Abstract
Background
The direct anterior approach (DAA) for total hip arthroplasty (THA) is a muscle-sparing approach thought to have less post-operative pain and quicker recovery, with similar functional outcomes to other approaches. However, it is technically challenging and transitioning surgeons may experience increased complication rates. The purpose of this systematic review is to identify reported learning curves associated with the DAA.
Methods
Three databases (MEDLINE, Embase, and Web of Science) were searched using terms including “total hip arthroplasty,” “direct anterior approach,” and “learning curve.” Study characteristics, patient demographics, learning curve analyses, and complications were abstracted.
Results
Twenty-one studies met inclusion criteria, with a total of 9738 patients (60% female), an average age of 63.7 years (range: 13–94), body mass index of 27.0 kg/m2 (range: 16.8–58.9), and follow-up of 19 months (range: 1.5–100). There were five retrospective cohort studies and 13 case series representing fair methodological quality. Six studies depicted a true learning curve, with mean operative time of 156.59 ± 41.71 minutes for the first case, 93.18 ± 14.68 minutes by case 30, and 80.45 ± 12.28 minutes by case 100. Mean complication rate was 20.8 ± 12.7% in early groups and decreased to 7.6 ± 7.1% in late groups.
Conclusion
This review demonstrated a substantial learning curve associated with the DAA to THA. Operative time plateaued after approximately 100 cases. Complication rates decreased substantially from early to late groups.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Total hip arthroplasty (THA) continues to be one of the most effective and commonly performed orthopaedic procedures, with over 500,000 performed each year in North America and a projected rise to almost 1.5 million by 2040 [1,2,3]. With the growing demand for minimally invasive surgical methods, there has been a recent resurgence of the direct anterior approach (DAA) to the hip in the context of THA [4]. Though originally described by Hueter, the DAA is more commonly known by the report published by Smith-Petersen in 1917 [5, 6]. This approach uses a true intermuscular, internervous plane to approach the hip, which, along with the size of the skin incision, contributes to its reputation as a minimally invasive technique.
Due to its muscle-sparing nature, proponents of the DAA believe it leads to less post-operative pain and faster recovery times [7,8,9,10,11,12]; however, it is thought that the functional advantages offered by the DAA are equivalent to other approaches by as early as two weeks post-operatively [7]. Additionally, it is suggested that the DAA leads to decreased post-operative dislocation rates, as the posterior capsule and soft tissues are preserved with this approach [13,14,15]. Lastly, with shorter average hospital stays due to enhanced early recovery, the use of the DAA has opened the door for outpatient THA [16,17,18]. Despite its many potential advantages, the use of the DAA is technically challenging and overall complication rates may be higher in DAA THA [19, 20], especially among surgeons new to the approach [21, 22]. This, along with the large number of surgeons transitioning to the DAA, has led to discussion regarding the procedure’s learning curve.
The concept of a learning curve for surgical procedures is not a new one. The relationship between operative procedures performed by a surgeon and lower mortality rates was reported in 1979 [23]. This concept of a learning curve in a surgical context was further described as having four stages: (1) a rapid ascent during the early stages of training; (2) a zone of decreasing improvement, where additional experience yields only marginal improvement; (3) a plateau, in which further experience has no effect on the measured outcome; and (4) possible age-related decline in the measured outcome [24]. Due to its increasing popularity and technical challenges, the learning curve of the DAA THA has garnered considerable attention [25,26,27,28,29]. Identifying this learning curve has considerable implications for patient safety, surgical training, and cost-effectiveness as it relates to operative time. The purpose of this systematic review is to identify the reported learning curves associated with the DAA to THA, primarily by analysis of operative time and complications. Additionally, the purpose was to determine, based on the best available evidence, a point on the learning curve after which the surgeon can be considered proficient.
Methods
This systematic review was conducted in accordance with the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) guidelines for conducting and reporting systematic reviews [30]. The study protocol was registered prospectively on The International Prospective Register of Systematic Reviews (PROSPERO) (ID: CRD42020195680).
Search strategy
Three online databases (MEDLINE, Embase, and Web of Science) were searched from database inception to June 25, 2020, for literature addressing the learning curve associated with the use of the DAA for THA. Search terms used to identify eligible studies included “direct anterior approach,” “total hip arthroplasty,” “learning curve,” “clinical competence,” “outcome assessment,” and “complication” (Appendix).
Study screening
Studies identified during the comprehensive literature search were screened at the title/abstract as well as full-text stages by two reviewers independently and in duplicate using the online software Rayyan (2010, Qatar Computing Research Institute, Doha, Qatar). Any discrepancies at the title/abstract stage were resolved with automatic inclusion into the next stage of screening for more in-depth review. At the full-text stage, discrepancies were discussed and resolved by consensus between the reviewers, and a more senior author was consulted for any remaining discrepancies. In addition, the references of relevant studies were screened manually to identify any eligible studies potentially missed by the database search.
