Abstract
Purpose
This study investigated whether Escherichia coli-derived bone morphogenetic protein (BMP)-2 (E-BMP-2) adsorbed onto β-tricalcium phosphate (β-TCP) granules can induce bone regeneration in critical-size femoral segmental defects in rabbits.
Methods
Bone defects 20 mm in size and stabilized with an external fixator were created in the femur of New Zealand white rabbits, which were divided into BMP-2 and control groups. E-BMP-2-loaded β-TCP granules were implanted into defects of the BMP-2 group, whereas defects in the controls were implanted with β-TCP granules alone. At 12 and 24 weeks after surgery, radiographs were obtained of the femurs and histological and biomechanical assessments of the defect area were performed. Bone regeneration was quantified using micro-computed tomography at 24 weeks.
Results
Radiographic and histologic analyses revealed bone regeneration in the BMP-2 group but not the control group; no fracturing of newly formed bone occurred when the external fixator was removed at 12 weeks. At 24 weeks, tissue mineral density, the ratio of bone volume to total volume, and volumetric bone mineral density of the callus were higher in the BMP-2 group than in control animals. In the former, ultimate stress, extrinsic stiffness, and failure energy measurements for the femurs were higher at 24 weeks than at 12 weeks.
Conclusion
E-BMP-2-loaded β-TCP granules can effectively promote bone regeneration in long bone defects.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Treating large bone defects caused by severe trauma, nonunion, infection, or tumor resection is a major clinical challenge. Autogenous bones or allografts are widely used to fill bone defects but have several disadvantages including donor site morbidity, limited quantities of graft material, and disease transmission. Bone tissue engineering technology—where bone regeneration is induced with osteoinductive growth factors, scaffolds, or both—can potentially overcome these problems.
Bone morphogenetic proteins (BMPs) have been widely studied for their osteoinductive properties and are considered as promising agents for generating bone graft substitutes. There are currently two commercially available BMPs—namely, recombinant human (rh)BMP-2 and -7. These have been shown in preclinical studies to have potent bone-inducing activity [1, 2] and have been evaluated in clinical trials for the treatment of various skeletal disorders such as open fractures, nonunion, and osteonecrosis [3,4,5,6,7,8]. However, the production of the large amounts of rhBMP required for this purpose is costly if mammalian cells such as Chinese hamster ovary (CHO) cells are used [3, 5]. Engineered Escherichia coli provide an alternative system for generating recombinant proteins; it has been shown that E. coli-derived BMP-2 (E-BMP-2) can be produced at relatively low cost [9] while having comparable biological activity to the protein produced in CHO cells [10].
Hydroxyapatite (HA) and β-tricalcium phosphate (β-TCP) have been proposed as carriers of rhBMP [11]. β-TCP is a biodegradable bone substitute that is replaced with newly regenerated bone at a high rate [12, 13] and is more bioabsorbable than HA [14]; it has thus been employed in clinical settings as a BMP delivery system [15]. However, the efficacy of E-BMP-2 in promoting bone regeneration has not yet been demonstrated. To this end, the present study investigated whether E-BMP-2-loaded β-TCP granules can induce bone regeneration in critical-sized femoral segmental defects in rabbits.
Materials and methods
Animals
A total of 28 female New Zealand white rabbits (Japan SLC, Hamamatsu, Japan) were used in this study. Surgeries were performed on animals that were 24 weeks old.
Surgical procedure
Surgery was performed under general anaesthesia with isoflurane and pentobarbital. After exposing the femur, two pairs of tip-threaded pins were placed in the proximal and distal femurs. Bone defects 20 mm in size were created between the proximal and distal pins using an oscillating saw, and an external fixator (MiniRail Fixator System; Orthofix SRL, Bussolengo, Italy) was used to connect the pairs of pins. Rabbits were divided into BMP-2 (n = 20) and (2) control (n = 8) groups. In the former, 0.67 g of β-TCP granules (a gift from HOYA Technosurgical Corp., Uenohara, Japan) loaded with 50 μg of E-BMP-2 (a gift from Osteopharma, Osaka, Japan) was implanted at the defect site. E-BMP-2-loaded β-TCP granules were prepared as follows: 50 μl of BMP-2 suspension (5 mg/ml) was thawed on ice and was diluted tenfold with 450 μl of 0.5 mM hydrochloric acid (HCl). After thorough pipetting, the suspension was stored in a refrigerator at 4 °C until the surgery. A 2000-μl volume of 0.5 mM HCl was added to dilute the suspension to 100 μg/ml, and 500 μl was implanted for a total of 0.67 g β-TCP granules. In the control group, 0.67 g of β-TCP granules alone was implanted. The pins were removed from the bones 12 weeks after surgery. Macroscopic examination and a manual stability test of the defect site were performed.
