Abstract
Sperm competition will be the inevitable consequence of polyandrous mating behavior if two or more males inseminate a single female. It has been demonstrated for a wide variety of animals that males adapt to this situation behaviorally, physiologically and morphologically, e.g. by evolving relatively large testes size to produce more sperm. All pair-living primates investigated so far were found to have relatively small testes, suggesting a monandrous mating system. We investigated the relationship between extra-pair paternity (EPP) rate as a measure of sperm competition intensity and relative testes size in a pair-living primate, the fork-marked lemur (Phaner furcifer). Paternity exclusion analyses for seven offspring using six polymorphic DNA-microsatellite markers suggested a high EPP rate. Female nocturnal travel distances were longer during the mating season, suggesting that females take an active role in achieving extra-pair copulations (EPCs). Surprisingly, fork-marked lemur testes size was relatively small compared to 23 other lemuroid primates, a result that is in contrast to predictions of sperm competition theory. Neither possible behavioral and morphological adaptations to an alternative paternity guard (i.e. mate guarding), nor sampling biases, phylogenetic constraints, and population density effects explain the absence of large testes in a species with high EPP, a phenomenon also known from birds with moderate to low EPP rates. We conclude that more data are needed on the frequency of EPCs, the timing of in-pair and extra-pair copulations, as well as the role of female choice, to explain why males of some species apparently do not adapt to sperm competition.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Sperm competition theory posits that, if the sperm of several males compete for fertilization of a female’s egg, and if fertilization success is positively related to the relative amount of sperm a male inseminates, then sexual selection will favor males producing large sperm quantities (Parker 1970; recent review in Birkhead and Kappeler 2003). Because larger testes generally produce larger ejaculates (Møller 1988) and more sperm (Møller 1989), males living in polyandrous mating systems should have larger testes than males living in monandrous mating systems. This prediction has been supported in comparative studies on primates (Short 1979; Harcourt et al. 1981; Harvey and Harcourt 1984; Kappeler 1997a) and many other taxa (review, e.g., in Møller and Briskie 1995). Due to a lack of socio-genetic work, in particular on pair-living taxa, comparative studies of primate testes-size variation have deduced the mating system of a species from their social organization (e.g. Harcourt et al. 1981; Harvey and Harcourt 1984). Assuming that mating takes place only within social units may lead to spurious results, however, if extra-pair paternity (EPP) occurs in pair-living species.
Extra-pair paternity has been found among pair-living birds (Barber et al. 1996; Møller and Ninni 1998), reptiles (Bull et al. 1998), fish (DeWoody et al. 2000) and mammals (e.g. Girman et al. 1997; Goossens et al. 1998; Wolff and Dunlap 2002), including fat-tailed dwarf lemurs (Fietz et al. 2000). It cannot be concluded, however, that EPP will occur in every pair-living species, because strict genetic monogamy has also been demonstrated in several pair-living non-passerine birds (e.g. Moreno et al. 2000; Michalek and Winkler 2001; Müller et al. 2001; Masello et al. 2002; Stanback et al. 2002), passerines (e.g. Haggerty et al. 2001), fish (Jones et al. 1998), and mammals (Ribble 1991; Heller et al. 1993; Brotherton et al. 1997; Sommer and Tichy 1999). If EPP is a consequence of females mating polyandrously, sperm competition intensity will vary with EPP rate. Accordingly, pair-living birds with high EPP rates exhibit relatively large testes (Møller 1991; Møller and Briskie 1995).
Genetic data to investigate the relationship between EPP rates and testes size in pair-living primates are scarce (but see Fietz et al. 2000; Oka and Takenaka 2001; Nievergelt et al. 2002). The potential variation in EPP rates among pair-living primates, however, has been attributed to differences in social organization (van Schaik and Kappeler 2003; Schülke 2003a). Pair-living among primates has long been thought of as a homogenous phenomenon involving long-lasting close bonds between pair partners reflected by affiliative interactions and highly cohesive movements (e.g. Mason 1974; Kleimann 1977; van Schaik and Dunbar 1990; Anzenberger 1992). Recent comparative analyses, however, revealed that pair-living primate species exhibit variation along two axes (bracketed terms refer to van Schaik and Kappeler 2003): (1) species are either strictly pair-living (uniform pairs) or some larger percentage (>10%) of social units are groups of more than two adults (variable pairs); (2) they either form cohesive units (associated pairs) or they spend a significant percentage of their activity time apart from their pair-partners (dispersed pairs). On the basis of phylogenetic reconstruction and reasoning about the causes of character shifts, it has been proposed that EPP rates are high among variable pairs and low among uniform pairs (van Schaik and Kappeler 2003). The small (330 g), nocturnal fork-marked lemur (Phaner furcifer) lives in dispersed variable pairs (Schülke and Kappeler 2003) and is, thus, expected to exhibit high levels of EPP. As a consequence, they are also expected to exhibit a relatively large testes size. However, neither the existence or extent of EPP, nor relative testes size have been examined in this species.
Fork-marked lemur pair-partners spend three-quarters of their activity time apart from each other (Schülke and Kappeler 2003). The key factor determining year-round low cohesion between pair-partners is avoidance of direct feeding competition. Frequent encounters at a limited number of food resources invariably lead to agonistic interactions between pair-partners, with females dominating males in all cases (Schülke 2003c; Schülke and Kappeler 2003). Nevertheless, pairs are stable over time and represented the modal grouping pattern in 18 out of 21 group years. Pair-partners use well-defined territories (5 ha) with small overlapping areas between pairs where neighbors meet regularly (once every 6 h). Fork-marked lemurs spend the day in hollow trees where pair-partners associated on average every 3rd day (Schülke and Kappeler 2003). Reproduction is highly seasonal and limited to a few weeks per year. Strong limits to group size, together with constraints on juvenile dispersal, lead to low reproductive rates with only 0.3 offspring per year and female (Schülke 2003c). These low reproductive rates among females induce a strong skew in operational sex ratio, increasing male intrasexual competition for females (Kvarnemo and Ahnesjö 1996).