Assessment of study eligibility
The research question and study eligibility criteria were established a priori. The inclusion criteria were as follows: (1) all levels of evidence, (2) studies performed on human patients, (3) operative studies using the DAA for THA, and (4) formal discussion or analysis of the learning curve based on the results of the study. Exclusion criteria were (1) review articles, opinion pieces, editorials, or basic science studies, and (2) multiple studies reporting on the same group of patients (only the most recent study is to be included).
Data abstraction
Three reviewers independently extracted data from included studies into a Google Sheet (Google, CA, USA) online collaborative spreadsheet, designed a priori, and piloted prior to its use. Collected data included study characteristics, patient demographics, data on the learning curve, and complications both intra-operative and post-operative. Learning curve data was extracted from figures using WebPlotDigitizer (Version 4.3, Pacifica, CA, USA) to estimate values for individual data points.
Quality assessment
The methodological quality of non-randomized studies was evaluated using the Methodological Index for Non-Randomized Studies (MINORS) criteria [31]. Using the MINORS checklist, non-comparative studies can achieve a maximum score of 16, while comparative studies can achieve a maximum score of 24. No literature currently exists for categorizing MINORS scores; however, we categorized the quality of evidence a priori based on a previous systematic review by our group: <5 indicated very low quality evidence, 6–9 low quality, 10–14 fair quality, and >14 good quality [32].
Statistical analysis
Inter-class correlation (ICC) was calculated to determine agreement between reviewers on MINORS assessments and studies were assigned the mean score in cases when there was disagreement between reviewers. Descriptive statistics including means, standard deviations, and ranges are presented where applicable. Due to the heterogeneity of existing literature and inconsistency in reported outcomes, meta-analysis was unable to be performed.
Results
Study characteristics
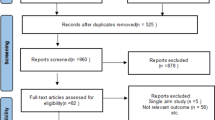
The initial search yielded 2083 studies, 21 of which met the inclusion criteria for this review (Fig. 1) [21, 25, 27,28,29, 33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48]. These studies, which were published between 2008 and 2020, included five retrospective cohort studies (level 3 evidence) and 13 case series (level 4 evidence) (Table 1). The included studies were completed in North America (10 studies), Europe (8 studies), Asia (2 studies), and Australia (1 study). These studies analyzed the learning curves of 63 surgeons. Main methodology and findings of included studies are described in Table 2.
Study quality
There was excellent overall agreement between assessors for quality assessment scores using the MINORS criteria (ICC=0.945, 95% CI: 0.868–0.977). The included studies had an average MINORS score of 14 ± 3 which indicates a fair quality of evidence of included studies.
Patient demographics
There were 9908 patients included, 60% of which were women. Included patients had an average age of 63.5 years (range: 13–94) and body mass index of 27.1 kg/m2 (range: 16.79–58.94). The average length of follow-up was 19 months (range: 1.5–100). Most studies used exclusively cementless stems, but four studies also used cemented stems for some cases (range: 1–5% of cases) [29, 33, 39, 40]. Twelve studies used cementless acetabular components [21, 25, 27, 28, 33, 34, 37,38,39, 41, 43, 48], two used cemented [29, 40], and seven studies did not report the type of acetabular component used [25, 35, 36, 42, 44, 46, 47]. Seven studies used intra-operative fluoroscopy to assist with component positioning [25, 36, 38, 39, 44,45,46]. Osteoarthritis was the most common indication for THA in most studies, with the exception of two in which femoral neck fractures [37] and osteonecrosis [38] were the most common indications for THA.
Learning curve groups and surgeon experience
All studies investigated the learning curve by grouping consecutive cases and comparing outcomes between the groups. Each study chose slightly different grouping variations, but most frequently studies compared the first number cases to subsequent cases or divided their cases into two equal groups. Groups ranged from 15 cases to 100 cases. Pooled analysis of early and late groups was performed when appropriate. Surgeon experience also varied between studies. Most studies investigated the learning curve in experienced surgeons who had performed hundreds of THAs via alternative approaches. One study compared the learning curve of a senior surgeon to junior surgeons, showing that the learning curve of junior surgeons joined that of the senior surgeon after ten cases [25].