Radiographic assessment of bone regeneration
At 12 and 24 weeks after surgery, 14 animals (BMP-2 n = 10 and control n = 4) were anaesthetized and fixed in a prone position with hip joints fully abducted, and radiographs of the defect sites were acquired to examine whether radiographic bone regeneration had occurred. Subsequently, bone union—which is defined as the absence of a bony gap or complete bony bridging between anterior and posterior cortices—was evaluated by four orthopaedic surgeons.
Histological assessment of bone regeneration
At 12 and 24 weeks after surgery, eight animals (n = 4 each in the BMP-2 and control groups) were anesthetized and sacrificed. The femur was harvested, and the tissue was fixed in 4% paraformaldehyde, decalcified with formic acid, and embedded in paraffin. Sagittal sections (5 μm thick) were cut and stained with haematoxylin and eosin for histological examination of the bone defect area.
Micro-computed tomography measurement of bone regeneration
To quantify bone regeneration, micro-computed tomography (μ-CT) imaging was performed at 24 weeks. The femurs were scanned using a R_mCT2 scanner (Rigaku, Tokyo, Japan). The region of interest was set as 7 mm proximal and distal from the midline of the defect site in the sagittal view (Fig. 1). Morphometric parameters including tissue mineral density (TMD), ratio of bone volume to total volume (BV/TV), and volumetric bone mineral density (vBMD) of the callus were measured with the TRI/3D-BON analysis system (Ratoc System Engineering, Tokyo, Japan).
Biomechanical assessment of bone regeneration
Six femurs each in the BMP-2 group were used for biomechanical testing at 12 and 24 weeks. After euthanasia, the femur was dissected and the muscle surrounding the defect site was removed. A standardized three-point bending test was performed with a load torsion and bending tester (MZ-500D, MZ-500S, Maruto Instrument Co., Tokyo, Japan). Ultimate stress (N), extrinsic stiffness (N/mm), and failure energy (N·mm) were evaluated.
Adverse events
Adverse events caused by implantation of E-BMP-2-loaded β-TCP granules in rabbits such as ectopic bone formation, skin abnormalities, and death from unknown cause were recorded.
Statistical analysis
Fisher’s exact test was used to compare radiographic results between the two groups at each time point. The Mann-Whitney U test was used to compare the μ-CT and biomechanical test results of the two groups at 24 weeks. Differences were considered significant at a P value < 0.05.
Results
Macroscopic findings and manual stability of the defect site
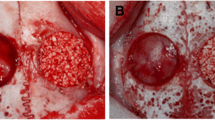
A macroscopic examination revealed no bone regeneration in the control group; the defect sites of all eight animals were highly unstable at 12 and 24 weeks, with obvious bone movement. In contrast, in the BMP-2 group, macroscopic bone regeneration was observed in all 20 samples at both time points; the bone was hard, and a manual test revealed no abnormal movement (Fig. 2). The newly formed bone did not fracture when the external fixator was removed at 12 weeks, indicating that the bone had considerable strength.
Images of macroscopic features of bone in control and BMP-2 groups. No bone regeneration was observed in the control group; the defect sites were highly unstable, with obvious bone movement. In contrast, in the BMP-2 group, macroscopic bone regeneration was observed; the bone was hard, and a manual test revealed no abnormal movement
Radiographic assessment of bone regeneration
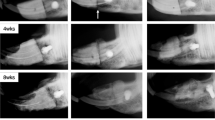
The lack of bone regeneration in the four control samples at 12 and 24 weeks was confirmed by radiographic assessment. On the other hand, newly formed bone was detected in each of the ten animals in the BMP-2 group at 12 and 24 weeks (Fig. 3 and Table 1), with four animals showing radiographic bone union at 12 weeks. In contrast, none of the femurs in the control group showed bone union after 12 weeks. Newly formed bone in the defect site was more evident at 24 weeks than at 12 weeks; nine of ten femurs in the BMP-2 group and none in the control group exhibited bone union at 24 weeks, representing a statistically significant difference (P < 0.01; Table 2).
Histological assessment of bone regeneration
Representative histological findings of control and BMP-2 groups at 12 and 24 weeks are shown in Fig. 4. In the control group, there was no evidence of bone regeneration, and defect sites were filled with fibrous tissue. In contrast, bone regeneration was detected in eight samples of the BMP-2 group at 12 and 24 weeks (n = 4 at each time point), and two cortical bones on each side of the defect site were nearly united, with formation of medullary canals. There was greater β-TCP absorption and cortical bone remodeling as well as formation of a marrow cavity at 24 weeks as compared to 12 weeks.