To test predictions about EPP rates and testes size, we present the first paternity data for fork-marked lemurs in this paper. To place these data in a sufficiently broad comparative perspective, we also analyze variation in relative testes size among 24 lemur species. If high EPP rates could be confirmed, we predicted that fork-marked lemurs should have large testes for a lemur of its body size. If males do not copulate frequently with their partner and do not inseminate large amounts of sperm, they may alternatively guard their paternity via mate guarding and compete directly for access to females (Møller and Birkhead 1991). We present data on morphological adaptations to pre-copulatory male competition, i.e. sexual dimorphism in body and canine size, as well as data on behavioral adaptations to high levels of EPP to evaluate these alternative hypotheses. With respect to EPP, we predict that: (1) if males guard their pair-partners during the mating season, they should increase the rate of association at the diurnal sleeping site during the mating season; (2) if male mate guarding opposes the interests of the female, frequencies of intersexual agonistic interaction should increase in the mating season; (3) if males and/or females actively seek extra-pair copulations (EPCs), they are expected to search for and assess additional mating partners in the vicinity of their territory. This effort should result in increased travel distances, increased proportions of time spent in the periphery of the territory, and increased rates of encounters with neighbors and strange conspecifics during the mating season.
Methods
Study site and animals
The study was conducted at Kirindy Forest, which is situated at 44°39′E/20°03′S in western Madagascar. On the forest concession of the Centre de Formation Professionelle Forestière de Morondava (C.F.P.F.), the Deutsches Primatenzentrum Göttingen, Germany operates a research station. Kirindy Forest is part of one of the largest remaining fragments of dry deciduous forest in Madagascar. The 900×700 m grid system (25 m squares) where the study was conducted is locally known as CS7. The region is characterized by a short, hot rainy season from December to March, with an average rainfall of 800 mm and 8 months of virtually no precipitation. Between June and September, temperatures may drop as low as 5°C at night (for a detailed description of the forest and climate, see Ganzhorn and Sorg 1996).
Between November 1998 and April 2001, a total of 30 fork-marked lemurs were captured 103 times (1–8 times per individual). The age-sex composition and grouping patterns of the study population that was sampled and genotyped completely are given elsewhere (Schülke 2003c; Schülke and Kappeler 2003). Tomahawk life-traps (ca. 15×15×40 cm) were fixed to the entrance of sleeping sites in hollow trees. Typically, animals entered the unbaited trap 10–20 min after dusk. Animals were brought to the forest camp immediately after capture and were briefly anesthetized (0.02–0.04 ml Ketanest Pharmacia Upjon, Erlangen, Germany, 100 mg Ketanime/ml). Animals were weighed and standard field measurements were taken by O.S. (Schülke and Kappeler 2003). Until April 2000, only harness-fitted radio tags (10 g) especially designed for this study by Biotrack (Wareham, UK) were used because males possess a large scent gland on their throat that might get irritated by collar tags. From June 2000 onwards, females were fitted with brass collar tags (14 g) because harness tags were often removed by the animals. Tag life-time was approximately 1 year and tags were replaced whenever necessary. The study is ongoing and animals are still wearing tags. Animals were released at their capture site later the same night. All sleeping trees where animals were captured once were reused by the same individuals after some time. All procedures caused no noticeable harm to the animals and were in compliance with current laws of Madagascar.
Data collection
Behavioral and spatial data were sampled between June 1999 and August 2000 and from February to April 2001. Animals were located and followed using a Telonics TR-4 receiver with a three-element yagi antenna, a head-lamp and, if fine details needed to be observed, a strong flashlight and binoculars. Focal animals were all adult males and females from eight fork-marked lemur families carrying radio collars at the respective time. Between November 1998 and April 2001, 12 different males and 8 females lived in these families (for details of demographic changes, see Schülke and Kappeler 2003). For most analyses, a subset of only six pairs was used because of bad observation conditions in one pair and lack of observations on the male in another pair. During 2-h follows, the focal animal’s (Altmann 1974) spatial position within the grid system of 25×25 m squares was recorded continuously. Focal follows were conducted in the first half of the night and were evenly distributed across this time period. Observations started with the first focal animal leaving the sleeping site (about 1745–1845 hours depending on season) and were terminated only after the third 2-h protocol was finished between approximately 0030 and 0215 hours (depending on season and search times between protocols). Behavioral data on all social interactions (affiliative and agonistic) were recorded continuously. Visual contact times were recorded instantaneously at 2.5-min intervals. The number of observation hours accumulated per animal varied between 12 and more than 120 and totaled 1,227 h for all individuals over the entire study period. During 65% of observation time (average across months and individuals), visual contact was maintained.
Sleeping sites of radio-tagged animals were determined between April 1999 and August 2000 and again between February and April 2001 on a total of 419 days. Animals were classified as adult or non-adult according to body length, body mass, cranium size, teeth wear and, in the case of males, testes development and size of the sexually dimorphic throat gland. It was assumed that animals not yet fully grown when first captured were natal to the territories they were caught in. DNA microsatellite analyses of relatedness (see below) did not contradict any of these assigned mother-offspring relationships.
Data on relative testes size were taken from an earlier review (Kappeler 1997a, eight species), gathered from the literature (nine species) and through personal communication with colleagues (six species). In case two or more independent references were found for one species, preference was given to data sampled from wild populations, and in case more than one wild population had been investigated, the larger sample was preferred. In one case, the value from the literature was corrected because original authors used a slightly wrong formula to calculated testes size (Pochron and Wright 2002). Species were classified as pair-living if more than 50% of observed social units consisted of pairs. Species living solitary or in dispersed groups are assumed to exhibit polygynandrous mating systems (e.g. Kappeler 1997b; Eberle and Kappeler 2002; Radespiel et al. 2002) and were thus lumped in one category with species living in cohesive groups. The classification of white-footed sportive lemurs (Lepilemur leucopus) as being solitary or dispersed group-living follows Müller and Thalmann (2000), whereas greater dwarf lemurs (Cheirogaleus major) were conservatively classified as being not pair-living.