Operative time learning curve
Only six studies reported individual case data to depict a true learning curve, with five reporting on operative time [34, 39, 40, 42, 43] and individual studies reporting on blood loss [43], setup time [40], and component placement [48]. Although five studies reported a learning curve for operative time, each study reported these results slightly differently. Individual studies reported on the first 30 cases [39], the first 50 cases [43], the first 100 cases [34], the first 210 cases [40], and the first 1000 patients [42]. Mean operative time for the first case was 156.59 ± 41.71 min but decreased to 93.18 ± 14.68 minutes by case 30 and 80.45 ± 12.28 min by case 100 (Fig. 2). Although mean operative time did not reach a true plateau, it remained less than 90 minutes for all cases after the 100th case.
All studies that evaluated operative time across the learning curve revealed a downward trend as surgeons became more experienced with the procedure. Most studies reported a statistically significant reduction in operative time in the late group compared to the early group [27, 29, 36,37,38,39,40, 45,46,47], though two did not reach significance [21, 42]. Mean operative time for all early and late groups was pooled (see Table 3). The grouping of early DAAs had a mean operating time of 109.5 ± 20.7 min (range: 64.7–135.9), whereas it was 82.6 ± 17.3 min (range: 47.4–113.9) in the late group.
Intra-operative outcomes
Seven studies reported on estimated blood loss across the learning curve [21, 27, 36, 37, 39, 41, 47][21, 27, 36, 37, 39, 40, 47]. Three of these studies demonstrated significant reductions in blood loss from early to late groups [27, 36, 37][27, 36, 37], three showed trends toward blood loss reduction that were not statistically significant [21, 39, 47], and one did not change [40]. When early and late groups were pooled, estimated blood loss decreased from 642.5 ± 219.0 mL (range: 400–1071) to 468.6 ± 115.9 mL (range: 347.5–643). Three studies reported on fluoroscopic time during the procedure [36, 38, 39]. Two studies showed a significant reduction in fluoroscopic time between early and late groups [38, 39] and one showed only a downward trend [36]. Mean fluoroscopic time for the early groups was 19.5 ± 13.7 s (range: 4.8–32.1) and decreased to 11.5 ± 7.5 s (range: 2.86–17.0).
Post-operative outcomes
Five studies reported on duration of hospitalization following DAA [21, 27, 38, 47, 48]. Experience performing DAA did not have a significant impact on length of stay in any of the studies. The mean length of stay for the early group of cases was 4.4 ± 1.7 days (range: 2.5–6.6) compared to 4.0 ± 1.3 days (range: 2.5–5.4) for the late group. Three studies reported on acetabular component placement between early and late procedures, though there was no substantial differential between groups [36, 39, 40]. Mean acetabular inclination of early DAAs was 46.8 ± 4.3° (range: 41.9–50) and 47.5 ± 2.9° (range: 44.4–50.1) for late DAAs. Mean acetabular anteversion was 14.3 ± 1.8° (range: 13–15.6) for early DAAs and 12.9 ± 1.6° (range: 11.7–14) for late DAAs. Six studies reported on leg length discrepancy (LLD) [33, 36, 38,39,40, 44]. The early groups of DAAs had a mean LLD of 3.2 ± 1.4 mm (range: 2–5.04) and late groups of DAAs had a mean LLD of 2.0 ± 1.2 mm (range: 1.07–3.74). Two studies only reported on the number of unacceptable LLDs. One showed a decrease in unacceptable LLDs in the late group from seven to two cases [33] while the other showed no difference between groups [44].
Functional outcomes
Two studies evaluated subjective pain using a visual analogue scale from 0–100 post-operatively, revealing a downward trend of post-operative pain with surgeon experience performing the DAA [29, 48]. Mean pain VAS scores decreased from 21.3 ± 0.5 (range: 20.9–21.6) in early DAAs to 14.2 ± 0.2 (range: 14.0–14.3) in late DAAs. Two studies also compared post-operative Harris Hip Scores (HHS) between early and late groups [36, 38]. Mean HHS did not change significantly, increasing slightly from 89.4 ± 8.8 (range: 83.2–95.7) in early groups to 90.3 ± 8.5 (range: 84.2–96.3) in late groups.
Complications
Eight studies reported increased rates of complications in early groups of DAA procedures compared to late groups [25, 27, 33, 35, 38, 40, 44, 48], while one study did not reveal any difference between groups [46]. Mean complication rate in early groups was 20.8 ± 12.7% (range: 7–44) whereas in late groups it decreased to 7.6 ± 7.1% (range: 0–20) (Fig. 3). Seven studies compared surgical revision rates between early and late procedures [11, 28, 29, 33, 35, 44, 48]. Across early groups, the mean revision rate was 7.1 ± 5.0% (range: 2–15) compared to 1.1 ± 0.9% (range: 0–3) for late groups. Revision rate decreased from early to late groups across all studies. Lateral femoral cutaneous nerve injury was more frequent in early procedures than late, decreasing from 15.0 ± 11.0% (range: 4–25) to 5.0 ± 4.8% (range: 2–12) in late groups [27, 33, 37, 47]. The most common complications during the DAA learning curve were fracture, dislocation, component malposition, and infection [25, 27, 33, 34, 38, 47, 48]. Five-year implant survival was evaluated by three studies, showing a rising trend in implant survival with DAA experience from 85.9 ± 8.7% (range: 78.9–95.6) in early groups to 96.4 ± 0.6% (range: 95.7–96.8) in late groups [34, 35, 41].
