Abstract
Purpose
Osteogrow, an osteoinductive device containing recombinant human Bone Morphogenetic Protein 6 (rhBMP6) in autologous blood coagulum, is a novel therapeutic solution for bone regeneration. This study aimed to evaluate different commercially available calcium phosphate synthetic ceramic particles as a compression-resistant matrix (CRM) added to Osteogrow implants to enhance their biomechanical properties.
Methods
Osteogrow implants with the addition of Vitoss, ChronOs, BAM, and Dongbo ceramics (Osteogrow-C, where C stands for ceramics) were evaluated in the rodent subcutaneous ectopic bone formation assay. Osteogrow-C device was prepared as follows: rhBMP6 was added to blood, and blood was mixed with ceramics and left to coagulate. Osteogrow-C was implanted subcutaneously in the axillary region of Sprague–Dawley rats and the outcome was analyzed 21 days following implantation using microCT, histology, morphometric analyses, and immunohistochemistry.
Results
Osteogrow-C implants with all tested ceramic particles induced the formation of the bone-ceramic structure containing cortical bone, the bone between the particles, and bone at the ceramic surfaces. The amount of newly formed bone was significant in all experimental groups; however, the highest bone volume was measured in Osteogrow-C implants with highly porous Vitoss ceramics. The trabecular number was highest in Osteogrow-C implants with Vitoss and ChronOs ceramics while trabeculae were thicker in implants containing BAM and Dongbo ceramics. The immunological response and inflammation were comparable among ceramic particles evaluated in this study.
Conclusion
Osteogrow-C bone regenerative device was effective with a broad range of commercially available synthetic ceramics providing a promising therapeutic solution for the regeneration of long bone fracture nonunion, large segmental defects, and spinal fusion surgeries.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Osteogrow is a novel osteoinductive device comprised of recombinant human bone morphogenetic protein 6 (rhBMP6) delivered within autologous blood coagulum (ABC) as a BMP carrier [1]. Osteogrow has been extensively evaluated in preclinical studies including animal models such as rat subcutaneous assay and spinal fusions in rabbit and sheep models [2,3,4]. Moreover, Osteogrow has been proven safe and effective in phase I/II clinical trials in patients with distal radial fracture (DRF) and patients undergoing high tibial osteotomy (HTO) [5, 6].
Biomechanical properties of the Osteogrow device might be enhanced by the addition of compression-resistant matrix (CRM) to Osteogrow implants [2, 7,8,9]. During Osteogrow preclinical studies, allograft was the first tested CRM and Osteogrow implants with allograft (Osteogrow-A) were successfully tested in rabbit and sheep PLF studies [2, 4]. However, due to allograft disadvantages such as immunogenicity and viral transmission risk, we aimed to substitute allograft with equally effective material without these issues.
Calcium phosphate (CaP) ceramics are widely used in regenerative medicine of bone tissue [10,11,12]. CaP ceramics are available in a broad range of formulations including macroporous blocks and particles ranging from 74 µm to several millimeters. Moreover, properties of CaP ceramics like porosity and pore size might be tailored during the sintering process [13]. Chemical compositions of CaP ceramics include highly resorbable tricalcium phosphate (TCP) and more stable hydroxyapatite (HA). Moreover, ceramics resorbabily might be adjusted by combining different ratios of TCP and HA in biphasic calcium phosphate (BCP) [14]. CaP ceramics might be used as a standalone BMP carrier or might be combined with natural and synthetic polymers to enhance their biomechanical properties [14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30]. Therefore, we have conducted preclinical studies to evaluate Osteogrow with CaP ceramics as a CRM (Osteogrow-C) and to elucidate how the chemical composition and size of ceramics affect the outcome [7,8,9, 31, 32]. In these studies, we showed that CaP ceramics promote BMP-mediated ectopic osteogenesis and spinal fusion between adjacent transverse processes in rabbits [8]. Moreover, we have also shown that the size of ceramic particles determines the structural properties of newly formed bone [7]. The aforementioned studies have been conducted using synthetic ceramics produced by CaP Biomaterials (East Troy, Wisconsin, USA). However, Osteogrow-C is intended to be a worldwide available product while the availability of ceramics differs among different markets. Therefore, there is an imminent need to explore the osteoinductive properties of Osteogrow-C implants with the addition of different synthetic ceramic particles available in specific global markets.
This study aimed to evaluate different commercially available calcium phosphate ceramic particles as a CRM in Osteogrow-C implants.