Data analyses
Spatial data were sub-sampled at 5-min intervals, thereby making consecutive data points independent because 5 min are enough for an individual to reach any point in its home range at highest locomotor speed (Rooney et al. 1998). Minimum convex polygons (MCPs: Mohr 1947) were calculated with Tracker 1.1 software (Camponotus and Radio Location Systems) with 5% outlier removal by the harmonic mean method (for details of home-range analyses, see Schülke and Kappeler 2003). The periphery of a home range was operationally defined as all grid squares that touched the border of the 95% MCP.
Testis volume was calculated from testis length (TL) and width (TW) assuming the form of a spherical ellipsoid: V=1/6×π×(TL×TW2). Values for right and left testis of each individual were averaged for these calculations.
In order to compare behavior between the mating and the non-mating season (Nms), the mating season (Ms) had to be defined. Despite good observation conditions in the dry season 1999, only one copulation (18.10.1999) and one case of mate guarding (03.11.99) were observed. Hence, the mating season could not be defined using the distribution of actual matings. Lemur breeding seasons are generally short (Pereira 1991). Births occurred in late February and in early March in 2000 and in late January in 2001 (Schülke 2003b). The female that mated in October 1999 gave birth between 2 and 5 March. Using this information and assuming gestation to be comparable in length to that of closely related, similar-sized species (e.g. Mirza coquereli: 90 days, Stanger et al. 1995), the mating season was inferred to take place in October or November.
DNA microsatellites and paternity analysis
DNA was obtained from small (ca. 3×3 mm) tissue samples taken from the animals’ earlobes at first capture. Samples were preserved in 70% ethanol and stored at 4°C for 3–13 months. DNA extraction was carried out using the a QIAamp Tissue Kit (Qiagen, Hilden, Germany) following the instructions of the manufacturer, and extracts were stored at −20°C. For details of PCR procedures and information about genotyping, see Hapke et al. (2003a, 2003b).
Paternity analyses were performed at 6 loci amplified with primers designed for C. medius (N=4) and Microcebus murinus (N=2), which were moderately polymorphic with 2–6 alleles (mean 3.7 alleles) in the sampled population of 30 fork-marked lemurs (Table 1). For details on Hardy-Weinberg-Equilibrium tests and linkage analyses, see Hapke et al. (2003b). Informative homozygotes were genotyped at least four times in independent PCRs to control for allelic dropout phenomena. Mother-offspring relations were known from detailed behavioral observation (1999–2001) or inferred for offspring born before 1999. Inferred mother-offspring relationships were never contradicted by the genetic data. All 12 males were included in paternity exclusion analysis (Table 1), irrespective of the date of first capture (November 1998 to February 2002). Throughout the study, there was no evidence for the existence of non-territorial or transient males in the population. Exclusion power for the second parent calculated with Cervus 2.0 software (Marshall et al. 1998) varied between 14% and 49% between loci, combining to a total paternity exclusion probability of 89%. Paternity exclusion analysis was carried out by hand, assuming that any mismatch excludes a male from paternity.
The time lag between the conception of the respective offspring and the first unequivocal identification of the social father varied between 0 and 24 months. According to demographic data from a 3-year period, the average male tenure in a pair is 22.3±11.8 months (N=9), which is an underestimation because the maximum of 36 months was observed in 2 of the 3 males that were caught during the first field season, and because it is unlikely that all tenures measured ended exactly when data sampling ended after 3 years. Although it cannot be verified retrospectively, we assumed for the subsequent paternity analyses that males residing in a pair at a given time have been residents at maximum 2 years before and are the social father of offspring sired during that time.
Paternity likelihood analysis was performed using Cervus 2.0 software (Marshall et al. 1998). Simulations (10,000) were run on the genotypes of 30 individuals at 6 loci under the assumption that 50% of candidate fathers have not been sampled and that typing errors occur at a rate of 1%. The likelihood that a given male is the true father was compared to the likelihood that this male is not the true father given the observed genotypes (likelihood ratio). The product of the likelihood ratios of all genotypes were log (base e) transformed (LOD score) and compared across males. Positive LOD scores denominate males that are more likely to be the true father than an arbitrary randomly chosen male. The male with the highest LOD score is the most likely father. Confidence limits of 80% and 95% for the differences in LOD scores between the most likely and the second most likely father were calculated from the simulation to describe whether these differences are likely to occur by chance.
Statistical analyses
Individual mean travel distances varied considerably (males: 1,345–2,335 m, females: 913–1,578 m per 6 h), which can be attributed to differences in territory size between pairs. The larger a female’s territory, the longer her travel distance per night (two-tailed Kendall’s Tau correlation: Tau6=1.00, P=0.008). Since territories of pair-partners are of similar size and travel distances are related to territory size, only males and females from the same pair can be compared between seasons and paired tests are applied. For behavioral data and data on space use, medians and inter-quartile ranges are given with the results of statistical tests, whereas morphometric data that were compared between males and females in parametric tests are presented as means and standard deviations. Because body mass may vary with season, only individuals captured within a 1-month period in June 2000 were compared to determine body-mass dimorphism. The alpha level was set at 0.05 and exact P-values are given to allow for a rough estimation of the beta error in case of non-significant results. Where not mentioned otherwise, tests were performed one-tailed in accordance with the directional predictions deduced in the introduction. Most comparisons of measures between the mating season and the non-mating season were performed as Wilcoxon Matched Pairs-tests (WMP-test as implemented in Statistica 6.0), which control for inter-individual differences. Other tests used are the Mann-Whitney U-test (MWU-test) and Kendall’s Tau correlation, all performed with Statistica 6.0.
