box-Cox transformed FA did not significantly deviate from normality according to a Kolmogorov-Smirnov test (Z=0.53, P=0.94).
As the percentage of young in the harem sired by the harem holder was not normally distributed, we tested whether the Spearman-rank correlation index significantly deviated from zero. As we tested for a correlation between reproductive success and both FL and FA, we based the analysis on a Bonferroni-corrected α of 2.5%. For standard statistical analysis, we used SPSS (1994). Means are quoted ±1 standard deviation and all tests were two-tailed.
Results
Parentage patterns
Between 1996 and 2001, 78 female sac-winged bats reproduced in the study colony. On average, 91±7% of the females within the colony gave birth to an offspring per cohort. All probable progeny of the colony could be assigned to a mother, either through genetic analysis alone or through both behavioural and genetic analysis. In total, we observed 129 adult males in the colony between 1996 and 2001. During the 3-year period of morphological measurements, 21 males defended a territory. The results of paternity assignments for all 209 tested juveniles are listed in Table 2. For 144 juveniles, only 1 male remained as putative father after exclusions. For 24 juveniles, the most likely male was regarded as father although more than 1 male showed either no or 1 exclusion. In total, 168 juveniles were assigned to a father. For 145 juveniles, likelihood calculations yielded a significant difference between the likelihood ratios of the 2 most likely males at the 95% significance level. In all cases where only one male was not excluded as putative father, the respective male was also the most likely father at the 95% level. For 31 juveniles, all males were excluded as putative father, and for 10 juveniles no father could be assigned as putative father because of lack of confidence. In total, 96 juveniles were assigned to the territorial males under study.
Male morphology and reproductive success
The forearm length of the 21 territorial males averaged 45.05±1.06 mm, which did not significantly deviate from the FL of non-territorial males (44.60±0.68 mm, Student t-test: t30=1.29, P=0.21). The average fluctuating asymmetry of forearms equalled 0.10±0.16 mm (0.2% of the average FL) for territorial males and 0.12±0.12 mm for non-territorial males. The difference in FA between territorial and non-territorial males was not significant (Student t-test, t30=0.82, P=0.42). The mean size of harems defended by these males equalled 1.46±1.56 females. On average, the 21 males sired 0.95±0.72 juveniles per year in the colony.
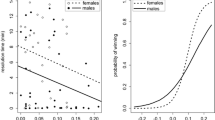
Mean reproductive success of territorial males did not correlate with the mean number of females defended by the male (weighted stepwise multiple regression: t=0.88, n=21, P=0.93), whereas reproductive success of males significantly decreased with increasing size and increasing asymmetry (see Table 3 for details of statistics; Fig. 2a, b). The level of determination of the regression model equalled r2=0.60. The probability of detecting an increase in r2 by including a second covariate in the regression model (adjusting for the first) equalled 96% for both variables FA and FL (Table 3).
Relationship between morphological parameters of males (forearm length: filled circles; fluctuating asymmetry of forearms: unfilled circles) and their mean reproductive success per year (a, b) and the percentage of juveniles sired within their harem (c, d) (individual circles are adjusted in size according to the number of seasons the individual was present in the colony). Fluctuating asymmetry of forearm length is expressed as non-signed Box-Cox transformed data (see Methods). Regression lines were calculated according to a weighted stepwise multiple regression analysis. The mean reproductive success of males was negatively related to both mean forearm length and asymmetry of the corresponding harem holders, whereas the percentage of intra-harem young (IHY) to total young born in the harem was significantly related only to mean forearm length and not to fluctuating asymmetry.
As we did not find a significant correlation between FL and FA of males (rs=0.08, P=0.37, n=3, Fig. 3), we conclude that small and symmetric males had a higher reproductive success than large and asymmetric males. We could not find a significant relationship between FA and FL for all males present in the colony during the study period (rs=0.06, n=32, P=0.76).
We hypothesized that small or symmetric males should be better able to monopolize a harem than large or asymmetric males, because of increased agility on the wing and lower energy costs. The percentage of the total number of young in the harems that were sired by the harem holder increased with decreasing FL (rs=−0.62, P=0.003, n=3, Fig. 2c), but not with decreasing FA (rs=−0.18, P=0.43, n=3, Fig. 2d).