Discussion
This systematic review revealed a steep learning curve for the DAA to THA over the first 30 cases and a relative plateau after approximately 100 cases. Mean operative time decreased by more than 50 minutes over the first 30 cases, showing a significant improvement in surgeon skill and comfort with the procedure. This is consistent with previous literature for other orthopaedic procedures, which have often defined the learning curve as the first 30 cases [32, 49, 50]. Although mean operative time was plotted for more than 200 cases, the learning curve never reached a true plateau or inflection point. This suggests that even after performing hundreds of DAA procedures, surgical technique may continue to improve. It should be noted, however, that the learning curve determined by this systematic review only involved the results of two surgeons past 50 cases.
The average operative time for the late groups in this review was 82.6 ± 17.3 minutes. A recent systematic review including 630 675 THA procedures showed that the average operative time for THAs of all approaches was between 90–99 minutes [51]. This review suggests that surgeons who are new to the DAA can reach the average operative time of more traditional THA approaches after approximately 50 cases.
While operative time has been used to evaluate learning curve in many procedures, there is some debate as to the utility of the measure [52, 53]. It is important to consider that there are many factors which may influence operative time, aside from surgeon proficiency. For example, as surgeons become more comfortable performing a procedure, they may take on more technically complex cases which take longer to complete [49]. While operative time is an important outcome with respect to cost-effectiveness of a procedure, small reductions in operative time do not directly lead to patient benefit [52, 54]. Recent literature has shown that each 20 minutes increase in operative time increases the rate of periprosthetic joint infection following total joint arthroplasty by 25% [55]. This suggests that the large reductions in operative time seen early in the learning curve may substantially impact patient outcomes and the small reductions seen past the first 50 cases are less significant. Increased operative time in surgeons who are learning a procedure is likely to represent lack of comfort from the entire surgical team and attention to detail, and thus does not necessarily correlate to patient-centered outcomes such as complication rates [32]. Learning curves may be better evaluated using multiple outcomes rather than operative time alone.
Another key finding from this review was that complication and revision rates showed considerable reductions from early to late groups. Although the specific number of cases needed to significantly reduce complication rates was unable to be determined, the decline in complications and revisions between early and late groups suggests that patient-centered outcomes improve with surgical experience. The average rate of complications in early groups was 20.8 ± 12.7% but ranged from 7 to 44% between individual studies. These complications include fracture, infection, and dislocation, which can require prolonged hospitalization or revision surgery. This trend is also seen in revision rates, five year implant survival, and leg length discrepancy, with improved outcomes as surgeons became more experienced with the procedure. Average rate of revision in the late DAA groups was 1.1 ± 0.9%, which is quite comparable to the rates demonstrated in the literature in alternative approaches beyond the learning curve [19]. The reductions in leg length discrepancy from 3.2 ± 1.4 mm (range: 2–5.04) to 2.0 ± 1.2 mm (range: 1.07–3.74) are especially important, as this is a common reason for litigation and patient dissatisfaction following THA [56, 57]. These increased risks during the learning curve are important for surgeons to discuss with patients when receiving informed consent and consideration should be made to select technically favourable cases as surgeons gain proficiency with the procedure. Female patients with low BMI have been considered technically favourable cases to maximize patient safety during initial procedures [35].
Proper component positioning in THA is important for minimizing the risk of component wear, instability, and impingement, leading to dislocation and revision [58, 59]. Acetabular anteversion is a key aspect of component positioning, with literature showing that ideal anteversion is 15° [60]. Excessive acetabular anteversion is a common concern with the DAA due to the limited view of the anterior acetabular wall [61, 62]. This review showed that mean acetabular anteversion was 14.3 ± 1.8° (range: 13–15.6) for early DAAs and 12.9 ± 1.6° (range: 11.7–14) for late DAAs, suggesting that early DAAs are not at risk of excessive anteversion and that with experience anteversion tends to decrease. The use of intra-operative fluoroscopy in seven of the included studies may help explain the precise component positioning seen in this review.
This systematic review was strengthened by its rigorous methodology. This includes a comprehensive search strategy that involved three major databases and criteria designed to be as inclusive as possible. Reviewer bias was minimized by independently completing each stage of the process in duplicate and automatically including any conflicts. This allowed for the inclusion of 21 studies and 63 surgeons.