Results
Paternity analyses
In paternity exclusion analyses, in four cases the social father was excluded as genetic father at one to three loci, and in one case the social father was one of two males not excluded. In only two cases was the social father the only male not excluded as the genetic father. Non-resident males not excluded as potential fathers were direct neighbors in all cases (Table 1). Paternity-likelihood analyses revealed that social fathers were the most likely fathers in three of seven cases analyzed. In none of the four cases where social fathers were excluded from paternity by at least one genotypic mismatch were they the most likely father among the males sampled. In 2001 and 1999, i.e. in three cases, the only males not excluded from paternity (which are also the most likely fathers) were direct neighbors and known to be present in their respective territories at the time the offspring were conceived. Hence, although it can only be inferred that the social fathers of the two 1999 offspring were present during conception, we know that the respective most likely fathers resided in territories neighboring those of the extra-pair young. Thus, the paternity-likelihood analysis strengthened the result of the paternity-exclusion analyses that social fathers are often not the genetic fathers.
The social father was directly observed during the mating season in only two cases and his presence was inferred in the other five cases. However, these five cases included two cases where the most likely father was a neighbor confirmed to be present in the neighboring territory when the offspring was conceived. Regarding only these four most robust cases (two with known social fathers, and two with known neighbors as genetic fathers) yields an EPP rate of 0.75. Finding such a high incidence of EPP in such a small sample would be highly improbable if the population-wide EPP rate was very low.
Paternities may have been falsely assigned to neighboring males if the infant’s mother was closely related to the neighboring male. However, all males that immigrated into study families came from outside the marked population (N=6), although three full-grown male offspring ready to disperse were among the marked individuals in the study area (Schülke and Kappeler 2003). One female gained a breeding position in her natal territory and formed a pair with an immigrant male after her mother and her mother’s mate had died. Female migration has not been observed so far. Thus, high relatedness between resident adult females and males at the sibling or mother-offspring level is unlikely to have affected the results.
Relative testes size
A new comparative data set on relative testes size for Malagasy primates was compiled. In contrast to earlier studies (Harcourt et al. 1981; Kappeler 1997a), most data included here stem from wild populations. Closely related African and Asian strepsirhines were not included in the analyses to reduce phylogenetic noise. A regression between testes volume and body mass was calculated using double logarithmic scales to identify the general trend among species (log testes size=2.03+0.38×log body size, Fig. 1). As a general rule, values for lemurs living solitarily or in (dispersed) groups lay above the regression line, indicating relatively large testes size. Lemurs living in (dispersed) pairs, including fork-marked lemurs, exhibited relatively small testes size, with values lying below the regression line. The only case deviating considerably from expected patterns was the group-living Propithecus verreauxi verreauxi, with much smaller testes size than expected (Kraus et al. 1999).
Comparison of relative testes size across 24 Malagasy primates (r=0.73, P=0.003). Species are classified as: (1) living in (dispersed) pairs: unfilled circles, or (2) as being solitary or living in (dispersed) groups: filled squares. All measures taken during the mating season unless stated otherwise [Al Avahi laniger (Petter-Rousseaux 1964, N=1, mating season?), Cma Cheirogaleus major (Petter-Rousseaux 1964, N=1, mating season?), Cm Cheirogaleus medius (Fietz 1999a, N=45), Ea Eulemur albocollaris (Johnson et al. 2002, S. Johnson, A. Gordon, B. Bradley and R. Kitko, unpublished data, N=10), Ec Eulemur coronatus (Kappeler 1997a, N=8), Efr Eulemur fulvus rufus (Johnson et al. 2002, S. Johnson, A. Gordon, R. Stumpf, D. Overdorff and A. Merenlender, unpublished data, N=6), Ema Eulemur macaco (Kappeler 1997a, N=8), Emo Eulemur mongoz (Kappeler 1997a, N=9), Er Eulemur rubriventer (Kappeler 1997a, N=4), Hg Hapalemur griseus (Kappeler 1997a, N=5), Ii Indri indri (Powzyk 1997, N=2), Lc Lemur catta (Kappeler 1997a, N=12), Ll Lepilemur leucopus (L. Nash, unpublished data, N=8), Lr Lepilemur ruficaudatus (measures closest to ms, D. Zinner and R. Hilgartner, unpublished data, N=15), Mb Microcebus berthae (Schwab 2000), Mc Mirza coquereli (Kappeler 1997a, N=15), Mm Microcebus murinus (Fietz 1999b, N=23), Mr Microcebus ravelobensis (N. Hagenah, U. Radespiel, B. Randrianambinina, B. and E. Zimmermann, unpublished data, N=40), Msp Mirza spec nov (Kappeler 1997b, Kappeler and Roos, unpublished data, N=28), Pdd Propithecus diadema diadema (Powzyk 1997, N=3), Pde Propithecus diadema edwardsi (value of Pochron and Wright 2002 divided by 8, N=19), Pf Phaner furcifer (largest testes size per male, this study, N=12), Pvv Propithecus verreauxi verreauxi (Lewis and Kappeler, unpublished data), Vv Varecia variegata (Kappeler 1997a, N=11)]
Sexual dimorphism in body and canine size
Sexual dimorphism in favor of males was absent in fork-marked lemurs. Instead, females were slightly heavier than males (Fig. 2; t-test: N m=8, N f=6, t=−2.37, two-tailed P=0.035). Body-mass differences between males and females are not just a matter of size but reflect better physical condition in females (Schülke 2003c). Canine size residuals did not differ between the sexes (Fig. 2; t-test: N m=12, N f=9, t=−0.011, two-tailed P=0.991).
Mobility and space use
Average male travel distances were similar during and outside the mating season (WMP-test: T 6=7.00, P=0.232). In contrast, 6 out of 7 females traveled further during the mating season, yielding a statistical trend (WMP-test: T 7=3.00, P=0.058). As a result, male (median: 1.76, 0.88–2.15 km) and female (1.77, 1.71–1.84 km) travel distances were similar during the mating season (two-tailed WMP-test: T 6=10.00, P=0.917). To test whether ecological differences may explain these effects, we compared travel distances during the mating season with those of the period we assume to be ecologically most similar, i.e. the 2 months preceding the mating season. For this comparison, data from only five females were available and males were not compared due to small sample size. Females traveled much further in the mating season (median: 1.80, 1.73–1.84 km) than during the 2 preceding months (median: 1.41, 1.22–1.49 km), indicating that social rather than ecological causes were responsible for the observed increase in locomotor activity (WMP-test: T 5=0.00, P=0.021). For both sexes, no difference between mating and non-mating season was found in time spent in the periphery of the home ranges (Table 2), even if only outlier positions were considered (i.e. the 5% locations outside the 95% MCP borders). Note that the proportion of time spent in peripheral areas and outlier positions was remarkably high for both sexes throughout the year (Table 2).