Material and methods
Experimental design
In this study, we evaluated Osteogrow-C implants with five different commercially available ceramic particles, namely: Vitoss (Stryker, Kalamazoo, Minessota, USA), ChronOs (DePuy Synthes, Oberdorf, Switzerland) in two different particle sizes, 700–1400 µm (ChronOs-M) and 1400–2800 µm (ChronOs-L), BAM (Engineering Research Center in Biomaterials, Sichuan University, Chengdu, China) and Dongbo (Hunan Gongchuang Biofunctional Materials Co., Hunan, China). As a control, we used Osteogrow-C implants with previously evaluated ceramics produced by CaP Biomaterials (East Troy, Wisconsin, USA). Tested ceramic particles were in the size range between 700 and 5400 µm, and they were composed of tricalcium phosphate (TCP) or biphasic calcium phosphate (BCP) with the predominance of TCP. Properties of tested ceramics are presented in Table 1.
Osteogrow-C implants were tested by conducting a well-established rat subcutaneous implant assay. The follow-up period was 21 days and it was chosen based on the findings from our previous studies which established bone formation parameters by undecalcified tissue histology, histomorphometry, microCT analyses, and immunolocalization of various molecules involved in osteogenesis [7, 9]. The number of implants was 5 per experimental group, and it was determined based on the known variability of this assay and recommendations for conducting studies in relevant animal models [33, 34]. Osteogrow-C implants were implanted bilaterally in the axillary regions (a total of two implants per animal). Different implants were placed on the right and left side of each animal to minimize the effect of biological variability on the study outcome.
Implant preparation
Lyophilized recombinant human bone morphogenetic protein 6 (rhBMP6) (Genera Research, Zagreb, Croatia) was dissolved in water for injection, and 20 µg of rhBMP6 was added to a tube containing 0.5 mL of freshly withdrawn autologous blood. Blood was subsequently mixed with ceramic particles (0.1 g per implant) placed in syringes and left to coagulate. Coagulation typically occurred after 5–10 min, and all Osteogrow-C devices were implanted within one hour following blood coagulation. Implant preparation was conducted in sterile conditions as shown in Fig. 1.
Preparation of Osteogrow-C implants. Osteogrow-C osteoinductive devices were prepared as follows: (1) Lyophilized rhBMP6 was dissolved in water for injection and added to freshly withdrawn rat autologous blood. Blood with rhBMP6 (2) was mixed with different ceramic particles and left to coagulate at room temperature (3). Following coagulation, Osteogrow-C devices were subcutaneously implanted in the axillary regions of Sprague–Dawley rats (4). Newly formed bone was evaluated (5) histologically and by microCT analysis
Experimental animals and surgical procedures
Subcutaneous implant assay was conducted on Sprague–Dawley laboratory rats (lat. Rattus norvegicus, male, six to 12 weeks old, bodyweight around 250–350 g). Experimental rats were bred and housed in standard laboratory conditions (temperature 18–24° C, relative humidity 40–70%, 12/12 h light/dark cycle, noise level 60 dB) at the Laboratory for Mineralized Tissues (University of Zagreb School of Medicine, Zagreb, Croatia; HR-POK-001).
At the beginning of the surgical procedure, laboratory rats were anaesthetized by a combination of xylazine 5 mg/kg (i.m.) and ketamine 100 mg/kg (s.c.). The skin over the thoracic region was sterilized and a vertical skin incision was made in the median line. Subcutaneous pockets were created bilaterally in the axillary region, and prepared Osteogrow-C implants were placed in the pockets as described [2, 3, 7, 31]. Animals were euthanized 21 days after surgery according to the experimental design. Following euthanasia, implants were recovered and prepared for microCT and histological analyses.
Animal care and conducted surgical procedures complied with the SOPs of the animal facility as well as with the European conventions for the Protection of Vertebrate Animals used for Experimental and other Scientific Purposes (ETS 123). The ethical principles of the study ensured compliance with European Directive 010/63/E, the Law on Amendments to Animal Protection Act (Official Gazette 37/13), the Animal Protection Act (Official Gazette 102/17), the Ordinance on the Protection of Animals Used for Scientific Purposes (Official Gazette 55/13), FELASA recommendations, and evaluation of the Ethics Committee at School of Medicine, University of Zagreb and the National Ethics Committee (EP 191/2019).
MicroCT analyses
To analyze newly formed bone-ceramic structures, extracted implants were scanned using a 1076 SkyScan µCT device (Bruker SkyScan, Billerica, Massachusetts, USA) using our standard protocol [7, 9, 31]. In brief, scanning parameters were set to a resolution of 18 µm, frame averaging of 2 with 0.5 mm aluminum filter, and rotation step of 0.5. Acquired images were reconstructed using NRecon software (Bruker SkyScan, Billerica, Massachusetts, USA). Following image reconstruction, CTAn software (Bruker SkyScan, Billerica, Massachusetts, USA) was used to calculate bone and ceramics volume as well as trabecular parameters (trabecular thickness, separation, and number) among experimental groups. 3D reconstructions of newly formed bone-ceramic structures were obtained using CTvox software (Bruker SkyScan, Billerica, Massachusetts, USA).