The findings of this review are limited by the overall low quality of evidence of included studies. Despite the broad search strategy utilized, the highest level of evidence was level 3 and most included studies were case series. The lack of consecutive case data presented in the included studies was another challenge for this review. Most included studies divided patients into distinct groups of patients with significant heterogeneity between groups. This heterogeneity made it difficult to draw conclusions about the slope of the learning curve beyond early versus late procedures. This limited our ability to analyze the early learning curve for outcomes other than operative time and prevented us from identifying the point at which surgeons reach proficiency.
Future studies should continue to investigate the learning curve beyond the first 50 cases to better characterize the point at which a plateau is reached, suggesting that mastery of the procedure has been achieved. Furthermore, including patient-reported outcomes in these learning curve studies, such as pain and functional scores, would clarify the impact of the DAA learning curve on patient benefit. By reporting continuous case data, future studies could improve the understanding of the learning curve and allow for the integration of risk mitigation strategies as surgeons transition to the procedure.
The DAA is a minimally invasive approach to THA that optimizes post-operative outcomes but is technically complex and has a significant learning curve. This learning curve means that there are increased risks for patients undergoing the procedure by surgeons who are new to the procedure. Operative time reached a relative plateau after approximately 100 cases, suggesting that it takes 100 cases for surgeons to develop proficiency in the DAA to THA.
Data and materials availability
This submission represents original work that has not been previously published and is not under consideration for publication elsewhere.
References
Wier L, Pfunter A, Maeda J, et al (2011) HCUP facts and figures: statistics on hospital-based care in the United States, 2009. Agency for Healthcare Research and Quality, Rockville, MD
Canadian Institute for Health Information (2019) Hip and knee replacements in Canada, 2017–2018: Canadian Joint Replacement Registry Annual Report. CIHI, Ottawa, ON
Singh JA, Yu S, Chen L, Cleveland JD (2019) Rates of total joint replacement in the United States: future projections to 2020-2040 using the national inpatient sample. J Rheumatol 46:1134–1140. https://doi.org/10.3899/jrheum.170990
Mikhail CM, Schwartz JT, Barbera J et al (2020) The most influential papers in direct anterior approach to total hip arthroplasty. Arthroplast Today 6:190–195. https://doi.org/10.1016/j.artd.2020.01.006
Rachbauer F, Kain MSH, Leunig M (2009) The history of the anterior approach to the hip. Orthop Clin North Am 40:311–320. https://doi.org/10.1016/j.ocl.2009.02.007
Smith-Petersen M (1917) A new supra-articular subperiosteal approach to the hip joint. J Bone Jt Surg s2-15:592–595
Rodriguez JA, Deshmukh AJ, Rathod PA et al (2014) Does the direct anterior approach in THA offer faster rehabilitation and comparable safety to the posterior approach? Clin Orthop Relat Res 472:455–463. https://doi.org/10.1007/s11999-013-3231-0
Goebel S, Steinert AF, Schillinger J et al (2012) Reduced postoperative pain in total hip arthroplasty after minimal-invasive anterior approach. Int Orthop 36:491–498. https://doi.org/10.1007/s00264-011-1280-0
Martusiewicz A, Delagrammaticas D, Harold RE, et al (2019) Anterior versus posterior approach total hip arthroplasty: patient-reported and functional outcomes in the early postoperative period. Hip Int Epub ahead of print. https://doi.org/10.1177/1120700019881413
Christensen CP, Jacobs CA (2015) Comparison of patient function during the first six weeks after direct anterior or posterior total hip arthroplasty (THA): a randomized study. J Arthroplasty 30:94–97. https://doi.org/10.1016/j.arth.2014.12.038
Berend KR, Lombardi AV, Seng BE, Adams JB (2009) Enhanced early outcomes with the anterior supine intermuscular approach in primary total hip arthroplasty. J Bone Jt Surg 91:107–120. https://doi.org/10.2106/JBJS.I.00525
Zhao HY, De Kang P, Xia YY et al (2017) Comparison of early functional recovery after total hip arthroplasty using a direct anterior or posterolateral approach: a randomized controlled trial. J Arthroplasty 32:3421–3428. https://doi.org/10.1016/j.arth.2017.05.056