Interactions within and between pairs
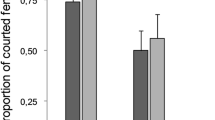
The mating season had a strong effect on the rate of intersexual agonistic interactions. In all pairs, conflicts occurred at higher rates during the mating than during the non-mating season (Fig. 3, WMP-test: T 6=0.00, P=0.014). Neither males nor females met more often with neighbors during the mating season. But meetings between neighbors involved more individuals during the mating season (median: 5.0, 3.0–6.0 individuals) than during the rest of the year (median: 4.0, 3.0–5.0; MWU-test: N Nms=67, N Ms=24, U=622, P=0.050). Sex ratio among meeting participants had a tendency to be more male-biased than in the non-mating season (median Ms: 1.0, 1–1.5, median Nms: 1.0, 0.5–1.0 males per female; Mann-Whitney U-test: N Nms=43, N Ms=21, U=350, P=0.061).
Sleeping associations
Pair-partners shared the same sleeping site at an average rate of 0.36±0.24 per day, i.e. about every 3rd day. When not sleeping in the same tree hole, pair-partners’ sleeping sites were on average 101± 60 m apart from one another, which is about half a territory diameter (Schülke and Kappeler 2003). The rate of association at the sleeping site did not differ between the mating season (median: 0.21, 0.0–0.72 per day) and non-mating season in all the 6 pairs (median: 0.28, 0.21–0.39 per day; WMP-test: T 6=10.0, P=0.458).
Discussion
Comparison of relative testes size across 24 lemur species revealed that fork-marked lemurs had small testes, as did all pair-living species in this sample and as do pair-living primates in general (Harcourt et al. 1981; Harvey and Harcourt 1984; Kappeler 1997a). This would be in accordance with sperm competition theory if pair-living females did not mate polyandrously. But high EPP levels found in pair-living fork-marked lemurs and fat-tailed dwarf lemurs (Cheirogaleus medius, Fietz 1999a; Fietz et al. 2000) suggest that females in some pair-living species indeed mate polyandrously, which implies a fundamental discrepancy with expectations derived from sexual selection and sperm competition theory. The question, therefore, is why fork-marked lemur males apparently did not adapt to sperm competition in this situation.
An alternative paternity guard
One possible explanation is that fork-marked lemurs rely on an alternative paternity guard (Møller and Birkhead 1991; Birkhead and Møller 1992) and use a mate guarding strategy instead of frequent copulations and insemination of large sperm quantities to gain paternity with their social mate. None of the results, however, are in accordance with intensive mate guarding: (1) sexual size dimorphism that would be indicative of intense male-male competition for access to mates (Darwin 1871) was absent although it can be found in other lemurs during the mating season (Kappeler 1997b; Schmid and Kappeler 1998), (2) mate guarding was observed only once during nocturnal activity, (3) mate guarding was evidently absent during the day with pair-partners sleeping at different sites 100 m apart, and (4) albeit increased agonistic interaction frequencies during the mating season that may indicate a conflict over mate guarding (Lijfeld et al. 1994), males lost in all conflicts.
In line with the lack of evidence for intensive mate guarding, several factors suggest that fork-marked lemur males should indeed not restrict their paternity guarding to mate guarding but invest (also) in sperm competition (van Schaik and Kappeler 2003). Female resistance has been shown to regulate male mate-guarding efficiency in some animals (Jormalainen and Merilaita 1995). Fork-marked lemur females are dominant (Schülke and Kappeler 2003) and slightly larger than males, which may constrain the male’s ability to physically force her to restrain from EPCs (Brotherton et al. 1997) or may prohibit male punishment of infidelity (Clutton-Brock and Parker 1995; Gowaty 1996). Instead of physically enforcing female fidelity, small and subordinate males may, however, simply deter rivals. The latter strategy may, however, be less viable in nocturnal species (Schülke 2003a). Nocturnal activity with low visibility allows a female to escape her guard easily while he is distracted by fighting off rivals.
The last factor potentially affecting the viability of male mate guarding as paternity guard in fork-marked lemurs is highly seasonal reproduction, which will produce opportunity costs (Grafen 1980) for males even if female fertile periods are not perfectly synchronized. The degree to which this opportunity cost reduces mate-guarding efficiency or effort depends on the degree of estrous synchronization among females (Emlen and Oring 1977) and on the amount of information males can gain over a female’s reproductive status (Eberle and Kappeler 2002; Reichert et al. 2002). Both factors are under female control and likely to play a role in determining male mate-guarding efficiency. Although conclusive analyses are lacking, the limited information available suggests that mate guarding may not be a viable paternity guard and thus may not explain small testes size in fork-marked lemurs.
Other explanations for small testes size in fork-marked lemurs
An alternative explanation for small testes size in fork-marked lemurs may be that the sample used to scale testes size of fork-marked lemurs is biased towards species with large testes. In this case, fork-marked lemur testes would appear to be relatively small although they are not. Comparison of testes size in lemurs and the sister taxon, the lorises, however, revealed no grade shift between the two groups of strepsirrhines in a previous study (Kappeler 1997a), making this explanation unlikely.
It may be argued that testes-size evolution in fork-marked lemurs is constrained. Pair-living evolved several times independently in the lemur clade (Kappeler 1998) and pair-living lemurs with small testes size come from seven different genera and from four of the five families of lemurs. In all cases, solitary or (dispersed) group-living species from the same family or even from the same genus as the pair-living ones exhibit large testes size in our sample, making phylogenetic constraints on testes-size evolution within this clade unlikely.