Histology and immunohistochemistry
Obtained specimens were immersed for 10 days in 4% formalin solution to achieve fixation followed by decalcification using 14% EDTA in 4% formalin. Samples were embedded in paraffin, cut at 6 µm, and subsequently stained by Goldner stain.
To evaluate the inflammation related to ceramics, we performed immunostaining to detect CD45-positive hematopoietic cells in bone marrow and CD68-positive macrophages. Immunohistochemistry (IHC) was performed according to our standard protocol [9]. In brief, IHC was conducted after deparaffinization and rehydration of tissue. In all procedures, we used mouse and rabbit-specific HRP/AEC IHC detection kit—micro-polymer (Abcam, UK). Sections were incubated with the rabbit anti-CD45 and anti-CD68 primary antibodies (Abcam, UK). As a secondary antibody, we used anti-rabbit antibody coupled with horseradish-peroxidase (HRP) and detected with diaminobenzidine (DAB) chromogen. Neutrophils and cells in bone marrow committed to granulomonocytic differentiations were identified by myeloperoxidase (MPO) stain. In addition, to test the immunogenicity of the synthetic ceramics, we stained histological sections with tartrate-resistant acid phosphatase (TRAP) to detect the presence of foreign body giant cells using commercially available kit (Sigma-Aldrich, St. Louis, Missouri, USA) according to the provided protocol.
Histological sections were analyzed and documented using an Olympus microscopy system—Olympus SZX10 Stereo Microscope and Olympus BX53 Upright Microscope equipped with a DP27 camera and operated by cellSens Dimension software (Olympus, Tokyo, Japan).
Data analysis
The Gaussian distribution was tested by the Kolmogorov–Smirnov test. In the case of normal distribution, one-way ANOVA was applied with Tukey post hoc test. In the case of unequal standard deviations, the Welch test was applied with the Dunnet T3 post hoc test. In the case of the non-Gaussian distribution, nonparametric tests (Kruskal–Wallis test) were applied with Dunn’s post hoc tests. Results are presented as mean with standard deviation or as a median ranging from minimum to maximum values within the experimental group. All P values below 0.05 were considered significant and are marked with asterisks as follows: * (P < 0.05), ** (P < 0.01), *** (P < 0.001). All statistical analyses were performed with the GraphPad Prism statistical program (GraphPad Software, San Diego, California, USA).
Results
Quantity of newly formed bone and residual ceramics
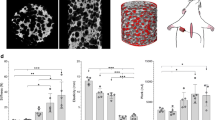
All tested Osteogrow-C implants induced ectopic bone regardless of the used ceramics as a CRM (Fig. 2). The newly formed bone along with ceramic particles formed a construct which we previously named bone-ceramic structure (BCS) [7]. Bone and ceramics were separated on microCT sections, and bone/CRM volume was determined.
MicroCT analyses of newly formed bone. A Bone volume (mm3), CRM volume (mm3), trabecular thickness (mm), trabecular separation (mm), and trabecular number (1/mm) among experimental groups. All P values below 0.05 were considered significant and are marked with asterisks as follows: * (P < 0.05), ** (P < 0.01), *** (P < 0.001). B MicroCT reconstruction of bone-ceramic structures (BCS) containing newly formed bone (green) and different ceramic particles (white)
The amount of induced bone was extensive in all experimental groups. However, the largest bone volume was induced by Osteogrow-C implants containing Vitoss ceramics as CRM. Importantly, the variability in the amount of formed bone was minimal among specimens in the same group.
Ceramic particles were mainly unresorbed 21 days following subcutaneous implantation. However, CRM volume differed among experimental groups and was highest for Dongbo ceramics and smallest for the Vitoss ceramics.
Trabecular parameters of ectopic bone
MicroCT analyses were conducted to determine trabecular parameters (trabecular number, trabecular thickness, and trabecular separation) of newly formed bone (Fig. 2). Trabecular parameters of newly formed bone differed among implants with different ceramic particles. The trabecular number was higher in BCS with Vitoss and ChronOs ceramics than in BCS with Dongbo and BAM ceramics. On the contrary, trabecular thickness and trabecular separation were higher in BCS with Dongbo and BAM ceramics than in BCS with Vitoss and ChronOs ceramics. Similar to the bone volume, the variability in trabecular parameters was minimal among the specimens within the same experimental group.
Histological structure of newly formed bone
Newly formed bone and formation of BCS were observed at all histological sections of Osteogrow-C implants with tested ceramic particles (Fig. 3). Regardless of the experimental group, BCS consisted of a thin cortical bone forming BCS boundaries, ceramic particles, and bone trabeculae between ceramic particles. Moreover, bone was present on the outer and inner surfaces (in the pores) of the ceramic particles in all experimental groups. There were only few portions of ceramic surfaces without visible bone formation. Importantly, the structural properties of newly formed bone and BCS with tested ceramic particles were comparable with the findings of our previous study in which we evaluated Osteogrow-C implants with CaP particles (500–1700 µm and 1000–4000 µm) [7].