Histological analysis of bone defect sites in control and BMP-2 groups at 12 and 24 weeks after surgery. Sections were stained with hematoxylin and eosin. New bone formation was detected in the BMP-2 group, while only fibroblast-like cells were observed in the control group. FC, fibroblast-like cell; MB, newly formed mineralized bone. Scale bar = 2 mm
μ-CT analysis of bone regeneration
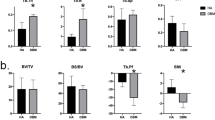
The TMD of the callus was higher in the BMP-2 group than in the control group at 24 weeks (702 vs. 612 mg/cm3, P < 0.05; Fig. 5a). The BV/TV ratio (33% vs. 27%, P < 0.05; Fig. 5b) and vBMD (235 vs. 165 mg/cm3, P < 0.05; Fig. 5c) of the callus were significantly higher in the BMP-2 group than in the control group.
μ-CT assessment of a callus at the bone defect site in control and BMP-2 groups at 24 weeks after surgery. Four animals each in the BMP-2 and control groups were examined. TMD (a), BV/TV ratio (b), and vBMD (c) were higher in the BMP-2 group as compared to the control group. *P < 0.05 vs. indicated groups
Biomechanical assessment of bone regeneration
Ultimate stress, extrinsic stiffness, and failure energy of the defected femurs (expressed as a percentage of the value in intact femora) were significantly higher at 24 weeks than at 12 weeks in the BMP-2 group (P < 0.05; Fig. 6). In the control group, neither bone regeneration nor bone union was observed, making it impossible to perform mechanical assessments.
Biomechanical assessment of the bone defect site in control and BMP-2 groups at 12 and 24 weeks after surgery. All three mechanical parameters including ultimate stress (a), extrinsic stiffness (b), and failure energy (c) are expressed as a percentage of the value in the intact femora; the values were higher in the BMP-2 group than in the control group. *P < 0.05 vs. indicated groups
Adverse events
Ectopic bone formation—an important local complication of BMP administration—was not observed, and there were no other complications such as skin abnormalities or death from unknown cause.
Discussion
Among growth factors involved in bone healing and regeneration, BMPs were originally identified as osteoinductive proteins; they are now known to modulate the complex biological process of osteogenesis [16] and are key factors in chondrogenic and skeletogenic functions [17]. Several BMPs have been cloned and have been used to promote bone healing and regeneration [18].
Autogenous bone grafting and artificial bone implantation are standard treatments for bone regeneration. The former involves elimination of healthy bone and can be invasive; it can also cause pain at the wound site and carries the risk of paralysis or infection. Additionally, the amount of bone that can be removed may be limited in some patients. Artificial bone can overcome some of these problems but has no osteoinductive capacity. BMP-2 products are expected to be useful for the treatment of fracture and nonunion as well as other procedures requiring bone grafting such as spinal fixation and tumor resection.
In the present study, we evaluated the efficacy of E-BMP-2 in promoting bone regeneration in vivo. E-BMP-2 is particularly attractive for biotechnological applications because large amounts can be generated using an E. coli expression system at a lower cost than when using CHO cell-derived BMP-2 [10]. A method for producing E-BMP-2 by converting BMP monomers to biologically active dimers has been reported [19]. Moreover, E-BMP-2 has no sugar chains, which facilitates protein quality control.
In this study, we investigated whether E-BMP-2-loaded β-TCP granules could induce bone regeneration in critical-sized femoral segmental defects in rabbits. E-BMP-2 has been used in previous studies to treat long tubular bone defects [20, 21], but this is the first demonstration that E-BMP-2 can be used for femoral bone defects. Posterolateral lumbar spinal fusion was previously performed in female New Zealand white rabbits with autogenous bone or β-TCP granules loaded with various doses of E-BMP-2 (0–150 μg) [15]. After eight and 16 weeks, defects implanted with rhBMP-2-adsorbed β-TCP scaffolds showed greater radiopacity than those implanted with unloaded β-TCP scaffolds; a histological analysis revealed that eight weeks after the operation, the bone mass obtained by treatment with 50 μg/side of E-BMP-2 was similar to that achieved by autogenous bone grafting, with peripheral cortical bone bridging the transverse processes. In another study, posterolateral lumbar spinal fusion was performed in Japanese white rabbits with β-TCP granules with or without adsorbed rhBMP-2 (30, 60, or 120 μg); in all L5–6 spinal segments harvested from the BMP60 and BMP120 groups eight weeks after implantation, bony hard masses bridging the interspinous process were visible, and passive motion between vertebrae was significantly restricted compared to sham and BMP0 groups [22]. Histological assessment of the L5–6 interspinous fusion mass in animals treated with rhBMP-2 (60 or 120 μg) revealed new bone bridging the spinous processes and interwoven with haematopoietic marrow.