Charney M, Paxton EW, Stradiotto R et al (2020) A comparison of risk of dislocation and cause-specific revision between direct anterior and posterior approach following elective cementless total hip arthroplasty. J Arthroplasty 35:1651–1657. https://doi.org/10.1016/j.arth.2020.01.033
Siguier T, Siguier M, Brumpt B (2004) Mini-incision anterior approach does not increase dislocation rate: a study of 1037 total hip replacements. Clin Orthop Relat Res 426:164–173. https://doi.org/10.1097/01.blo.0000136651.21191.9f
Sariali E, Leonard P, Mamoudy P (2008) Dislocation after total hip arthroplasty using Hueter anterior approach. J Arthroplasty 23:266–272. https://doi.org/10.1016/j.arth.2007.04.003
Crampet C, Common H, Bajeux E et al (2019) Does performing outpatient total hip arthroplasty contribute to early complications and readmissions? Retrospective case-control study of 50 patients. Orthop Traumatol Surg Res 105:1245–1249. https://doi.org/10.1016/j.otsr.2019.07.015
Goyal N, Chen AF, Padgett SE et al (2017) Otto Aufranc Award: a multicenter, randomized study of outpatient versus inpatient total hip arthroplasty. Clin Orthop Relat Res 475:364–372. https://doi.org/10.1007/s11999-016-4915-z
Toy PC, Fournier MN, Throckmorton TW, Mihalko WM (2018) Low rates of adverse events following ambulatory outpatient total hip arthroplasty at a free-standing surgery center. J Arthroplasty 33:46–50. https://doi.org/10.1016/j.arth.2017.08.026
Pincus D, Jenkinson R, Paterson M et al (2020) Association between surgical approach and major surgical complications in patients undergoing total hip arthroplasty. JAMA 323:1070–1076. https://doi.org/10.1001/jama.2020.0785
Brismar BH, Hallert O, Tedhamre A, Lindgren JU (2018) Early gain in pain reduction and hip function, but more complications following the direct anterior minimally invasive approach for total hip arthroplasty: a randomized trial of 100 patients with 5 years of follow up. Acta Orthop 89:484–489. https://doi.org/10.1080/17453674.2018.1504505
Spaans AJ, Van Den Hout JAAM, Bolder SBT (2012) High complication rate in the early experience of minimally invasive total hip arthroplasty by the direct anterior approach. Acta Orthop 83:342–346. https://doi.org/10.3109/17453674.2012.711701
Bhandari M, Matta JM, Dodgin D et al (2009) Outcomes following the single-incision anterior approach to total hip arthroplasty: a multicenter observational study. Orthop Clin North Am 40:329–342. https://doi.org/10.1016/j.ocl.2009.03.001
Luft HS, Bunker JP, Enthoven AC (1979) Should operations be regionalized? N Engl J Med 301:1364–1369. https://doi.org/10.1056/nejm197912203012503
Hopper AN, Jamison MH, Lewis WG (2007) Learning curves in surgical practice. Postgrad Med J 83:777–779. https://doi.org/10.1136/pgmj.2007.057190
Foissey C, Fauvernier M, Fary C, et al (2020) Total hip arthroplasty performed by direct anterior approach – does experience influence the learning curve? Sicot-J 6. https://doi.org/10.1051/sicotj/2020015
Foissey C, Batailler C, Fary C, et al (2020) Transitioning the total hip arthroplasty technique from posterior approach in lateral position to direct anterior approach in supine position—risk factors for acetabular malpositioning and the learning curve. Int Orthop Epub ahead of print. https://doi.org/10.1007/s00264-020-04583-0
van Den Eeden Y, van Den Eeden F (2018) Learning curve of direct anterior total hip arthroplasty: a single surgeon experience. Acta Orthop Belg 84:321–330
de Steiger RN, Lorimer M, Solomon M (2015) What is the learning curve for the anterior approach for total hip arthroplasty? Clin Orthop Relat Res 473:3860–3866. https://doi.org/10.1007/s11999-015-4565-6
Brun OCL, Månsson L, Nordsletten L (2018) The direct anterior minimal invasive approach in total hip replacement: a prospective departmental study on the learning curve. HIP Int 28:156–160. https://doi.org/10.5301/hipint.5000542
Moher D, Shamseer L, Clarke M et al (2015) Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst Rev 4:1–9. https://doi.org/10.1186/2046-4053-4-1
Slim K, Nini E, Forestier D et al (2003) Methodological index for non-randomized studies (Minors): development and validation of a new instrument. ANZ J Surg 73:712–716. https://doi.org/10.1046/j.1445-2197.2003.02748.x
Ekhtiari S, Horner NS, Bedi A et al (2018) The learning curve for the Latarjet procedure: a systematic review. Orthop J Sport Med 6. https://doi.org/10.1177/2325967118786930