Alternatively, the discrepancy between small testes and high EPP levels may be explained by density effects. EPP might be exaggerated in the population because the population density is high, territories are compressed in size and the availability of extra-pair mates in the vicinity of a female is increased (Westneat and Sherman 1997). If this situation is unusual and has occurred only recently, fork-marked lemur males might not have adapted to the new situation yet in terms of increased sperm production. Fork-marked lemurs are apparently not distributed uniformly across Kirindy forest. Census data from ten 1-km2 plots in Kirindy forest indicate that fork-marked lemur densities vary between 153 and 555 individuals per km2 (Ganzhorn and Kappeler 1996). Density in the study area was intermediate on this scale and compared to other areas of the Malagasy dry forests, densities at Kirindy are intermediate as well (Ausilio and Raveloanrinoro 1998; Zinner et al. 2001). Thus, there is no indication for an artificially increased population density.
In conclusion, there is currently no simple explanation for the absence of male testes-size adaptation to a situation with potentially intense sperm competition in fork-marked lemurs. Although testes size was positively associated with EPP rate in a sample of 53 pair-living bird species, it varied tremendously among species with low EPP rates (<30% EPP, Møller and Briskie 1995), so that, just as these lemurs, several bird species with significant EPP rates exhibited relatively small testes. This lack of adaptation to sperm competition may be either due to an evolutionary disequilibrium with behavior evolving faster than morphological traits, or a current extreme in the evolutionary arms race between the sexes about the control of mating and paternity (Birkhead and Møller 1993; Davies et al. 1996), or because we still lack some important insight into the mechanisms of sperm competition. Paternity data of fork-marked lemurs and fat-tailed dwarf lemurs suggest that males may sire extra-pair young while being cuckolded in their own territory (sometimes in one season) and that (extra-pair) paternity is not concentrated upon a small number of males, which implies that female choice is not about absolute male quality and/or that paternity is not only controlled by females. More data are needed on mating behavior, especially on the timing of pair-copulations, on the efficiency of male mate-guarding, and on female choice for both deviating birds and lemurs before the lack of testes-size adaptation can be explained conclusively.
References
Altmann J (1974) Observational study of behavior: sampling methods. Behaviour 39:193–267
Anzenberger G (1992) Monogamous social systems and paternity in primates. In: Martin RD, Dixson AF, Wickings EJ (eds) Paternity in primates: genetic tests and theories. Karger, Basel, pp 203–224
Ausilio E, Raveloanrinoro G (1998) Les lémuriens de la région de Bemaraha: forêts de Tsimembo, de l’Antsingy et de la région Tsiandro. Lemur News 3:4–7
Barber CA, Robertson RJ, Boag PT (1996) The high frequency of extra-pair paternity in the tree-swallow is not an artefact of nestboxes. Behav Ecol Sociobiol 38:425–430
Birkhead TR, Kappeler PM (2003) Post-copulatory sexual selection in birds and mammals. In: Kappeler PM, van Schaik CP (eds) Sexual selection in primates. Cambridge University Press, Cambridge
Birkhead TR, Møller AP (1992) Sperm competition in birds: evolutionary causes and consequences. Academic, London
Birkhead TR, Møller AP (1993) Female control of paternity. TREE 8:100–104
Brotherton PNM, Pemberton JM, Komers PE, Malarky G (1997) Genetic and behavioural evidence for monogamy in a mammal Kirk’s dik-dik (Madoqua kirkii). Proc R Soc Lond B 164:675–681
Bull CM, Cooper SJB, Baghurst BC (1998) Social monogamy and extra-pair fertilization in an Australian lizard Tiliqua rugosa. Behav Ecol Sociobiol 44:63–72
Clutton-Brock TH, Parker GA (1995) Punishment in animal societies. Nature 373:209–219
Darwin C (1871) The descent of man, and selection in relation to sex. Murray, London
Davies NB, Hartley LR, Hatchwell BJ, Langmore NE (1996) Female control of copulations to maximize male help: a comparison of polygynandrous alpine accentors Prunella collaris and dunnocks P. modularis. Anim Behav 51:27–47
DeWoody JA, Fletcher DE, Wilkins SD, Nelson WS, Avise JC (2000) Genetic monogamy and biparental care in an externally fertilizing fish the largemouth brass (Micropterus salmoides). Proc R Soc Lond B 267:2431–2437
Eberle M, Kappeler PM (2002) Mouse lemurs in space and time—a test of the socioecological model. Behav Ecol Sociobiol 51:131–139
Emlen ST, Oring LW (1977) Ecology sexual selection and the evolution of mating systems. Science 197:215–223
Fietz J (1999a) Monogamy as a rule rather than exception in nocturnal lemurs: the case of the fat-tailed dwarf lemur (Cheirogaleus medius). Ethology 105:259–272
Fietz J (1999b) Mating system of Microcebus murinus. Am J Primatol 48:127–133
Fietz J, Zischler H, Schwiegk C, Tomiuk J, Dausmann KH, Ganzhorn JU (2000) High rates of extra-pair young in the pair-living fat-tailed dwarf lemur Cheirogaleus medius. Behav Ecol Sociobiol 49:8–17
Ganzhorn JU, Kappeler (1996) Lemurs of the Kirindy Forest. Primate Rep 46:257–274
Ganzhorn JU, Sorg J-P (1996) Ecology and economy of a tropical dry forest in Madagascar. Primate Rep 46:1–382
Girman DJ, Mills MGL, Geffen E, Wayne RK (1997) A molecular genetic analysis of social structure dispersal and interpack relationships of the african wild dog (Lycaon pictus). Behav Ecol Sociobiol 40:187–198