Discussion
Male size and harem monopolization
The present study analysed the relationship between morphological traits, i.e. body size and fluctuating asymmetry, and reproductive success in territorial male sac-winged bats (S. bilineata). Small male sac-winged bats sired, on average, more offspring per juvenile cohort than large males, and small males sired more offspring within their harem than large males. This implies that small males were better able to monopolize the harem group, either by preventing competing males from sneaking into the territory or by preventing females from mating with a male of a different territory. Prior to and during the mating season, female S. bilineata occasionally switch between territories (Tannenbaum 1975, personal observation), and harem holders follow their potential mates, trying to prevent them from landing at any place other than within their territory. As other males may take over the territory in the meantime (Voigt and Streich 2003), harem males are constrained in the time they can devote to the pursuit of a mate. Thus, the ability of harem males to regain control over their females as fast as possible may reduce the percentage of extra-harem young within their harems, and small size via increased aerial agility could contribute to the success of harem monopolization.
In addition to increased agility and manoeuvrability, small individuals also encounter lower flight costs than large individuals (Norberg 1995; Norberg and Rayner 1987). Previous studies in male sac-winged bats showed that the field metabolic rate of males scaled with an exponent of 2 with body mass (Voigt et al. 2001). This scaling exponent is similar to that of the intraspecific scaling relationship of flight power with body mass (Voigt 2000; Voigt and Winter 1999). In the current data set, the range of body size equalled 10% of the mean value. According to the scaling factor of 2, a 10% difference in body mass translates into a 20% difference in flight power. Thus, small males probably have lower flight costs and this could enable them to allocate relatively more energy to harem defence and aerial courtship displays. This energetic benefit of small size may add to the advantage of increased agility.
If small-sized males have a competitive advantage over large males, other selective forces act against small size to prevent the evolution of smaller males. For example, large size may be beneficial during adverse climatic conditions because large animals lose relatively less heat as a consequence of their beneficial surface to volume ratio (Kleiber 1966). Large individuals may also be capable of storing relatively more fat than small individuals. Both physiological aspects may lead to an increased likelihood of survival for large males during adverse conditions.
In some birds, small males are chosen by females either because they are better hunters or because they are more economical in breeding. In the kestrel (Falco tinnunculus), small-sized males are better providers in courtship and offspring feeding (Hakkarainen et al. 1996). The authors suggested that female kestrels may gain direct fitness benefits from choosing small males because of their superior flight performance. In moorhens (Gallinula chloroplus), females prefer small fat males because they enable females to initiate more breeding attempts (Petrie 1983). In both species, the evolutionary benefit to select small males is related to parental care. Male sac-winged bats, however, do not provide parental care, e.g. we never observed males defending the juveniles within their harem; harem young never profited from clustering with the territory holder, and harem males never fed the harem offspring. Thus, regarding parental care, male size should be irrelevant in the mating system of S. bilineata.
Male asymmetry and sexual selection
Fluctuating asymmetry is considered to be related to an individual’s ability to withstand environmental or genetic instability during ontogenesis (summarized in Møller and Swaddle 1997). According to this theory, females select males with a low degree of FA, as these males are genetically superior to males with a high degree of FA. In addition, symmetry could also directly benefit males because of lower costs for locomotion and increased agility (Swaddle et al. 1996; Witter et al. 1994). In S. bilineata, symmetric males had a higher reproductive success than asymmetric individuals, and FA was not related to the percentage of intra-harem young. Studies of the relationship between FA and male reproductive success in natural populations of mammals are rare. Kruuk et al. (2003) found a significant negative correlation between the breeding success of male red deer (Cervus elaphus) and the FA of antlers. As only one of four measurements of FA correlated with reproductive success and as antlers are not under the influence of female choice in red deer, the authors concluded that their finding was merely a mechanistic effect rather than a proof of FA indicating male quality. As outlined in Møller (1980), FA and trait size should be negatively correlated as only high-quality males are able to sustain the costs of exaggerated size in sexually selected trait. A proof of correlation is controversial and not supported, for example, by Kruuk et al. (2003). The degree of inbreeding in the studied males was not the focus of our experiment and we, therefore, cannot address the question of higher levels of FA in inbred individuals at this point. In addition, we are also not able to prove whether FA is heritable in sac-winged bats. Our data show, nonetheless, a significant negative correlation between FA and the number of sired offspring per cohort, and no correlation between FA and the percentage of young sired within the harem of the male. We argue that, in contrast to forearm size, wing symmetry may not be relevant for a male’s ability to monopolize his harem. Instead, female S. bilineata possibly choose symmetric males as their mating partners and, as a consequence, symmetric males could be able to sire more offspring than asymmetric males. An important role of female choice in the mating system of S. bilineata is supported by the reversed sexual size dimorphism in this species, with females being larger than males (Bradbury and Emmons 1974), a high rate of extra-harem young (Heckel and von Helversen 2002) and diverse and multimodal courtship displays (Behr and von Helversen 2004; Bradbury and Emmons 1974; Davidson and Wilkinson 2002, 2004; Voigt and von Helversen 1999). Summarizing, we argue that male asymmetry could possibly be involved in female choice in sac-winged bats, whereas small size seems to be more important for harem monopolization.