Histological features of newly formed bone. Histological sections of bone-ceramic structure (BCS) induced by Osteogrow-C implants with different ceramic particles: Vitoss (A1,B1), ChronOs-L (A2,B2), ChronOs-M (A3,B3), Dongbo (A4,B4), BAM (A5,B5), and CaP Biomaterials (A6,B6) 21 days following implantation. BCS consisted of newly formed bone (yellow arrows) and residual ceramic particles (black asterisks). New bone was uniformly present in the pores inside ceramic particles. Sections were stained with Goldner stain. Scale bars are shown in the lower-right corner
Bone marrow was present between bone trabeculae in all implants. Bone marrow contained CD45 and MPO-positive haematopoietic cells (Fig. 4), adipocytes, and numerous blood vessels. Importantly, blood vessels were also present inside the pores providing vascularization to the newly formed bone throughout the implant.
Localization of haematopoietic cells. A CD45 positive haematopoietic cells (red arrows) and B neutrophils and cells in bone marrow committed to granulomonocytic differentiations (black arrows) identified by myeloperoxidase (MPO) stain were predominantly present in the bone marrow surrounding bone trabeculae. Selected sections of bone-ceramic structure containing Vitoss (A2,B1), ChronOs-L (A4,B3), BAM (A3,B4), and CaP Biomaterials (A1,B2). Scale bars are shown in the lower-right corner
Histological features and immunogenicity of residual ceramics
Ceramic particles in all groups were unresorbed 21 days following Osteogrow-C subcutaneous implantation. However, Vitoss particles appeared more resorbed compared to other ceramics which was confirmed by microCT analyses. Tested ceramics contained large pores inside the particles. The number of pores appeared highest in Vitoss and ChronOs ceramic particles. Pores were well interconnected allowing ingrowth of bone and blood vessels. Newly formed bone was uniformly present in the pores through ceramic particles regardless of the pore position inside the particle. Inside the pores, bone was predominantly present on the surfaces encircling the bone marrow, and few trabeculae were observed in the central part of the pores.
Importantly, ceramic surfaces without visible bone formation contained lined macrophages and foreign body giant cells as evidenced by IHC and TRAP staining (Fig. 5). The inflammatory and imunogenic reactions to different ceramics evaluated in this study were comparable between experimental groups (Figs. 4 and 5), and the number of macrophages and foreign body giant cells per square millimeter was not statistically different (dana not shown).
Localization of macrophages (CD68) and foreign body giant cells (FBGC). A CD68 positive cells (blue arrows) and B tartrate-resistant acid phosphatase (TRAP) positive FBGC (red arrows) were present at the ceramic surfaces without newly formed bone. Selected sections of bone-ceramic structure containing Vitoss (A1,B1), Dongbo (A3,B3), BAM (A4,B4), and CaP Biomaterials (A2,B2). Scale bars are shown in the lower-right corner
Discussion
Calcium phosphate ceramics are widely used in bone regenerative medicine due to their osteoconductive properties. To address the specific needs of different clinical indications, a broad range of ceramics have been developed and are commercially available. However, ceramics lack osteoinductive properties and are unable to rebridge large segmental defects or achieve fusion between two vertebral transverse processes. Bone morphogenetic proteins are potent osteoinductive molecules used to induce spinal fusions, promote rebridgment of segmental defects, and enhance fracture healing [35,36,37]. We have recently developed Osteogrow-C, an osteoinductive device comprised of rhBMP6 within autologous blood coagulum with the addition of calcium phosphate ceramic particles [1, 38]. Moreover, we have conducted extensive preclinical studies to demonstrate the safety and efficacy of Osteogrow-C implants using ceramics acquired from one specific vendor [7,8,9, 31,32,33]. In the present study, we have for the first time tested Osteogrow-C implants with a broad range of commercially available synthetic ceramic particles.
The main finding of this study is that all tested commercially available ceramic particles promote bone induction by rhBM6 delivered within ABC. Moreover, all tested Osteogrow-C implants induced the formation of a bone-ceramic structure with similar microarchitecture and a significant amount of new bone. Newly formed BCS in all experimental groups was well vascularized since blood vessels were formed throughout the implants including the pores inside the ceramic particles. The amount of bone induced by osteoinductive devices containing ceramics is affected by different properties of ceramic particles including particle and pore size, porosity, chemical composition, and the surface topography of ceramics [7, 14, 22]. We tested commercially available ceramic particles in the size range between 500 and 5400 µm since these particles are easier distributed homogenously in the implant as compared to the smallest particles (74–420 µm) [8] with the highest osteoinductive properties [7]. The amount of bone was highest in Osteogrow-C implants with Vitoss ceramics (1000–2000 µm), while it was comparable among other experimental groups supporting our previous finding that in general rhBMP6/ABC induce a larger amount of bone with smaller particles than with larger particles [7]. Moreover, implants with Vitoss induced a larger amount of bone than other particles of the same size indicating that the amount of formed bone correlates as well with the ceramics porosity which was highest in Vitoss ceramics (90 compared to 60%). The size of the ceramic particles also affected the trabecular parameters since the trabecular number was higher while trabecular thickness was lower in BCS with smaller particles (Vitoss and ChronOs) than with larger particles (Bam and Dongbo). Importantly, we explored inflammatory and immunogenic response to synthetic ceramics and found that they were comparable among ceramic particles evaluated on day 21 following implantation.