The above studies examined posterolateral lumbar spinal fusion, while we used an external fixator for long tubular bone defects. Nonetheless, both models showed that E-BMP-2-adsorbed β-TCP granules can effectively induce bone regeneration and union, and that even bioactive materials with osteoconductive ability such as β-TCP are not sufficient in themselves to promote bone regeneration in critical bone defects, requiring additional factors such as BMP-2.
In a study using Japanese white rabbits in which porous β-TCP cylinders with or without rhBMP-2 (50 μg) were implanted into long bone defects, calcification was detected using radiography after four weeks, and newly formed bone connecting both ends of defects appeared to have been remodeled into cortical bone with a bone marrow cavity [23]. The BMP treatment group showed a time-dependent increase in callus formation to nearly 100% at six weeks. The histological assessment revealed that bone tissue occupying the defects had been remodeled, such that cortical bone and haematopoietic marrow-like tissue were clearly visible after eight weeks. These authors used porous cylindrical β-TCP, but granules such as those used in our study are easier to handle and thus have broader applicability.
There was some discrepancy in bone formation observed by radiographic or histological assessment as compared to μ-CT. Tissue with a vBMD of ≥ 100 mg/cm3 was defined as a callus in μ-CT analysis. As a result, calluses were identified even in parts where there was no obvious bone formation by macroscopic observation. On the other hand, in radiographic and histological assessments, macroscopic observation did not reveal and did not show obvious bone formation in the control group, in contrast to results obtained by μ-CT.
This study had some limitations. Firstly, in the standardized three-point bending test, we did not compare control and BMP-2 groups, since control animals showed no evidence of bone regeneration. We therefore compared the 12- and 24-week samples of the BMP-2 group to evaluate the increase in bone strength. Secondly, it has been reported that E-BMP-2 has similar biological activity to CHO cell-derived BMP-2 [10]; however, we did not confirm this in the present study.
In conclusion, we showed that E-BMP-2-loaded β-TCP granules induced bone regeneration in critical-sized femoral segmental defects in rabbits. Our results suggest that E-BMP-2 combined with β-TCP granules can be used to effectively promote bone regeneration in clinical settings.
References
Keskin DS, Tezcaner A, Korkusuz P, Korkusuz F, Hasirci V (2005) Collagen-chondroitin sulfate-based PLLA-SAIB-coated rhBMP-2 delivery system for bone repair. Biomaterials 26:4023–4034
Zara JN, Siu RK, Zhang X et al (2011) High doses of bone morphogenetic protein 2 induce structurally abnormal bone and inflammation in vivo. Tissue Eng Part A 17(9–10):1389–1399
Govender S, Csimma C, Genant HK et al (2002) Recombinant human bone morphogenetic protein-2 for treatment of open tibial fractures: a prospective, controlled, randomized study of four hundred and fifty patients. J Bone Joint Surg Am 84-A:2123–2134
Calori GM, Tagliabue L, Gala L, d’Imporzano M, Peretti G, Albisetti W (2008) Application of rhBMP-7 and platelet-rich plasma in the treatment of long bone non-unions: a prospective randomised clinical study on 120 patients. Injury 39:1391–1402
Friedlaender GE, Perry CR, Cole JD et al (2001) Osteogenic protein-1 (bone morphogenetic protein-7) in the treatment of tibial nonunions. J Bone Joint Surg Am 83-A(Suppl 1):S151–S158
Giannoudis PV, Tzioupis C (2005) Clinical applications of BMP-7: the UK perspective. Injury 36(Suppl 3):S47–S50
Swiontkowski MF, Aro HT, Donell S et al (2006) Recombinant human bone morphogenetic protein-2 in open tibial fractures. A subgroup analysis of data combined from two prospective randomized studies. J Bone Joint Surg Am 88:1258–1265
Sun W, Li Z, Gao F, Shi Z, Zhang Q, Guo W (2014) Recombinant human bone morphogenetic protein-2 in debridement and impacted bone graft for the treatment of femoral head osteonecrosis. PLoS One 9:e100424
Lee JH, Jang SJ, Koo TY (2011) Expression, purification and osteogenic bioactivity of recombinant human BMP-2 derived by Escherichia coli. J Tissue Eng Regen Med 8:8–15