References
Anderson SS, Fedak MA (1985) Grey seal males: energetic and behavioural links between size and sexual success. Anim Behav 33:928–838
Andersson M (1994) Sexual selection. Princeton University Press, Princeton
Backhaus K, Erichson B, Plinke W, Weiber R (1996) Multivariate Analysemethoden. Springer, Berlin Heidelberg New York
Behr O, Helversen O von (2004) Bat serenades—complex courtship songs of the sac-winged bat (Saccopteryx bilineata). Behav Ecol Sociobiol 56:106–115
Bradbury JW, Emmons L (1974) Social organization of some Trinidad bats. I. Emballonuridae. Z Tierpsychol 36:137–183
Bradbury JW, Vehrencamp SL (1976) Social organization and foraging in Emballonurid bats. I. Field studies. Behav Ecol Sociobiol 1:337–381
Bradbury JW, Vehrencamp SL (1977) Social organization and foraging in Emballonurid bats. III. Mating systems. Behav Ecol Sociobiol 2:1–17
Davidson SM, Wilkinson GS (2002) Geographic and individual variation in vocalizations by male Saccopteryx bilineata (Chiroptera: Emballonuridae). J Mammal 83:526–535
Davidson SM, Wilkinson GS (2004) Function of male song in the greater white-lined bat, Saccopteryx bilineata. Anim Behav 67:883–891
Elashoff JD (1995): nQuery Adviser Version 4.0
Hakkarainen H, Huhta E, Lathi K, Lundvall P, Mappes T, Tolonen P, Wiehn J (1996) A test of male mating and hunting success in the kestrel: the advantages of smallness. Behav Ecol Sociobiol 39:375–380
Heckel G, Helversen O von (2002) Male tactics and reproductive success in the harem polygynous bat Saccopteryx bilineata. Behav Ecol 13:750–756
Heckel G, Helversen O von (2003) Genetic mating system, relatedness and the significance of harem associations in the bat Saccopteryx bilineata. Mol Ecol 12:219–227
Heckel G, Voigt CC, Mayer F, Helversen O von (1999) Extra-harem paternity in the white-lined bat Saccopteryx bilineata. Behaviour 136:1173–1185
Heckel G, Achmann R, Mayer F (2000) Highly polymorphic microsatellite markers in the white-lined bat (Saccopteryx bilineata). Mol Ecol 9:242–244
Kleiber M (1966) The fire of life. Wiley, New York
Kruuk LEB, Slate J, Pemberton JM, Clutton-Brock TH (2003) Fluctuating asymmetry in a secondary sexual trait: no associations with individual fitness, environmental stress or inbreeding, and no heritability. J Evol Biol 16:101–113
Marshall TC, Slate J, Kruuk LEB, Pemberton JM (1998) Statistical confidence for likelihood-based paternity inference in natural populations. Mol Ecol 7:639–655
Møller AP (1980) Fluctuating asymmetry in male sexual ornaments may reliably reveal male quality. Anim Behav 40:1185–1187
Møller AP, Swaddle JP (1997) Asymmetry, developmental stability, and evolution. Oxford University Press, Oxford
Norberg UM (1995) How a long tail and changes in mass and wing shape affect the cost for flight in animals. Funct Ecol 9:48–54
Norberg UM, Rayner JMV (1987) Ecological morphology and flight in bats (Mammalia; Chiroptera): wing adaptations, flight performance, foraging strategy and echolocation. Philos Trans R Soc Lond B 316:335–427
Petrie M (1983) Female moorhens compete for small fat males. Science 220:413–414
Rose KE, Clutton-Brock TH, Guinness FE (1998) Cohort variation in male survival and lifetime breeding success in red deer. J Anim Ecol 67:979
Say L, Naulty F, Hayden TJ (2003) Genetic and behavioural estimates of reproductive skew in male fallow deer. Mol Ecol 12:2793