Another finding of this study was that the variability regarding the induced bone volume was small among the implants within the same experimental group. This finding might be very important for the clinical application of Osteogrow-C since the applied osteoinductive device must induce a sufficient and predictable amount of bone in every treated patient.
The follow-up period in this study was too short to observe significant resorption of the ceramic particles. However, resorption of ceramics began and differed among the experimental groups since the largest residual ceramic volume was observed for Dongbo ceramics, while the smallest volume was observed in Vitoss ceramics. These results indicate that the highest residual CRM volume is present in large particles that have relatively smaller surfaces at which resorption can occur compared to the smaller particles. Moreover, Vitoss ceramics had the largest porosity (up to 90%) which indicates that the resorption rate correlates with the porosity of ceramics. CRM resorbability and how it affects the longevity of newly formed bone is one of the most important questions in the development of osteoinductive devices containing ceramics. Therefore, in the continuation of this study, we will evaluate Osteogrow-C implants after a prolonged period of time at both ectopic sites conducting rat subcutaneous assay and functionally active site in the rabbit posterolateral spinal fusion (PLF) model.
Conclusions
Osteogrow-C osteoinductive device is intended to be the globally available product for use in various orthopaedic indications including large segmental long bone defects and nonunions as well as spinal fusions. On the other hand, the medical device market is strictly regulated and only a limited number of bioceramic products are approved and available in each targeted market. In this study, we have demonstrated that Osteogrow-C is effective with a broad range of commercial ceramics available in different markets across the world and may successfully replace allograft in various bone surgery indications. We have also shown that a larger amount of bone was formed when rhBMP6/ABC was combined with smaller and more porous particles like Vitoss ceramics that provide a large ceramic surface. Therefore, the findings in the present study are important for the development of the clinical formulations of the Osteogrow-C device which is currently undergoing clinical trials in patients with a tibial fracture nonunion (EudraCT number 2021–004,034-11).
Data availability
Raw data were generated at the Laboratory for Mineralized Tissues. Derived data supporting the findings of this study are available from the corresponding author, S.V., upon request.
References
Vukicevic S, Oppermann H, Verbanac D, Jankolija M, Popek I, Curak J, Brkljacic J, Pauk M, Erjavec I, Francetic I, Dumic-Cule I, Jelic M, Durdevic D, Vlahovic T, Novak R, Kufner V, Bordukalo Niksic T, Kozlovic M, Banic Tomisic Z, Bubic-Spoljar J, Bastalic I, Vikic-Topic S, Peric M, Pecina M, Grgurevic L (2014) The clinical use of bone morphogenetic proteins revisited: a novel biocompatible carrier device OSTEOGROW for bone healing. Int Orthop 38:635–647. https://doi.org/10.1007/s00264-013-2201-1
Vukicevic S, Grgurevic L, Erjavec I, Pecin M, Bordukalo-Niksic T, Stokovic N, Lipar M, Capak H, Maticic D, Windhager R, Sampath TK, Gupta M (2020) Autologous blood coagulum is a physiological carrier for BMP6 to induce new bone formation and promote posterolateral lumbar spine fusion in rabbits. J Tissue Eng Regen Med 14:147–159. https://doi.org/10.1002/term.2981
Grgurevic L, Oppermann H, Pecin M, Erjavec I, Capak H, Pauk M, Karlovic S, Kufner V, Lipar M, Bubic Spoljar J, Bordukalo-Niksic T, Maticic D, Peric M, Windhager R, Sampath TK, Vukicevic S (2019) Recombinant human bone morphogenetic protein 6 delivered within autologous blood coagulum restores critical size segmental defects of ulna in rabbits. JBMR Plus 3:e10085. https://doi.org/10.1002/jbm4.10085