Hartford JM, Bellino MJ (2017) The learning curve for the direct anterior approach for total hip arthroplasty: a single surgeon’s first 500 cases. HIP Int 27:483–488. https://doi.org/10.5301/hipint.5000488
Berndt K, Rahm S, Dora C, Zingg PO (2019) Total hip arthroplasty with accolade/trident through the direct minimally invasive anterior approach without traction table: learning curve and results after a minimum of 5 years. Orthop Traumatol Surg Res 105:931–936. https://doi.org/10.1016/j.otsr.2019.05.008
Gofton WT, Ibrahim MM, Kreviazuk CJ et al (2020) Ten-year experience with the anterior approach to total hip arthroplasty at a tertiary care center. J Arthroplasty 35:1281–1289.e1. https://doi.org/10.1016/j.arth.2019.12.025
Goytia RN, Jones LC, Hungerford MW (2012) Learning curve for the anterior approach total hip arthroplasty. J Surg Orthop Adv 21:78–83. https://doi.org/10.3113/JSOA.2012.0078
Kim C-Y, Chung Y-Y, Shim S-W et al (2020) Early experience of direct anterior approach total hip arthroplasty: analysis of the first 53 cases. Hip Pelvis 32:78. https://doi.org/10.5371/hp.2020.32.2.78
Kong X, Grau L, Ong A et al (2019) Adopting the direct anterior approach: experience and learning curve in a Chinese patient population. J Orthop Surg Res 14:1–7. https://doi.org/10.1186/s13018-019-1272-0
Masonis J, Thompson C, Odum S (2008) Safe and accurate: learning the direct anterior total hip arthroplasty. Orthopedics 31:129–134
Melman WPR, Mollen BP, Kollen BJ, Verheyen CCPM (2015) First experiences with the direct anterior approach in lateral decubitus position: Learning curve and 1 year complication rate. HIP Int 25:251–257. https://doi.org/10.5301/hipint.5000221
Müller DA, Zingg PO, Dora C (2014) Anterior minimally invasive approach for total hip replacement: five-year survivorship and learning curve. HIP Int 24:277–283. https://doi.org/10.5301/hipint.5000108
Pirruccio K, Evangelista PJ, Haw J et al (2020) Safely implementing the direct anterior total hip arthroplasty. J Am Acad Orthop Surg:1–7. https://doi.org/10.5435/jaaos-d-19-00752
Pogliacomi F, Paraskevopoulos A, Costantino C et al (2012) Influence of surgical experience in the learning curve of a new approach in hip replacement: anterior mini-invasive vs. standard lateral. HIP Int 22:555–561. https://doi.org/10.5301/HIP.2012.9710
Schwartz BE, Sisko ZW, Mayekar EM et al (2016) Transitioning to the direct anterior approach in total hip arthroplasty: is it safe in the current health care climate? J Arthroplasty 31:2819–2824. https://doi.org/10.1016/j.arth.2016.05.045
Seng BE, Berend KR, Ajluni AF, Lombardi AV (2009) Anterior-supine minimally invasive total hip arthroplasty: defining the learning curve. Orthop Clin North Am 40:343–350. https://doi.org/10.1016/j.ocl.2009.01.002
Stone AH, Sibia US, Atkinson R et al (2018) Evaluation of the learning curve when transitioning from posterolateral to direct anterior hip arthroplasty: a consecutive series of 1000 cases. J Arthroplasty 33:2530–2534. https://doi.org/10.1016/j.arth.2018.02.086
York PJ, Logterman SL, Hak DJ et al (2017) Orthopaedic trauma surgeons and direct anterior total hip arthroplasty: evaluation of learning curve at a level I academic institution. Eur J Orthop Surg Traumatol 27:421–424. https://doi.org/10.1007/s00590-017-1937-5
Zawadsky MW, Paulus MC, Murray PJ, Johansen MA (2014) Early outcome comparison between the direct anterior approach and the mini-incision posterior approach for primary total hip arthroplasty: 150 consecutive cases. J Arthroplasty 29:1256–1260. https://doi.org/10.1016/j.arth.2013.11.013
Hoppe DJ, De Sa D, Simunovic N et al (2014) The learning curve for hip arthroscopy: a systematic review. Arthrosc - J Arthrosc Relat Surg 30:389–397. https://doi.org/10.1016/j.arthro.2013.11.012
Zhang Q, Zhang Q, Guo W et al (2014) The learning curve for minimally invasive Oxford phase 3 unicompartmental knee arthroplasty: cumulative summation test for learning curve (LC-CUSUM). J Orthop Surg Res 9:1–9. https://doi.org/10.1186/s13018-014-0081-8
Cantrell WA, Samuel LT, Sultan AA, et al (2019) Operative times have remained stable for total hip arthroplasty for >15 years systematic review of 630,675 procedures. JBJS Open Access 4. https://doi.org/10.2106/JBJS.OA.19.00047
Darzi A, Smith S, Taffinder N (1999) Assessing operative skill. BMJ 318:887–888
Subramonian K, Muir G (2004) The “learning curve” in surgery: what is it, how do we measure it and can we influence it? BJU Int 93:1173–1174. https://doi.org/10.1111/j.1464-410X.2004.04890