Goossens B, Graziani L, Waits LP, Farand E, Magnolon S, Coulon J, Bel M-C, Taberlet P, Allainé D (1998) Extra-pair paternity in the monogamous alpine marmot revealed by nuclear DNA microsatellite analysis. Behav Ecol Sociobiol 43:281–288
Gowaty PA (1996) Battles of the sexes and origins of monogamy. In: Black JM (ed) Partnership in birds—the study of monogamy. Oxford University Press, Oxford, pp 21–52
Grafen A (1980) Opportunity cost benefit and the degree of relatedness. Anim Behav 28:967–968
Haggerty TM, Morton ES, Fleischer RC (2001) Genetic monogamy in carolina wrens (Thryothorus ludovicianus). Auk 118:215–219
Hapke A, Eberle M, Zischler H (2003a) Isolation of new microsatellite markers and application in four species of mouse lemurs (Microcebus sp). Mol Ecol Notes 3:205–208
Hapke A, Schülke O, Zischler H (2003b) Microsatellite markers for paternity testing in fork-marked lemurs (Phaner furcifer). Mol Ecol Notes 3:438–440
Harcourt AH, Harvey PH, Larson SG, Short R (1981) Testis weight body weight and breeding system in primates. Nature 293:55–56
Harvey PH, Harcourt AH (1984) Sperm competition, testes size, and breeding systems in primates. In: Smith RL (ed) Sperm competition and the evolution of animal mating systems. Academic, San Diego, pp 589–600
Heller KG, Achmann R, Witt K (1993) Monogamy in the bat Rhinolophus sedulus? Z Säugetierkde 58:376–377
Johnson S, Gordon A, Stumpf R (2002) Sexual dimorphism and testicle size in white-collared lemurs (Eulemur albocollaris). Am J Phys Anthropol 117:90–91
Jones AG, Kvarnemo C, Moore GI, Simmons LW, Avise JC (1998) Microsatellite evidence for monogamy and sex-biased recombination in the Western Australian seahorse Hippocampus angustsus. Mol Ecol 7:1497–1505
Jormalainen V, Merilaita S (1995) Female resistance and duration of mate-guarding in three aquatic peracarids (Crustaceae). Behav Ecol Sociobiol 36:43–48
Kappeler PM (1997a) Intrasexual selection and testis size in strepsirrhine primates. Behav Ecol 8:10–19
Kappeler PM (1997b) Intrasexual selection in Mirza coquereli: evidence for scramble competition polygeny in a solitary primate. Behav Ecol Sociobiol 45:115–127
Kappeler PM (1998) Nests, tree holes, and the evolution of primate life histories. Am J Primatol 46:7–33
Kleimann DG (1977) Monogamy in mammals. Q Rev Biol 52:39–69
Kraus C, Heistermann M, Kappeler PM (1999) Physiological suppression of sexual function of subordinate males: a subtle form of intrasexual competition among male sifakas (Propithecus verreauxi)? Physiol Behav 66:855–861
Kvarnemo C, Ahnesjö I (1996) The dynamics of operational sex ratios and competition for mates. TREE 11:404–408
Lifjeld JT, Dunn PO, Westneat DF (1994) Sexual selection by sperm competition in birds: male-male competition or female choice? J Avian Biol 25:244–250
Marshall TC, Slate J, Kruuk LEB, Pemberton JM (1998) Statistical confidence for likelihood-based paternity inference in natural populations. Mol Ecol 7:639–655
Masello JF, Sramkova A, Quillfeldt P, Epplen JT, Lubjuhn T (2002) Genetic monogamy in burrowing parrots Cyanoliseus patagonus? J Avian Biol 33:99–103
Mason WA (1974) Comparative studies of social behavior in Callicebus and Saimiri: behaviour of male-female pairs. Folia Primatol 22:1–8
Michalek KG, Winkler H (2001) Parental care and parentage in monogamous great spotted woodpeckers (Picoides major) and middle spotted woodpeckers (Picoides medius). Behaviour 138:1259–1285
Mohr CO (1947) Table of equivalent populations of North American small mammals. Am Midl Nat 37:223–249
Møller AP (1988) Testis size, ejaculate quality, and sperm competition in birds. Biol J Linn Soc 33:273–283
Møller AP (1989) Ejaculate quality, testes size and sperm competition in mammals. Funct Ecol 3:91–96
Møller AP (1991) Sperm competition, sperm depletion, paternal care, and relative testis size in birds. Am Nat 137:882–906
Møller AP, Birkhead TR (1991) Frequent copulations and mate guarding as alternative paternity guards. Behaviour 118:170–186
Møller AP, Briskie JV (1995) Extra-pair paternity, sperm competition and the evolution of testes size in birds. Behav Ecol Sociobiol 36:357–365
Møller AP, Ninni P (1998) Sperm competition and sexual selection: a meta-analysis of paternity studies in birds. Behav Ecol Sociobiol 43:345–358
Moreno J, Boto L, Fargallo JA, de Leon A, Potti J (2000) Absence of extra-pair fertilizations in the chinstrap penguin Pygoscelis antarctica. J Avian Biol 31:580–583
Müller AE, Thalmann U (2000) Origin and evolution of primate social organisation: a reconstruction. Biol Rev 75:405–435
Müller W, Epplen JT, Lubjuhn T (2001) Genetic paternity analyses in little owls (Athene noctua): does the high rate of paternal care select against extra-pair young? J Ornithol 142:195–203
Nievergelt CM, Digby LJ, Ramakrishna U, Woodruff DS (2002) Genetic analysis of group composition and breeding system in a wild common marmoset (Callithrix jacchus) population. Int J Primatol 21:1–20
Oka T, Takenaka O (2001) Wild gibbons’s parentage tested by non-invasive sampling and PCR-amplified polymorphic microsatellites. Primates 42:67–73
Parker GA (1970) Sperm competition and its evolutionary consequences in the insects. Biol Rev 45:525–567
Pereira ME (1991) Asynchrony within estrous synchrony among ringtailed lemurs (Primates: Lemuridae). Physiol Behav 49:47–52
Petter-Rousseaux A (1964) Reproductive physiology and behavior of the lemuroidea. In: Buettner-Janusch J (ed) Evolutionary and genetic biology of primates. Academic, New York, pp 91–132