Bessho K, Konishi Y, Kaihara S, Fujimura K, Okubo Y, Iizuka T (2000) Bone induction by Escherichia coli-derived recombinant human bone morphogenetic protein-2 compared with Chinese hamster ovary cell-derived recombinant human bone morphogenetic protein-2. Br J Oral Maxillofac Surg 38:645–649
Hong SJ, Kim CS, Han DK, Cho IH, Jung UW, Choi SH, Kim CK, Cho KS (2006) The effect of a fibrin-fibronectin/beta-tricalcium phosphate/recombinant human bone morphogenetic protein-2 system on bone formation in rat calvarial defects. Biochemistry 27:3810–3816
Jensen SS, Broggini N, Weibrich G, Hjorting-Hansen E, Schenk R, Buser D (2005) Bone regeneration in standardized bone defects with autografts or bone substitutes in combination with platelet concentrate: a histologic and histomorphometric study in the mandibles ofminipigs. Int J Oral Maxillofac Implants 20:703–712
Zerbo IR, Bronckers AL, de Lange GL, van Beek GJ, Burger EH (2001) Histology of human alveolar bone regeneration with a porous tricalcium phosphate. A report of two cases. Clin Oral Implants Res 12:379–384
Jensen SS, Broggini N, Hjorting-Hansen E, Schenk R, Buser D (2006) Bone healing and graft resorption of autograft, anorganic bovine bone and betatricalcium phosphate. A histologic and histomorphometric study in the mandibles of minipigs. Clin Oral Implants Res 17:237–243
Dohzono S, Imai Y, Nakamura H, Wakitani S, Takaoka K (2009) Successful spinal fusion by E. coli-derived BMP-2-adsorbed porous β-TCP granules: a pilot study. Clin Orthop Relat Res 467:3206–3212
Urist MR (1965) Bone: formation by autoinduction. Science 150:893–899
Ronga M, Fagetti A, Canton G, Paiusco E, Surace MF, Cherubino P (2013) Clinical applications 350 of growth factors in bone injuries: experience with BMPs. Injury 44(Suppl 1):S34–S39
Wang EA, Rosen V, Cordes P, Hewick RM, Kriz MJ, Luxenberg DP, Sibley BS, Wozney JM (1988) Purification and characterization of other distinct bone-inducing factors. Proc Natl Acad Sci U S A 85:9484–9488
Ruppert R, Hoffmann E, Sebald W (1996) Human bone morphogenetic protein 2 contains a heparin-binding site which modifies its biological activity. Eur J Biochem 237:295–302
Itoi T, Harada Y, Irie H et al (2016) Escherichia coli-derived recombinant human bone morphogenetic protein-2 combined with bone marrow-derived mesenchymal stromal cells improves bone regeneration in canine segmental ulnar defects. BMC Vet Res 12:201
Harada Y, Itoi T, Wakitani S et al (2012) Effect of Escherichia coli-produced recombinant human bone morphogenetic protein 2 on the regeneration of canine segmental ulnar defects. J Bone Miner Metab 30:388–399
Matsumoto T, Toyoda H, Dohzono S, Yasuda H, Wakitani S, Nakamura H, Takaoka K (2012) Efficacy of interspinous process lumbar fusion with recombinant human bone morphogenetic protein-2 delivered with a synthetic polymer and β-tricalcium phosphate in a rabbit model. Eur Spine J 21:1338–1345
Yoneda M, Terai H, Imai Y, Okada T, Nozaki K, Inoue H, Miyamoto S, Takaoka K (2005) Repair of an intercalated long bone defect with a synthetic biodegradable bone-inducing implant. Biomaterials 26:5145–5152
Acknowledgments
The authors thank Mr. T. Ueha, Ms. M. Yasuda, Ms. K. Tanaka, Ms. M. Nagata (Department of Orthopaedic Surgery, Kobe University Graduate School of Medicine), and Mr. H. Irie (Osteopharma) for excellent technical assistance.
Funding
This study was supported by a grant from the Hyogo Science and Technology Association.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
β-TCP granules were provided free of charge by HOYA Technosurgical Corporation (Mashiko, Japan). E-BMP-2 was provided free of charge by Osteopharma.
Ethical approval
Procedures involving animals were performed with approval and under the guidance of the Animal Care and Use Committee of Kobe University Graduate School of Medicine.
Rights and permissions
About this article
Cite this article
Kuroiwa, Y., Niikura, T., Lee, S.Y. et al. Escherichia coli-derived BMP-2-absorbed β-TCP granules induce bone regeneration in rabbit critical-sized femoral segmental defects. International Orthopaedics (SICOT) 43, 1247–1253 (2019). https://doi.org/10.1007/s00264-018-4079-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00264-018-4079-4