Childers CP, Maggard-Gibbons M (2018) Understanding costs of care in the operating room. JAMA Surg 153. https://doi.org/10.1001/jamasurg.2017.6233
Wang Q, Goswami K, Shohat N et al (2019) Longer operative time results in a higher rate of subsequent periprosthetic joint infection in patients undergoing primary joint arthroplasty. J Arthroplasty 34:947–953. https://doi.org/10.1016/j.arth.2019.01.027
Upadhyay A, York S, Macaulay W et al (2007) Medical malpractice in hip and knee arthroplasty. J Arthroplasty 22. https://doi.org/10.1016/j.arth.2007.05.003
Wylde V, Whitehouse SL, Taylor AH et al (2009) Prevalence and functional impact of patient-perceived leg length discrepancy after hip replacement. Int Orthop 33:905–909. https://doi.org/10.1007/s00264-008-0563-6
Barrack RL, Krempec JA, Clohisy JC et al (2013) Accuracy of acetabular component position in hip arthroplasty. J Bone Jt Surg 95:1760–1768. https://doi.org/10.2106/JBJS.L.01704
Jolles BM, Zangger P, Leyvraz PF (2002) Factors predisposing to dislocation after primary total hip arthroplasty: a multivariate analysis. J Arthroplasty 17:282–288. https://doi.org/10.1054/arth.2002.30286
Elkins JM, Callaghan JJ, Brown TD (2015) The 2014 Frank Stinchfield Award: the ‘landing zone’ for wear and stability in total hip arthroplasty is smaller than we thought: a computational analysis. Clin Orthop Relat Res 473:441–452. https://doi.org/10.1007/s11999-014-3818-0
Kobayashi H, Homma Y, Baba T et al (2016) Surgeons changing the approach for total hip arthroplasty from posterior to direct anterior with fluoroscopy should consider potential excessive cup anteversion and flexion implantation of the stem in their early experience. Int Orthop 40:1813–1819. https://doi.org/10.1007/s00264-015-3059-1
Barton C, Kim PR (2009) Complications of the direct anterior approach for total hip arthroplasty. Orthop Clin North Am 40:371–375. https://doi.org/10.1016/j.ocl.2009.04.004
Author information
Authors and Affiliations
Contributions
LN and LG performed study screening and data extraction and assessed study quality. LN and KG drafted the manuscript. SE designed the study and coordinated data extraction and manuscript preparation. VK edited the manuscript and provided key expert input. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval
Ethics approval was not required for this systematic review.
Consent to participate and consent for publication
As this was a systematic review, data from individual participants was not obtained and will not be published.
Competing interests
Author VK is a paid consultant for Stryker Canada and Zimmer Biomet. The other authors have no competing interests to disclose.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Level of Evidence: IV
Appendix
Appendix
MEDLINE | Embase | Web of Science | |
|---|---|---|---|
Search strategy | Total hip arthroplasty Direct anterior approach Direct anterior Anterior approach Smith Petersen Smith-Petersen Learning curve Learning Clinical competence Treatment outcome Experience Motor skills Outcome Outcome assessment Complication Intraoperative complications Postoperative complications 2 OR 3 OR 4 OR 5 OR 6 7 OR 8 OR 9 OR 10 OR 11 OR 12 OR 13 OR 14 OR 15 OR 16 OR 17 1 AND 18 AND 19 | Total hip arthroplasty Direct anterior approach Direct anterior Anterior approach Smith Petersen Smith-Petersen Learning curve Learning Clinical competence Treatment outcome Experience Motor skills Outcome Outcome assessment Complication Intraoperative complications Postoperative complications 2 OR 3 OR 4 OR 5 OR 6 7 OR 8 OR 9 OR 10 OR 11 OR 12 OR 13 OR 14 OR 15 OR 16 OR 17 1 AND 18 AND 19 | (((((direct anterior approach) OR direct anterior) OR anterior approach) OR Smith Petersen) OR Smith-Petersen) AND (total hip arthroplasty) AND (((((((((((learning curve) OR learning) OR clinical competence) OR treatment outcome) OR experience) OR motor skills) OR outcome) OR outcome assessment) OR complication) OR intraoperative complications) OR postoperative complications) |
Number of articles retrieved | 307 | 528 | 947 |
Rights and permissions
About this article
Cite this article
Nairn, L., Gyemi, L., Gouveia, K. et al. The learning curve for the direct anterior total hip arthroplasty: a systematic review. International Orthopaedics (SICOT) 45, 1971–1982 (2021). https://doi.org/10.1007/s00264-021-04986-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00264-021-04986-7