Grgurevic L, Erjavec I, Gupta M, Pecin M, Bordukalo-Niksic T, Stokovic N, Vnuk D, Farkas V, Capak H, Milosevic M, Spoljar JB, Peric M, Vuckovic M, Maticic D, Windhager R, Oppermann H, Sampath TK, Vukicevic S (2020) Autologous blood coagulum containing rhBMP6 induces new bone formation to promote anterior lumbar interbody fusion (ALIF) and posterolateral lumbar fusion (PLF) of spine in sheep. Bone 138:115448. https://doi.org/10.1016/j.bone.2020.115448
Chiari C, Grgurevic L, Bordukalo-Niksic T, Oppermann H, Valentinitsch A, Nemecek E, Staats K, Schreiner M, Trost C, Kolb A, Kainberger F, Pehar S, Milosevic M, Martinovic S, Peric M, Sampath TK, Vukicevic S, Windhager R (2020) Recombinant human BMP6 applied within autologous blood coagulum accelerates bone healing: randomized controlled trial in high tibial osteotomy patients. J Bone Miner Res 35:1893–1903. https://doi.org/10.1002/jbmr.4107
Durdevic D, Vlahovic T, Pehar S, Miklic D, Oppermann H, Bordukalo-Niksic T, Gavrankapetanovic I, Jamakosmanovic M, Milosevic M, Martinovic S, Sampath TK, Peric M, Grgurevic L, Vukicevic S (2020) A novel autologous bone graft substitute comprised of rhBMP6 blood coagulum as carrier tested in a randomized and controlled Phase I trial in patients with distal radial fractures. Bone 140:115551. https://doi.org/10.1016/j.bone.2020.115551
Stokovic N, Ivanjko N, Erjavec I, Milosevic M, Oppermann H, Shimp L, Sampath KT, Vukicevic S (2020) Autologous bone graft substitute containing rhBMP6 within autologous blood coagulum and synthetic ceramics of different particle size determines the quantity and structural pattern of bone formed in a rat subcutaneous assay. Bone 141:115654. https://doi.org/10.1016/j.bone.2020.115654
Stokovic N, Ivanjko N, Pecin M, Erjavec I, Karlovic S, Smajlovic A, Capak H, Milosevic M, Bubic Spoljar J, Vnuk D, Maticic D, Oppermann H, Sampath TK, Vukicevic S (2020) Evaluation of synthetic ceramics as compression resistant matrix to promote osteogenesis of autologous blood coagulum containing recombinant human bone morphogenetic protein 6 in rabbit posterolateral lumbar fusion model. Bone 140:115544. https://doi.org/10.1016/j.bone.2020.115544
Stokovic N, Ivanjko N, Milesevic M, Matic Jelic I, Bakic K, Rumenovic V, Oppermann H, Shimp L, Sampath TK, Pecina M, Vukicevic S (2020) Synthetic ceramic macroporous blocks as a scaffold in ectopic bone formation induced by recombinant human bone morphogenetic protein 6 within autologous blood coagulum in rats. Int Orthop 45:1097–1107. https://doi.org/10.1007/s00264-020-04847-9
Best SM, Porter AE, Thian ES, Huang J (2008) Bioceramics: past, present and for the future. J Eur Ceram Soc 28:1319–1327. https://doi.org/10.1016/j.jeurceramsoc.2007.12.001
LeGeros RZ, Lin S, Rohanizadeh R, Mijares D, LeGeros JP (2003) Biphasic calcium phosphate bioceramics: preparation, properties and applications. J Mater Sci Mater Med 14:201–209. https://doi.org/10.1023/a:1022872421333
Habraken W, Habibovic P, Epple M, Bohner M (2016) Calcium phosphates in biomedical applications: materials for the future? Mater Today 19:69–87. https://doi.org/10.1016/j.mattod.2015.10.008
Dorozhkin SV (2010) Bioceramics of calcium orthophosphates. Biomaterials 31:1465–1485. https://doi.org/10.1016/j.biomaterials.2009.11.050
Alam MI, Asahina I, Ohmamiuda K, Takahashi K, Yokota S, Enomoto S (2001) Evaluation of ceramics composed of different hydroxyapatite to tricalcium phosphate ratios as carriers for rhBMP-2. Biomaterials 22:1643–1651. https://doi.org/10.1016/s0142-9612(00)00322-7
El Bialy I, Jiskoot W, Reza Nejadnik M (2017) Formulation, delivery and stability of bone morphogenetic proteins for effective bone regeneration. Pharm Res 34:1152–1170. https://doi.org/10.1007/s11095-017-2147-x
Seeherman H, Wozney JM (2005) Delivery of bone morphogenetic proteins for orthopedic tissue regeneration. Cytokine Growth Factor Rev 16:329–345. https://doi.org/10.1016/j.cytogfr.2005.05.001
Haidar ZS, Hamdy RC, Tabrizian M (2009) Delivery of recombinant bone morphogenetic proteins for bone regeneration and repair. Part B: Delivery systems for BMPs in orthopaedic and craniofacial tissue engineering. Biotechnol Lett 31:1825–1835. https://doi.org/10.1007/s10529-009-0100-8