Schulte-Hostedde A, Millar JS, Gibbs HL (2003) Female-biased sexual size dimorphism in the yellow-pine chipmunk (Tamias amoenus): sex-specific patterns of annual reproductive success and survival. Evolution 56:2519–2529
Shuster SM, Wade MJ (2003) Mating systems and strategies. Princeton University Press, Princeton
SPSS (1994) SPSS Trends 6.1. SPSS
Stockwell EF (2001) Morphology and flight manoeuvrability in New World leaf-nosed bats (Chiroptera: Phyllostomidae). J Zool 254:505–514
Swaddle JP (1997) Within-individual changes in developmental stability affect flight performance. Behav Ecol 8:601
Swaddle JP, Witter MS, Cuthill IC (1994) The analysis of fluctuating asymmetry. Anim Behav 48:986–989
Swaddle JP, Witter MS, Cuthill IC, Budden A, McCowen P (1996) Plumage condition affects flight performance in common starlings: implications for developmental homeostasis, abrasion and moult. J Avian Biol 27:103–111
Tannenbaum R (1975) Reproductive strategies in the white-lined Bat. PhD Thesis, Cornell University
Thomas ALR (1993) The aerodynamic costs of asymmetry in the wings and tail of birds: asymmetric birds can’t fly round tight corners. Proc R Soc Lond B 254:181–189
Voigt CC (2000) Intraspecific scaling of flight costs in the bat Glossophaga soricina (Phyllostomidae). J Comp Physiol B 170:403–410
Voigt CC, Helversen O von (1999) Storage and display of odor by male Saccopteryx bilineata (Chiroptera; Emballonuridae). Behav Ecol Sociobiol 47:29–40
Voigt CC, Streich WJ (2003) Queuing for harem access in colonies of the sac-winged bat. Anim Behav 65:149–156
Voigt CC, Winter Y (1999) The energetic costs of hovering flight in nectar-feeding bats (Phyllostomidae, Glossophaginae) and its scaling in sphingid moths, hummingbirds and bats. J Comp Physiol B 169:38–48
Voigt CC, Helversen O von, Michener RH, Kunz TH (2001) The economics of harem maintenance in the sac-winged bat Saccopteryx bilineata. Behav Ecol Sociobiol 50:31–36
Voigt CC, Heckel G, Helversen O von (2003) Conflicts and strategies in the harem-polygynous mating system of the sac-winged bat Saccopteryx bilineata. In: McCracken G, Zubaid A, Kunz TH (eds) Functional and evolutionary ecology of bats, Oxford University Press, Oxford
Witter MS, Cuthill IC, Bonser RHC (1994) Experimental investigations of mass-dependent predation risk in the European starling, Sturnus vulgaris. Anim Behav 48:201–222
Worthington-Wilmer J, Barrat E (1996) A non-lethal method of tissue sampling for genetic studies of chiropterans. Bat Res News 37:1–3
Worthington-Wilmer J, Overall AJ, Pomeroy PP, Twis SD, Amos W (2000) Patterns of paternal relatedness in British grey seal colonies. Mol Ecol 9:283–292
Acknowledgements
We wish to express our thanks to Martina Brandt, Anne Brunner, Sonja Meister, Oliver Behr, Otto von Helversen, Marion East, and Heribert Hofer. We also thank the Costa Rican authorities for permission to conduct research in Costa Rica, and the Organization for Tropical Studies for permission to work on their property. Financial support was provided by the Deutsche Forschungsgemeinschaft (DFG Vo 890/3).
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by G. Wilkinson
Rights and permissions
About this article
Cite this article
Voigt, C.C., Heckel, G. & Mayer, F. Sexual selection favours small and symmetric males in the polygynous greater sac-winged bat Saccopteryx bilineata (Emballonuridae, Chiroptera). Behav Ecol Sociobiol 57, 457–464 (2005). https://doi.org/10.1007/s00265-004-0874-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00265-004-0874-6