Pochron ST, Wright PC (2002) Dynamics of testes size compensates for variation in male body size. Evol Ecol Res 4:577–585
Powzyk JA (1997) The socio-ecology of two sympatric indrids: Propithecus diadema diadema and Indri indri. A comparison of feeding strategies and their possible repercussions on species-specific behaviors. PhD Thesis, Duke University
Radespiel U, Del Secco V, Drögemüller C, Braune P, Labes E, Zimmermann E (2002) Sexual selection, multiple mating and paternity in grey mouse lemurs, Microcebus murinus. Anim Behav 63:259–268
Reichert KE, Heistermann M, Hodges JK, Boesch C, Hohmann G (2002) What females tell males about their reproductive status: are morphological and behavioural cues reliable signals of ovulation in bonobos (Pan paniscus)? Ethology 108:583–600
Ribble DO (1991) The monogamous mating system of Peromyscus californicus as revealed by DNA fingerprinting. Behav Ecol Sociobiol 29:161–166
Rooney SM, Wolfe A, Hayden TJ (1998) Autocorrelational data in telemetry studies: time to independence and the problem of behavioural effects. Mammal Rev 27:89–98
Schaik CP van, Dunbar RIM (1990) The evolution of monogamy in large primates: a new hypothesis and some crucial tests. Behaviour 115:30–62
Schaik CP van, Kappeler PM (2003) The evolution of pair living in primates. In: Reichard U, Boesch C (eds) Monogamy: partnership in birds humans and other mammals. University of Cambridge Press, Cambridge
Schmid J, Kappeler PM (1998) Fluctuating sexual dimorphism and hibernation by sex in a primate, the gray mouse lemur (Microcebus murinus). Behav Ecol Sociobiol 43:125–132
Schülke O (2003a) Living apart together—patterns, ecological basis, and reproductive consequences of life in dispersed pairs of fork-marked lemurs (Phaner furcifer, Primates). PhD Thesis, Würzburg. http://opus.bibliothek.uni-wuerzburg.de/opus/volltexte/2003/502/
Schülke O (2003b) The life history of Phaner furcifer. In: Goodman SM, Benstead JP (eds) The natural history of Madagascar. Chicago University Press, Chicago
Schülke O (2003c) To breed or not to breed: food competition and other factors influencing female reproductive decisions in pair-living nocturnal fork-marked lemurs (Phaner furcifer). Behav Ecol Sociobiol 55:11–21
Schülke O, Kappeler PM (2003) So near and yet so far: territorial pairs but low cohesion between pair-partners in a nocturnal lemur Phaner furcifer. Anim Behav 65:331–343
Schwab D (2000) A preliminary study of spatial distribution and mating system of pygmy mouse lemurs (Microcebus cf myoxinus). Am J Primatol 51:41–60
Short RV (1979) Sexual selection and its component parts, somatic and genital selection as illustrated by man and the great apes. Adv Stud Behav 9:131–158
Sommer S, Tichy H (1999) Major histocompatibility complex (MHC) class II polymorphism and paternity in the monogamous Hypogeomy antimena the endagered largest endemic Malagasy rodent. Mol Ecol 8:1259–1272
Stanback M, Richardson DS, Boix-Hinzen C, Mendelson J (2002) Genetic monogamy in Monteiro’s hornbill Tockus monteiri. Anim Behav 63:787–793
Stanger KF, Coffmann BS, Izard MK (1995) Reproduction in Coquerel’s dwarf lemur (Mirza coquereli). Am J Primatol 36:223–237
Westneat DF, Sherman PW (1997) Density and extra-pair fertilizations in birds: a comparative analysis. Behav Ecol Sociobiol 41:205–215
Wolff JO, Dunlap AS (2002) Multi-male mating probability of conception and litter size in the prairie vole (Microtus ochrogaster). Behav Proc 57:105–110
Zinner D, Ostner J, Dill A, Razafimanantsoa L, Rasoloarison R (2001) Results of a reconnaissance expedition in the western dry forests between Morondava and Morombe. Lemur News 6:16–18
Acknowledgements
The study is part of the dissertation work of O.Schülke presented to the University of Würzburg. We thank the Commission Tripartite of the Malagasy Government, the Direction des Eaux et Forêts Madagascar for research permits, the Centre de Formation Professionelle Forestiére de Morondava for hospitality and Professor B. Rakotosamimanana from the Université d’Antananarivo and Felix Rakotondraparany at PBZT Antananarivo for their co-operation. R. Rasoloarison and L. Razafimanantsoa provided invaluable logistic support. We gratefully acknowledge the help of Jean-Claude from Beroboka, Alpha from Marofandilia and many fellow researchers at Kirindy during capture sessions—without them the study would have been impossible. We thank B. Bradley, A. Gordon, N. Hagenah, R. Hilgartner, S. Johnson, R. Kitko, R. Lewis, A. Merenlender, L. Nash, D. Overdorff, U. Radespiel, B. Randrianambinina, R. Stumpf, E. Zimmermann, and D. Zinner for providing us with previously unpublished data. R. Hilgartner, M. Port, A. Perez-Mélis and T. Adrianjanahary contributed with their observations. A. Hapke introduced O.S. to the genetics laboratory and J. Fietz, A. Hapke and M. Eberle shared their DNA primers with us. We thank S. Alberts, C. Borries, A. Koenig, J. Ostner and D. Zinner for discussion and/or helpful comments. Financial support was generously provided by Deutsches Primatenzentrum Göttingen. The study complies with the current laws of the countries where it was conducted.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by S. Alberts
Rights and permissions
About this article
Cite this article
Schülke, O., Kappeler, P.M. & Zischler, H. Small testes size despite high extra-pair paternity in the pair-living nocturnal primate Phaner furcifer . Behav Ecol Sociobiol 55, 293–301 (2004). https://doi.org/10.1007/s00265-003-0709-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00265-003-0709-x