Kato M, Namikawa T, Terai H, Hoshino M, Miyamoto S, Takaoka K (2006) Ectopic bone formation in mice associated with a lactic acid/dioxanone/ethylene glycol copolymer-tricalcium phosphate composite with added recombinant human bone morphogenetic protein-2. Biomaterials 27:3927–3933. https://doi.org/10.1016/j.biomaterials.2006.03.013
Roldan JC, Detsch R, Schaefer S, Chang E, Kelantan M, Waiss W, Reichert TE, Gurtner GC, Deisinger U (2010) Bone formation and degradation of a highly porous biphasic calcium phosphate ceramic in presence of BMP-7, VEGF and mesenchymal stem cells in an ectopic mouse model. J Craniomaxillofac Surg 38:423–430. https://doi.org/10.1016/j.jcms.2010.01.003
Liang G, Yang Y, Oh S, Ong JL, Zheng C, Ran J, Yin G, Zhou D (2005) Ectopic osteoinduction and early degradation of recombinant human bone morphogenetic protein-2-loaded porous beta-tricalcium phosphate in mice. Biomaterials 26:4265–4271. https://doi.org/10.1016/j.biomaterials.2004.10.035
Kuboki Y, Takita H, Kobayashi D, Tsuruga E, Inoue M, Murata M, Nagai N, Dohi Y, Ohgushi H (1998) BMP-induced osteogenesis on the surface of hydroxyapatite with geometrically feasible and nonfeasible structures: topology of osteogenesis. J Biomed Mater Res 39:190–199. https://doi.org/10.1002/(sici)1097-4636(199802)39:2<190::aid-jbm4>3.0.co;2-k
Tsuruga E, Takita H, Itoh H, Wakisaka Y, Kuboki Y (1997) Pore size of porous hydroxyapatite as the cell-substratum controls BMP-induced osteogenesis. J Biochem 121:317–324. https://doi.org/10.1093/oxfordjournals.jbchem.a021589
Jung UW, Choi SY, Pang EK, Kim CS, Choi SH, Cho KS (2006) The effect of varying the particle size of beta tricalcium phosphate carrier of recombinant human bone morphogenetic protein-4 on bone formation in rat calvarial defects. J Periodontol 77:765–772. https://doi.org/10.1902/jop.2006.050268
Kim JW, Choi KH, Yun JH, Jung UW, Kim CS, Choi SH, Cho KS (2011) Bone formation of block and particulated biphasic calcium phosphate lyophilized with Escherichia coli-derived recombinant human bone morphogenetic protein 2 in rat calvarial defects. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 112:298–306. https://doi.org/10.1016/j.tripleo.2010.10.025
Pelletier MH, Oliver RA, Christou C, Yu Y, Bertollo N, Irie H, Walsh WR (2014) Lumbar spinal fusion with beta-TCP granules and variable Escherichia coli-derived rhBMP-2 dose. Spine J 14:1758–1768. https://doi.org/10.1016/j.spinee.2014.01.043
Toth JM, Wang M, Lawson J, Badura JM, DuBose KB (2016) Radiographic, biomechanical, and histological evaluation of rhBMP-2 in a 3-level intertransverse process spine fusion: an ovine study. J Neurosurg Spine 25:733–739. https://doi.org/10.3171/2016.4.SPINE151316
Suh DY, Boden SD, Louis-Ugbo J, Mayr M, Murakami H, Kim HS, Minamide A, Hutton WC (2002) Delivery of recombinant human bone morphogenetic protein-2 using a compression-resistant matrix in posterolateral spine fusion in the rabbit and in the non-human primate. Spine (Phila Pa 1976) 27:353–360. https://doi.org/10.1097/00007632-200202150-00006
Akamaru T, Suh D, Boden SD, Kim HS, Minamide A, Louis-Ugbo J (2003) Simple carrier matrix modifications can enhance delivery of recombinant human bone morphogenetic protein-2 for posterolateral spine fusion. Spine (Phila Pa 1976) 28:429–434. https://doi.org/10.1097/01.BRS.0000048644.91330.14
Vukicevic S, Stokovic N, Pecina M (2019) Is ceramics an appropriate bone morphogenetic protein delivery system for clinical use? Int Orthop 43:1275–1276. https://doi.org/10.1007/s00264-019-04322-0
Kuroiwa Y, Niikura T, Lee SY, Oe K, Iwakura T, Fukui T, Matsumoto T, Matsushita T, Nishida K, Kuroda R (2019) Escherichia coli-derived BMP-2-absorbed beta-TCP granules induce bone regeneration in rabbit critical-sized femoral segmental defects. Int Orthop 43:1247–1253. https://doi.org/10.1007/s00264-018-4079-4
Stokovic N, Ivanjko N, Erjavec I, Breski A, Peric M, Vukicevic S (2021) Zoledronate bound to ceramics increases ectopic bone volume induced by rhBMP6 delivered in autologous blood coagulum in rats. Biomed 9.https://doi.org/10.3390/biomedicines9101487
Stokovic N, Ivanjko N, Pecin M, Erjavec I, Smajlovic A, Milesevic M, Karlovic S, Capak H, Vrbanac Z, Maticic D, Vukicevic S (2022) Long-term posterolateral spinal fusion in rabbits induced by rhBMP6 applied in autologous blood coagulum with synthetic ceramics. Sci Rep 12:11649. https://doi.org/10.1038/s41598-022-14931-2
Stokovic N, Ivanjko N, Maticic D, Luyten FP, Vukicevic S (2021) Bone morphogenetic proteins, carriers, and animal models in the development of novel bone regenerative therapies. Materials 14:3513. https://doi.org/10.3390/ma14133513
Peric M, Dumic-Cule I, Grcevic D, Matijasic M, Verbanac D, Paul R, Grgurevic L, Trkulja V, Bagi CM, Vukicevic S (2015) The rational use of animal models in the evaluation of novel bone regenerative therapies. Bone 70:73–86. https://doi.org/10.1016/j.bone.2014.07.010
Dumic-Cule I, Pecina M, Jelic M, Jankolija M, Popek I, Grgurevic L, Vukicevic S (2015) Biological aspects of segmental bone defects management. Int Orthop 39:1005–1011. https://doi.org/10.1007/s00264-015-2728-4
Grgurevic L, Pecina M, Vukicevic S (2017) Marshall R. Urist and the discovery of bone morphogenetic proteins. Int Orthop 41:1065–1069. https://doi.org/10.1007/s00264-017-3402-9
Dumic-Cule I, Peric M, Kucko L, Grgurevic L, Pecina M, Vukicevic S (2018) Bone morphogenetic proteins in fracture repair. Int Orthop 42:2619–2626. https://doi.org/10.1007/s00264-018-4153-y
Vukicevic S, Peric M, Oppermann H, Stokovic N, Ivanjko N, Erjavec I, Kufner V, Vnuk D, BubicSpoljar J, Pecin M, Novak R, MaticJelic I, Bakic K, Milesevic M, Rumenovic V, Popek I, Pehar S, Martinovic S, Blazevic V, Rogina L, Vikic-Topic S, Bozic T, Verbanac D, BordukaloNiksic T, Sampath K, Pecina M, Maticic D, Grgurevic L (2020) Bone morphogenetic proteins: From discovery to development of a novel autologous bone graft substitute consisting of recombinant human BMP6 delivered in autologous blood coagulum carrier. Rad CASA - Med Sci 544:26–41. https://doi.org/10.21857/mnlqgc5vgy
Acknowledgements
We are grateful to Stryker Corporation (Kalamazoo, Minessota, USA) for providing Vitoss ceramic particles evaluated in this study. We thank to Mirjana Marija Renic and Djurdjica Car for their excellent technical assistance in animal experiments and excellent preparation of histology sections.
Funding
This research was funded by the FP7 Health Program (FP7/2007–2013) under grant agreement HEALTH-F4-2011–279239 (Osteogrow), H2020 Health GA 779340 (OSTEOproSPINE), and European Regional Development Fund—Scientific Center of Excellence for Reproductive and Regenerative Medicine (project “Reproductive and regenerative medicine—exploration of new platforms and potentials,” GA KK.01.1.1.01.0008 funded by the EU through the ERDF).
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection, and analysis were performed by NI, NS, VR, and AB. The first draft of the manuscript was written by NS and SV, and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval
Study has received ethics approval from the Ethics Committee at School of Medicine, University of Zagreb and the Croatian National Ethics Committee (EP 191/2019 and EP 296/2020).
Animal care complied with the SOPs of the Animal Facility and the European conventions for the Protection of Vertebrate Animals used for Experimental and other Scientific Purposes (ETS 123). The ethical principles of the study ensured compliance with European Directive 010/63/E, the Law on Amendments to Animal Protection Act (Official Gazette 37/13, the Animal Protection Act (Official Gazette 102/17), the Ordinance on the Protection of Animals used for Scientific Purposes (Official Gazette 55/13), and FELASA recommendations.
Consent to participate
Not applicable.
Consent to publish
Not applicable.
Competing interests
Slobodan Vukicevic is a founder of Genera Research and a coordinator of the EU HORIZON 2020 grant OSTEOproSPINE, funding clinical studies for new bone repair drugs (patent WO2019076484A1).
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Stokovic, N., Ivanjko, N., Rumenovic, V. et al. Comparison of synthetic ceramic products formulated with autologous blood coagulum containing rhBMP6 for induction of bone formation. International Orthopaedics (SICOT) 46, 2693–2704 (2022). https://doi.org/10.1007/s00264-022-05546-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00264-022-05546-3